Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Why Some People are Sda-?
3. Which are the Evolutionary Forces Which Led to the Selection of the Sda Phenotypes?
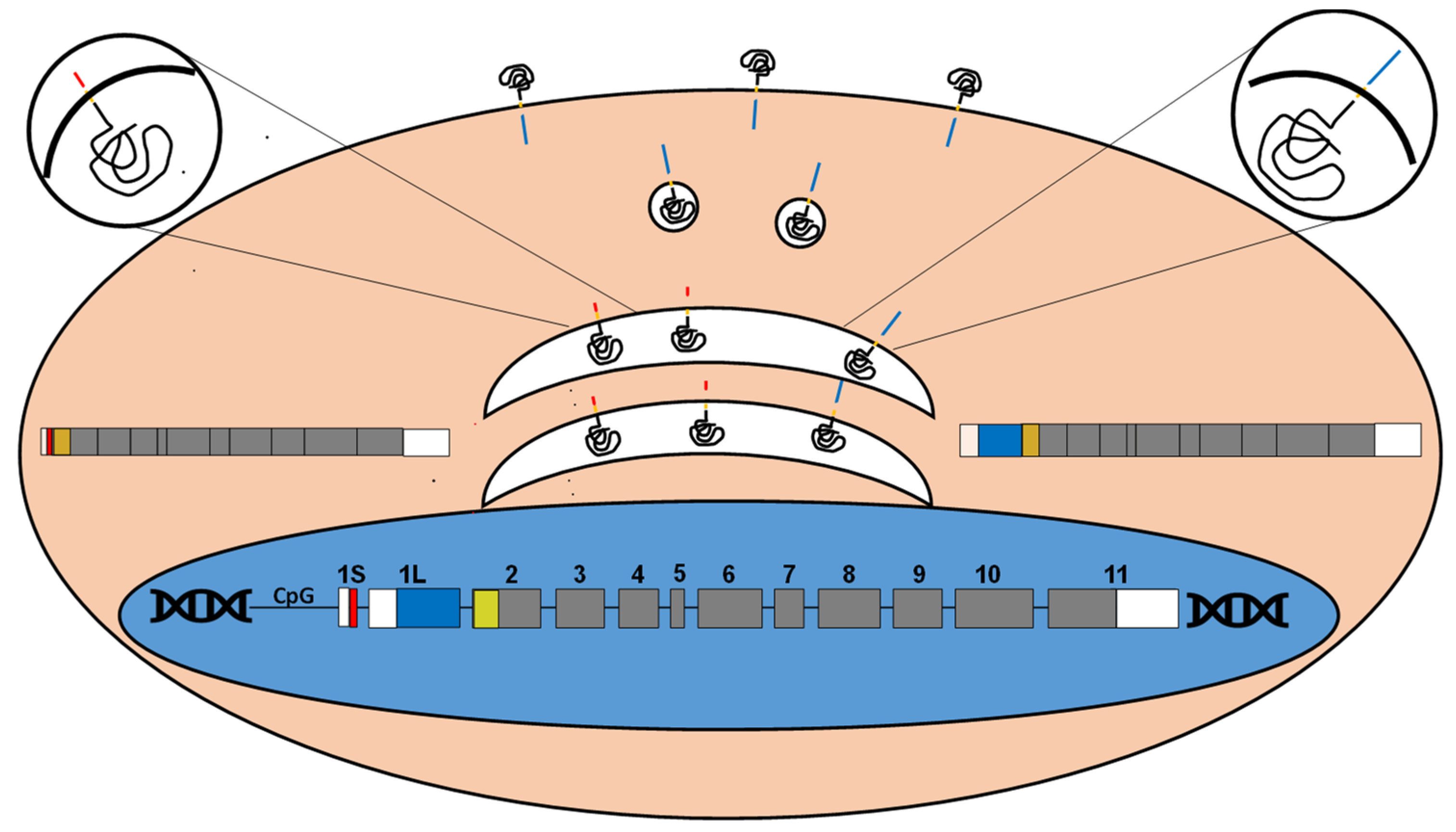
3.1. How B4GALNT2/Sda Regulate Microbe Infections
3.1.1. Viral Infections
3.1.2. Bacterial Infections
3.1.3. Worm Infections
3.2. How B4GALNT2/Sda Antigen Regulates Reproduction
3.2.1. Gametes
3.2.2. Implantation
3.2.3. Regulation of the Mother’s Immune Response
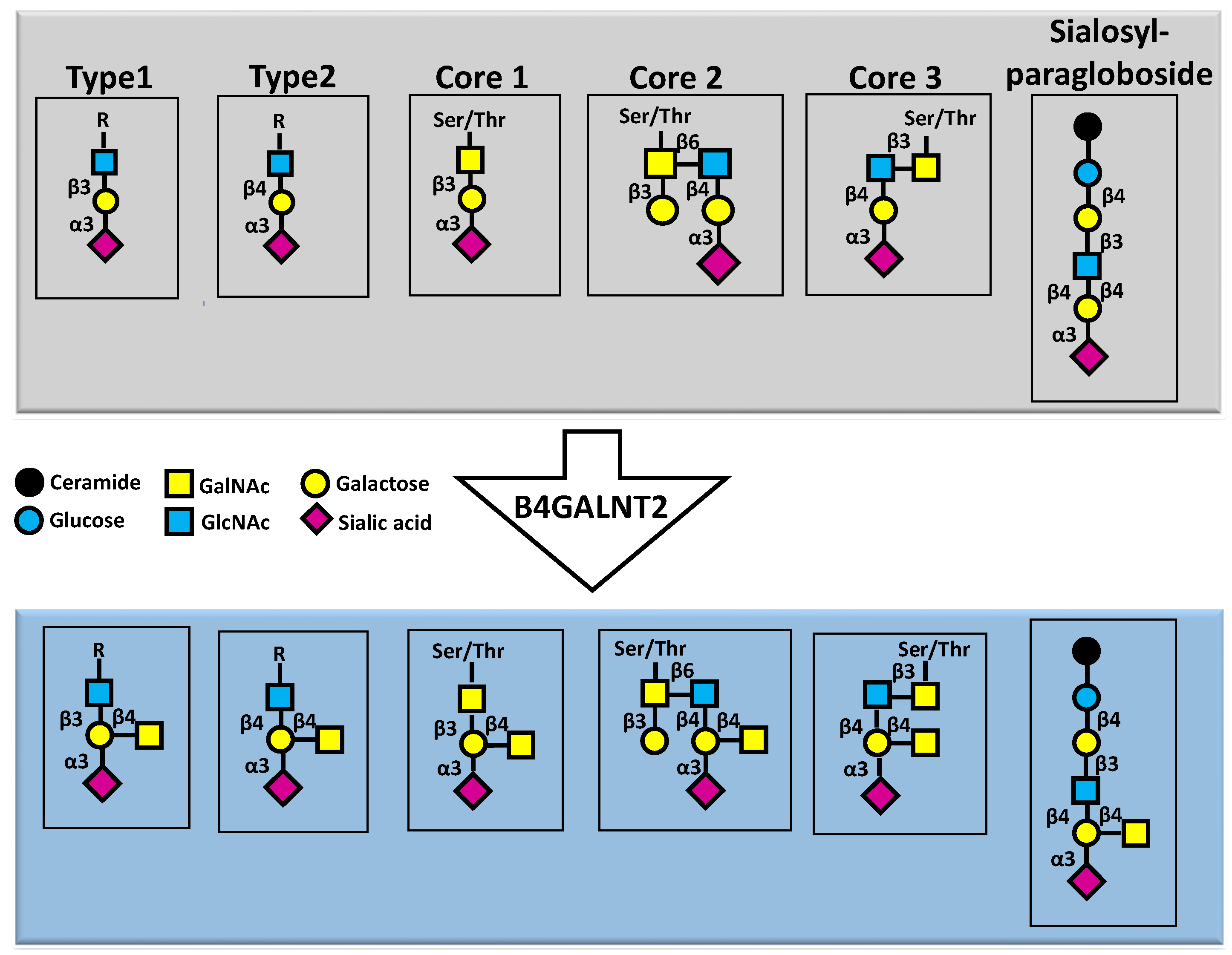
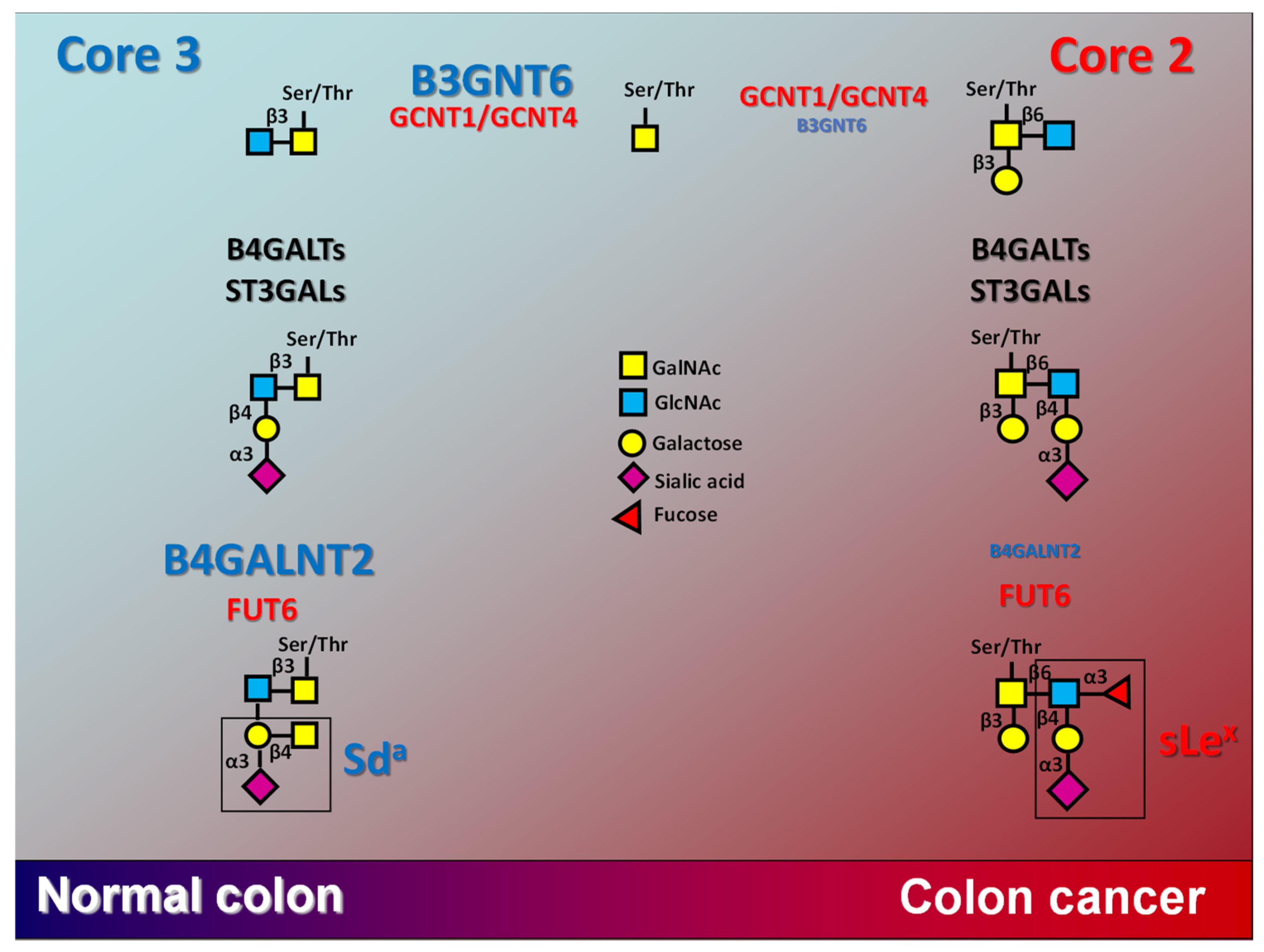
4. How B4GALNT2/Sda Play a Role in Cancer
5. How B4GALNT2/Sda Could Cure Duchene Muscular Dystrophy
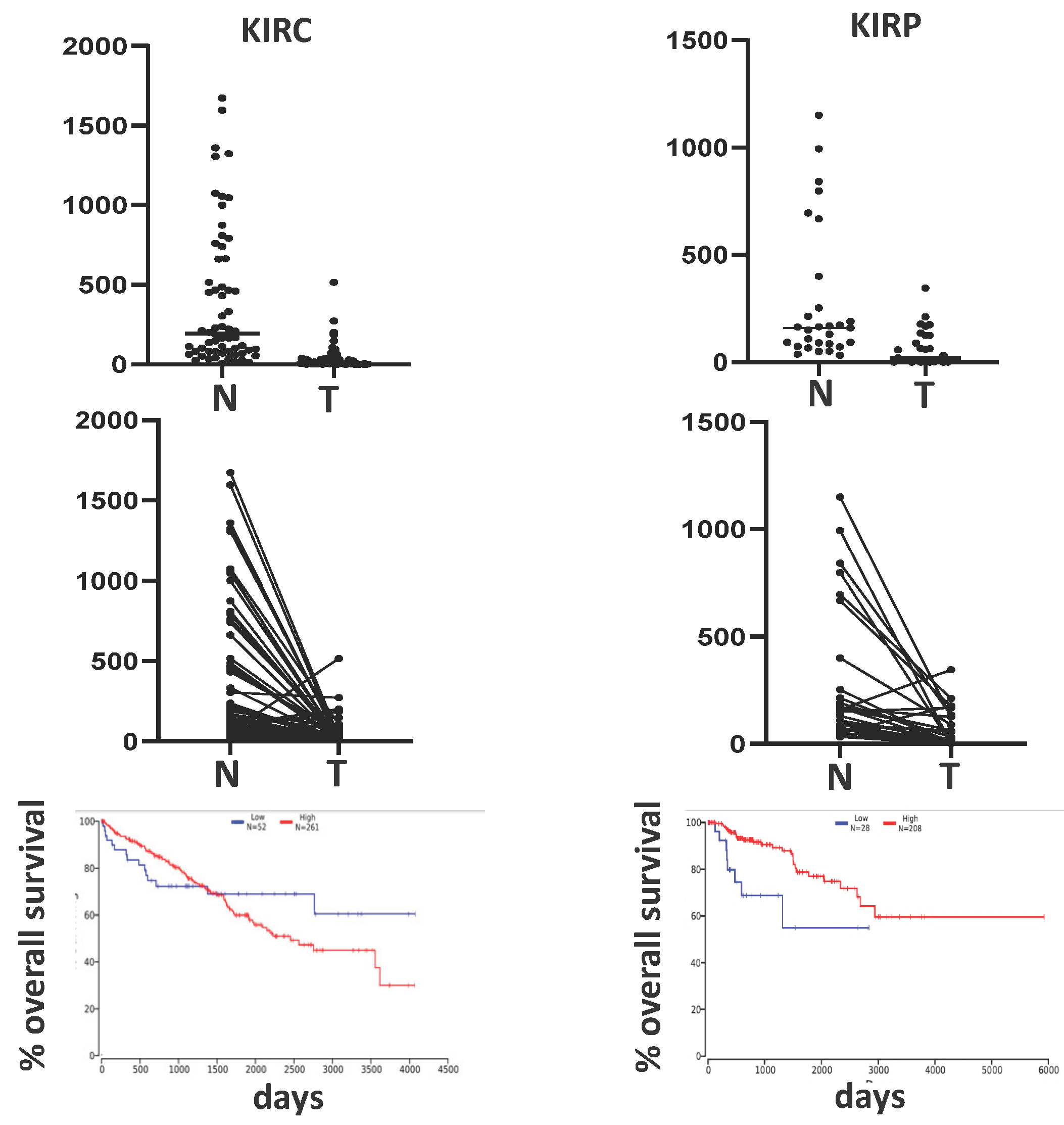
6. How B4GALNT2/Sda May Play a Role in Kidney Disease
7. How B4GALNT2/Sda Affects Xenotransplantation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, J.A.; Pickles, M.M.; Terry, A.M. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970, 19, 472–482. [Google Scholar]
- Morton, J.A.; Pickles, M.M.; Vanhegan, R.I. The Sda antigen in the human kidney and colon. Immunol. Invest 1988, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Renton, P.H.; Howell, P.; Ikin, E.W.; Giles, C.M.; Goldsmith, K.L. Anti Sda: a new blood group antibody. Vox Sang. 1967, 13, 493–501. [Google Scholar] [CrossRef]
- Macvie, S.I.; Morton, J.A.; Pickles, M.M. The reactions and inheritance of a new blood group antigen. Vox Sang. 1967, 13, 485–492. [Google Scholar]
- Sanger, R.; Gavin, J.; Tippett, P.; Teesdale, P.; Eldon, K. Plant agglutinin for another human blood-group. Lancet 1971, 1, 1130. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, L.; Hellberg, A.; Olsson, M.L. SID: a new carbohydrate blood group system based on a well-characterized but still mysterious antigen of great pathophysiologic interest. Immunohematology. 2023, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Donald, A.S.; Yates, A.D.; Soh, C.P.; Morgan, W.T.; Watkins, W.M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem. Biophys. Res. Commun. 1983, 115, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Dall'Olio, F.; Malagolini, N.; Chiricolo, M.; Trinchera, M.; Harduin-Lepers, A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta 2014, 1840, 443–453. [Google Scholar] [CrossRef]
- Blanchard, D.; Piller, F.; Gillard, B.; Marcus, D.; Cartron, J.P. Identification of a novel ganglioside on erythrocytes with blood group Cad specificity. J. Biol. Chem. 1985, 260, 7813–7816. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Cessi, F.; Dall'Olio, F. Guinea-pig kidney β-N-acetylgalactosaminyltransferase towards Tamm- Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem. J. 1983, 215, 483–489. [Google Scholar] [CrossRef]
