Introduction
Tree rings are natural archives containing encoded information about plant-environment interactions and the climate of the past. This information is (to a large extent) inaccessible to manipulation and monitoring experiments, and dendrochronologists are striving to decipher it to contribute to a better understanding of the climate system, regional and global biogeochemical cycles, and plant functioning. Stable carbon isotope (
12C,
13C) analysis across tree-ring series is among the most advanced dendrochronological tools available today. This tool has (
inter alia) been used to reconstruct leaf intrinsic water-use efficiency (CO
2 uptake relative to H
2O loss,
iWUE), air temperature, solar radiation, air relative humidity, precipitation, and drought over past centuries at various locations worldwide [
1,
2,
3,
4,
5,
6].
Seventy years ago, tree-ring
13C/
12C ratios were measured for the first time [
7,
8]. While early studies analysed whole-wood samples, most recent studies analyse cellulose, a glucose polymer extracted from tree rings to preclude error due to variation in wood composition (arguments given below apply to cellulose but not necessarily to wood) [
6]. Tree-ring cellulose
13C/
12C data are commonly expressed in terms of
13C discrimination,
Δtrc, denoting carbon isotope changes caused by physiological processes [
9]. Current data interpretations invoke a simple mechanistic model accounting for
13C discrimination accompanying two processes: CO
2 diffusion from ambient air into leaf intercellular air spaces (or chloroplasts) and carbon assimilation by rubisco [
6,
10,
11], collectively termed diffusion-rubisco (DR) discrimination [
12]. Carbon assimilated by leaf phospho
enolpyruvate carboxylase (PEPC) is thought to not enter cellulose biosynthesis or significantly affect its isotope composition [
13].
Variation in DR discrimination depends on the ratio of intercellular-to-ambient CO
2 concentration [
10,
14]. Intercellular CO
2 concentration, in turn, varies with the rate of CO
2 supply through leaf stomata and the rate of CO
2 assimilatory demand. Since stomata respond to moisture conditions,
Δtrc correlations with humidity parameters are thought to derive from CO
2-supply-side effects on DR discrimination [
6]. By contrast, CO
2 assimilation responds to air temperature and solar radiation, and corresponding
Δtrc correlations are thought to derive form CO
2-demand-side effects on DR discrimination [
6]. Moreover, there is a mechanistic relationship between DR discrimination and
iWUE [
9,
10] which forms the basis of
iWUE reconstructions from
Δtrc [
6,
11]. Note, all current
Δtrc interpretations assume that DR discrimination governs
Δtrc variation [
6]. Discrimination downstream of rubisco, denoted post-rubisco (PR) discrimination [
12], is consider constant for any given species [
11].
Recently, nuclear magnetic resonance spectroscopy was used (for the first time in dendrochronology) to measure intramolecular
13C discrimination,
Δi’, in glucose extracted across a tree-ring series (
i denotes glucose carbon position, C-1 to C-6) [
12]. This provided 6-fold higher resolution than (whole-molecule)
Δtrc analysis. The dataset was shown to contain multiple independent
13C signals implying that DR discrimination is not the only variable component of
Δtrc [
12]. Follow-up studies elucidated signal properties and put forward theories on the signals’ metabolic origins (see below, [
13,
15,
16]). Here, the relative contribution of intramolecular
13C signals to
Δtrc were estimated by variance component analysis (
SI Appendix, Supporting text T1). Furthermore, the capabilities of intramolecular versus whole-molecule carbon isotope analysis to investigate intramolecular
13C signals were compared by multiple linear regression analysis. The results from these analyses form the basis for a critical reassessment of the classical concepts and practices of carbon-isotope dendrochronology and recommendations on the future research direction of the field.
Intramolecular 13C Signals in Pinus nigra Tree-Ring Glucose
Previously, we measured intramolecular and whole-molecule
13C discrimination of glucose (
Δi’ and
Δglu, respectively) across an annually resolved series of
Pinus nigra tree rings [
12]. The dataset covers the period 1961 to 1995 but lacks measurements for 1977, 1978, 1981, and 1982 (
n = 31*6).
Δi’ was corrected for carbon redistribution by heterotrophic triose phosphate cycling (indicated by prime, 12). Results reported for tree-ring glucose (
Δi’ and
Δglu) presumably apply to tree-ring cellulose (
Δtrc) because the former can be expected to largely derive from the latter.
We analysed
Δ1’,
Δ2’, and
Δ3’