- Smith, P.L.; Lowe, J.B. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J. Biol. Chem. 1994, 269, 15162–15171. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Cabuy, E.; Chiricolo, M.; Dall'Olio, F. Molecular Cloning of the Human β1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. (Tokyo) 2003, 134, 675–682. [Google Scholar] [CrossRef]
- Montiel, M.D.; Krzewinski-Recchi, M.A.; Delannoy, P.; Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acα2-3Galβ-R β1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003, 373, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Schulz, C.; Cogez, V.; Noel, M.; Portier, L.; Vicogne, D.; Solorzano, C.; Dall'Olio, F.; Steenackers, A.; Mortuaire, M.; Gonzalez-Pisfil, M.; Henry, M.; Foulquier, F.; Heliot, L.; Harduin-Lepers, A. The extended cytoplasmic tail of the human B4GALNT2 is critical for its Golgi targeting and post-Golgi sorting. FEBS J. 2018, 285, 3442–3463. [Google Scholar] [CrossRef] [PubMed]
- Cogez, V.; Vicogne, D.; Schulz, C.; Portier, L.; Venturi, G.; de, R.J.; Decloquement, M.; Lensink, M.F.; Brysbaert, G.; Dall'Olio, F.; Groux-Degroote, S.; Harduin-Lepers, A. N-Glycan on the Non-Consensus N-X-C Glycosylation Site Impacts Activity, Stability, and Localization of the Sda Synthase B4GALNT2. Int. J. Mol. Sci. 2023, 24, 4139. [Google Scholar] [CrossRef]
- Kawamura, Y.I.; Toyota, M.; Kawashima, R.; Hagiwara, T.; Suzuki, H.; Imai, K.; Shinomura, Y.; Tokino, T.; Kannagi, R.; Dohi, T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 2008, 135, 142–151. [Google Scholar] [CrossRef]
- Wang, H.R.; Hsieh, C.Y.; Twu, Y.C.; Yu, L.C. Expression of the human Sda β-1,4-N-acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology 2008, 18, 104–113. [Google Scholar] [CrossRef]
- Dall'Olio, F.; Pucci, M.; Malagolini, N. The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation. Cancers. (Basel) 2021, 13, 5273. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Malagolini, N.; Dall'Olio, F. Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. Int. J. Mol. Sci. 2021, 22, 4331. [Google Scholar] [CrossRef]
- Wavelet-Vermuse, C.; Groux-Degroote, S.; Vicogne, D.; Cogez, V.; Venturi, G.; Trinchera, M.; Brysbaert, G.; Krzewinski-Recchi, M.A.; Bachir, E.H.; Schulz, C.; Vincent, A.; van, S.; Harduin-Lepers, A. Analysis of the proximal promoter of the human colon-specific B4GALNT2 (Sda synthase) gene: B4GALNT2 is transcriptionally regulated by ETS1. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864. 194747. S1874-9399(21)00065-1 [pii]. [Google Scholar] [CrossRef]
- Duca, M.; Malagolini, N.; Dall'Olio, F. The story of the Sda antigen and of its cognate enzyme B4GALNT2: What is new? Glycoconj. J. 2023, 40, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, L.; Hellberg, A.; Moller, M.; Thornton, N.; Larson, G.; Olsson, M.L. Missense mutations in the C-terminal portion of the B4GALNT2-encoded glycosyltransferase underlying the Sda- phenotype. Biochem. Biophys. Rep. 2019, 19, 100659. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, L.; Nilsson, J.; Hellberg, A.; Liew, Y.W.; Morrison, J.; Larson, G.; Olsson, M.L. Glycoproteomic and Phenotypic Elucidation of B4GALNT2 Expression Variants in the SID Histo-Blood Group System. Int. J. Mol. Sci. 2022, 23, 3936. [Google Scholar] [CrossRef]
- Heaton, B.E.; Kennedy, E.M.; Dumm, R.E.; Harding, A.T.; Sacco, M.T.; Sachs, D.; Heaton, N.S. A CRISPR Activation Screen Identifies a Pan-avian Influenza Virus Inhibitory Host Factor. Cell Rep. 2017, 20, 1503–1512. [Google Scholar] [CrossRef]
- Wong, H.H.; Fung, K.; Nicholls, J.M. MDCK-B4GalNT2 cells disclose a α2,3-sialic acid requirement for the 2009 pandemic H1N1 A/California/04/2009 and NA aid entry of A/WSN/33. Emerg. Microbes. Infect. 2019, 8, 1428–1437. [Google Scholar] [CrossRef]
- Park, J.S.; Woo, S.J.; Song, C.S.; Han, J.Y. Modification of surface glycan by expression of β-1,4-N-acetyl-galactosaminyltransferase (B4GALNT2) confers resistance to multiple viruses infection in chicken fibroblast cell. Front Vet. Sci. 2023, 10, 1160600. [Google Scholar] [CrossRef] [PubMed]
- Galeev, A.; Suwandi, A.; Cepic, A.; Basu, M.; Baines, J.F.; Grassl, G.A. The role of the blood group-related glycosyltransferases FUT2 and B4GALNT2 in susceptibility to infectious disease. Int. J. Med. Microbiol. 2021, 311, 151487. [Google Scholar] [CrossRef] [PubMed]
- Staubach, F.; Kunzel, S.; Baines, A.C.; Yee, A.; McGee, B.M.; Backhed, F.; Baines, J.F.; Johnsen, J.M. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME. J. 2012, 6, 1345–1355. [Google Scholar] [CrossRef]
- Vallier, M.; Suwandi, A.; Ehrhardt, K.; Belheouane, M.; Berry, D.; Cepic, A.; Galeev, A.; Johnsen, J.M.; Grassl, G.A.; Baines, J.F. Pathometagenomics reveals susceptibility to intestinal infection by Morganella to be mediated by the blood group-related B4galnt2 gene in wild mice. Gut Microbes. 2023, 15, 2164448. [Google Scholar] [CrossRef] [PubMed]
- Suwandi, A.; Alvarez, K.G.; Galeev, A.; Steck, N.; Riedel, C.U.; Puente, J.L.; Baines, J.F.; Grassl, G.A. B4galnt2-mediated host glycosylation influences the susceptibility to Citrobacter rodentium infection. Front Microbiol. 2022, 13, 980495. [Google Scholar] [CrossRef]
- Johnsen, J.M.; Levy, G.G.; Westrick, R.J.; Tucker, P.K.; Ginsburg, D. The endothelial-specific regulatory mutation, Mvwf1, is a common mouse founder allele. Mamm. Genome 2008, 19, 32–40. [Google Scholar] [CrossRef]
- Johnsen, J.M.; Teschke, M.; Pavlidis, P.; McGee, B.M.; Tautz, D.; Ginsburg, D.; Baines, J.F. Selection on cis-regulatory variation at B4galnt2 and its influence on von Willebrand factor in house mice. Mol. Biol. Evol. 2009, 26, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Linnenbrink, M.; Johnsen, J.M.; Montero, I.; Brzezinski, C.R.; Harr, B.; Baines, J.F. Long-term balancing selection at the blood group-related gene B4galnt2 in the genus Mus (Rodentia; Muridae). Mol. Biol. Evol. 2011, 28, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Cessi, F.; Monti, A.; Cavallone, D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj. J. 2005, 22, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Dall'Olio, F.; Malagolini, N.; Di Stefano, G.; Ciambella, M.; Serafini-Cessi, F. Postnatal development of rat colon epithelial cells is associated with changes in the expression of the β 1,4-N- acetylgalactosaminyltransferase involved in the synthesis of Sda antigen and of α 2,6-sialyltransferase activity towards N-acetyllactosamine. Biochem. J. 1990, 270, 519–524. [Google Scholar] [CrossRef]
- Robbe-Masselot, C.; Maes, E.; Rousset, M.; Michalski, J.C.; Capon, C. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj. J. 2009, 26, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, N.G.; Olson, F.J.; Jovall, P.A.; Andersch, Y.; Enerback, L.; Hansson, G.C. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem. J. 2000, 350, 805–814. [Google Scholar] [CrossRef]
- Holmen, J.M.; Olson, F.J.; Karlsson, H.; Hansson, G.C. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj. J. 2002, 19, 67–75. [Google Scholar] [CrossRef]
- Tsubokawa, D.; Goso, Y.; Kawashima, R.; Ota, H.; Nakamura, T.; Nakamura, K.; Sato, N.; Kurihara, M.; Dohi, T.; Kawamura, Y.I.; Ichikawa, T.; Ishihara, K. The monoclonal antibody HCM31 specifically recognises the Sda tetrasaccharide in goblet cell mucin. FEBS Open. Bio 2012, 2, 223–233. [Google Scholar] [CrossRef]
- Easton, R.L.; Patankar, M.S.; Lattanzio, F.A.; Leaven, T.H.; Morris, H.R.; Clark, G.F.; Dell, A. Structural Analysis of Murine Zona Pellucida Glycans. Evidence for the expression of core 2-type o-glycans and the sd(a) antigen. J. Biol. Chem. 2000, 275, 7731–7742. [Google Scholar] [CrossRef]
- Klisch, K.; Contreras, D.A.; Sun, X.; Brehm, R.; Bergmann, M.; Alberio, R. The Sda/GM2-glycan is a carbohydrate marker of porcine primordial germ cells and of a subpopulation of spermatogonia in cattle, pigs, horses and llama. Reproduction. 2011, 142, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Drouilhet, L.; Mansanet, C.; Sarry, J.; Tabet, K.; Bardou, P.; Woloszyn, F.; Lluch, J.; Harichaux, G.; Viguie, C.; Monniaux, D.; Bodin, L.; Mulsant, P.; Fabre, S. The highly prolific phenotype of Lacaune sheep is associated with an ectopic expression of the B4GALNT2 gene within the ovary. PLoS. Genet. 2013, 9, e1003809. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemaa, S.; Ruesche, J.; Sarry, J.; Woloszyn, F.; Lassoued, N.; Fabre, S. The high prolificacy of D'man sheep is associated with the segregation of the FecL(L) mutation in the B4GALNT2 gene. Reprod. Domest. Anim 2019, 54, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Liang, B.; Di, R.; Liu, Q.; Hu, W.; He, X.; Zhang, J.; Zhang, X.; Chu, M. Molecular Cloning of the B4GALNT2 Gene and Its Single Nucleotide Polymorphisms Association with Litter Size in Small Tail Han Sheep. Animals. (Basel) 2018, 8, 160. [Google Scholar] [CrossRef]
- Ji, X.; Cao, Z.; Hao, Q.; He, M.; Cang, M.; Yu, H.; Ma, Q.; Li, X.; Bao, S.; Wang, J.; Tong, B. Effects of New Mutations in BMPRIB, GDF9, BMP15, LEPR, and B4GALNT2 Genes on Litter Size in Sheep. Vet. Sci. 2023, 10, 258. [Google Scholar] [CrossRef]
- Li, P.T.; Liao, C.J.; Wu, W.G.; Yu, L.C.; Chu, S.T. Progesterone-regulated B4galnt2 expression is a requirement for embryo implantation in mice. Fertil. Steril. 2011, 95, 2409. [Google Scholar] [CrossRef]
- Li, P.T.; Liao, C.J.; Yu, L.C.; Wu, W.G.; Chu, S.T. Localization of B4GALNT2 and its role in mouse embryo attachment. Fertil. Steril. 2012, 97, 1206–1212. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, F.; Freitag, N.; Borowski, S.; Wang, Y.; Harms, C.; Pang, P.C.; Desforges, J.; Wen, T.; Schwedhelm, E.; Singh, M.; Dechend, R.; Dell, A.; Haslam, S.M.; Dveksler, G.; Garcia, M.G.; Blois, S.M. Maternal-derived galectin-1 shapes the placenta niche through Sda terminal glycosylation: Implication for preeclampsia. PNAS. Nexus. 2023, 2, ad247. pgad247. [Google Scholar] [CrossRef]
- Lee, C.L.; Pang, P.C.; Yeung, W.S.; Tissot, B.; Panico, M.; Lao, T.T.; Chu, I.K.; Lee, K.F.; Chung, M.K.; Lam, K.K.; Koistinen, R.; Koistinen, H.; Seppala, M.; Morris, H.R.; Dell, A.; Chiu, P.C. Effects of differential glycosylation of glycodelins on lymphocyte survival. J. Biol. Chem. 2009, 284, 15084–15096. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiu, P.C.; Pang, P.C.; Chu, I.K.; Lee, K.F.; Koistinen, R.; Koistinen, H.; Seppala, M.; Morris, H.R.; Tissot, B.; Panico, M.; Dell, A.; Yeung, W.S. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus: evidence from reduced α2-6 sialylation and impaired immunomodulatory activities of pregnancy-related glycodelin-A. Diabetes 2011, 60, 909–917. [Google Scholar] [CrossRef]
- Malagolini, N.; Dall'Olio, F.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Expression of UDP-GalNAc:NeuAc α2,3Gal β-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989, 49, 6466–6470. [Google Scholar] [PubMed]
- Dohi, T.; Yuyama, Y.; Natori, Y.; Smith, P.L.; Lowe, J.B.; Oshima, M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer 1996, 67, 626–631. [Google Scholar] [CrossRef]
- Malagolini, N.; Santini, D.; Chiricolo, M.; Dall'Olio, F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology 2007, 17, 688–697. [Google Scholar] [CrossRef]
- Robbe-Masselot, C.; Herrmann, A.; Maes, E.; Carlstedt, I.; Michalski, J.C.; Capon, C. Expression of a core 3 disialyl-Lex hexasaccharide in human colorectal cancers: a potential marker of malignant transformation in colon. J. Proteome. Res. 2009, 8, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Gomes Ferreira, I.; Orlandani, M.; Malagolini, N.; Ferracin, M.; Dall'Olio, F. High Expression of the Sda Synthase B4GALNT2 Associates with Good Prognosis and Attenuates Stemness in Colon Cancer. Cells 2020, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Low, E.N.D.; Mokhtar, N.M.; Wong, Z.; Raja Ali, R.A. Colonic Mucosal Transcriptomic Changes in Patients with Long-Duration Ulcerative Colitis Revealed Colitis-Associated Cancer Pathways. J. Crohns. Colitis. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Vicogne, D.; Cogez, V.; Schulz, C.; Harduin-Lepers, A. B4GALNT2 Controls Sda and SLex Antigen Biosynthesis in Healthy and Cancer Human Colon. Chembiochem. 2021, 22, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, M.; Aronica, A.; Dall'Olio, F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology (Basel) 2017, 6, 16. [Google Scholar] [CrossRef]
- Capon, C.; Maes, E.; Michalski, J.C.; Leffler, H.; Kim, Y.S. Sda-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J 2001, 358, 657–664. [Google Scholar] [CrossRef]
- Madunic, K.; Mayboroda, O.A.; Zhang, T.; Weber, J.; Boons, G.J.; Morreau, H.; van, V.R.; van, W.T.; Lageveen-Kammeijer, G.S.M.; Wuhrer, M. Specific (sialyl-)Lewis core 2 O-glycans differentiate colorectal cancer from healthy colon epithelium. Theranostics. 2022, 12, 4498–4512. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Wavelet, C.; Krzewinski-Recchi, M.A.; Portier, L.; Mortuaire, M.; Mihalache, A.; Trinchera, M.; Delannoy, P.; Malagolini, N.; Chiricolo, M.; Dall'Olio, F.; Harduin-Lepers, A. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014, 53, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Ohta, S.; Hanai, N.; Yamaguchi, K.; Oshima, M. Sialylpentaosylceramide detected with anti-GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosa. J. Biol. Chem. 1990, 265, 7880–7885. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Duca, M.; Malagolini, N.; Dall'Olio, F. Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models. Cancers. (Basel) 2022, 14, 2128. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Kawashima, R.; Fukunaga, R.; Hirai, K.; Toyama-Sorimachi, N.; Tokuhara, M.; Shimizu, T.; Dohi, T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005, 65, 6220–6227. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Adachi, Y.; Curiel, D.T.; Kawashima, R.; Kannagi, R.; Nishimoto, N.; Dohi, T. Therapeutic adenoviral gene transfer of a glycosyltransferase for prevention of peritoneal dissemination and metastasis of gastric cancer. Cancer Gene Ther. 2014, 21, 427–433. [Google Scholar] [CrossRef]
- Trinchera, M.; Malagolini, N.; Chiricolo, M.; Santini, D.; Minni, F.; Caretti, A.; Dall'Olio, F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011, 43, 130–139. [Google Scholar] [CrossRef]
- Pucci, M.; Gomes, F.; Malagolini, N.; Ferracin, M.; Dall'Olio, F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020, 21, 6558. [Google Scholar] [CrossRef]
- Qusa, M.H.; Abdelwahed, K.S.; Siddique, A.B.; El Sayed, K.A. Comparative Gene Signature of (-)-Oleocanthal Formulation Treatments in Heterogeneous Triple Negative Breast Tumor Models: Oncological Therapeutic Target Insights. Nutrients 2021, 13, 1706. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, L.; Cui, K.; Du, Y.; Zhang, C.; Ma, W.; Guo, J. B4GALNT2 Gene Promotes Proliferation, and Invasiveness and Migration Abilities of Model Triple Negative Breast Cancer (TNBC) Cells by Interacting With HLA-B Protein. Front Oncol. 2021, 11, 722828. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Jayasinha, V.; Xia, B.; Hoyte, K.; Martin, P.T. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc. Natl. Acad. Sci. U. S. A 2002, 99, 5616–5621. [Google Scholar] [CrossRef]
- Xu, R.; Chandrasekharan, K.; Yoon, J.H.; Camboni, M.; Martin, P.T. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am. J. Pathol. 2007, 171, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Camboni, M.; Martin, P.T. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: evidence for a utrophin-independent mechanism. Neuromuscul. Disord. 2007, 17, 209–220. [Google Scholar] [CrossRef]
- Xu, R.; Devries, S.; Camboni, M.; Martin, P.T. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am. J. Pathol. 2009, 175, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Xu, R.; Martin, P.T. B4GALNT2 (GALGT2) Gene Therapy Reduces Skeletal Muscle Pathology in the FKRP P448L Mouse Model of Limb Girdle Muscular Dystrophy 2I. Am. J. Pathol. 2016, 186, 2429–2448. [Google Scholar] [CrossRef] [PubMed]
- Jayasinha, V.; Hoyte, K.; Xia, B.; Martin, P.T. Overexpression of the CT GalNAc transferase inhibits muscular dystrophy in a cleavage-resistant dystroglycan mutant mouse. Biochem. Biophys. Res. Commun. 2003, 302, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.T.; Xu, R.; Rodino-Klapac, L.R.; Oglesbay, E.; Camboni, M.; Montgomery, C.L.; Shontz, K.; Chicoine, L.G.; Clark, K.R.; Sahenk, Z.; Mendell, J.R.; Janssen, P.M. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am. J. Physiol Cell Physiol 2009, 296, C476–C488. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.T.; Zygmunt, D.A.; Ashbrook, A.; Hamilton, S.; Packer, D.; Birch, S.M.; Bettis, A.K.; Balog-Alvarez, C.J.; Guo, L.J.; Nghiem, P.P.; Kornegay, J.N. Short-term treatment of golden retriever muscular dystrophy (GRMD) dogs with rAAVrh74.MHCK7.GALGT2 induces muscle glycosylation and utrophin expression but has no significant effect on muscle strength. PLoS. One. 2021, 16, e0248721. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, K.M.; Vetter, T.A.; Simmons, T.R.; Iammarino, M.; Frair, E.C.; Rinaldi, F.; Chicoine, L.G.; Harris, J.; Cheatham, J.P.; Cheatham, S.L.; Boe, B.; Waldrop, M.A.; Zygmunt, D.A.; Packer, D.; Martin, P.T. A first-in-human phase I/IIa gene transfer clinical trial for Duchenne muscular dystrophy using rAAVrh74.MCK.GALGT2. Mol. Ther. Methods Clin. Dev. 2022, 27, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Y.; Consolato, F.; Schiano, G.; Chong, M.R.; Pietzner, M.; Nguyen, N.Q.H.; Scherer, N.; Biggs, M.L.; Kleber, M.E.; Haug, S.; Gocmen, B.; Pigeyre, M.; Sekula, P.; Steinbrenner, I.; Schlosser, P.; Joseph, C.B.; Brody, J.A.; Grams, M.E.; Hayward, C.; Schultheiss, U.T.; Kramer, B.K.; Kronenberg, F.; Peters, A.; Seissler, J.; Steubl, D.; Then, C.; Wuttke, M.; Marz, W.; Eckardt, K.U.; Gieger, C.; Boerwinkle, E.; Psaty, B.M.; Coresh, J.; Oefner, P.J.; Pare, G.; Langenberg, C.; Scherberich, J.E.; Yu, B.; Akilesh, S.; Devuyst, O.; Rampoldi, L.; Kottgen, A. Genome-wide studies reveal factors associated with circulating uromodulin and its relations with complex diseases. JCI. Insight. 2022, 7, e157035. [Google Scholar] [CrossRef]
- Byrne, G.; Ahmad-Villiers, S.; Du, Z.; McGregor, C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation. 2018, 25, e12394. [Google Scholar] [CrossRef]
- Zhao, C.; Cooper, D.K.C.; Dai, Y.; Hara, H.; Cai, Z.; Mou, L. The Sda and Cad glycan antigens and their glycosyltransferase, beta1,4GalNAcT-II, in xenotransplantation. Xenotransplantation. 2018, 25, e12386. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.W.; Du, Z.; Stalboerger, P.; Kogelberg, H.; McGregor, C.G. Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014, 21, 543–554. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015, 22, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, T.; Du, J.; Xia, Q.; Wang, L.; Chen, S.; Zhu, L.; Pan, D.; Wang, Y.; Chen, G. Both Natural and Induced Anti-Sda Antibodies Play Important Roles in GTKO Pig-to-Rhesus Monkey Xenotransplantation. Front Immunol. 2022, 13, 849711. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Rieblinger, B.; Hein, R.; Sfriso, R.; Zuber, J.; Fischer, A.; Klinger, B.; Liang, W.; Flisikowski, K.; Kurome, M.; Zakhartchenko, V.; Kessler, B.; Wolf, E.; Rieben, R.; Schwinzer, R.; Kind, A.; Schnieke, A. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation. 2020, 27, e12560. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, P.; Butler, J.R.; Blankenship, R.L.; Downey, S.M.; Montgomery, J.B.; Nagai, S.; Estrada, J.L.; Tector, M.F.; Tector, A.J. Immunogenicity of Renal Microvascular Endothelial Cells From Genetically Modified Pigs. Transplantation 2016, 100, 533–537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




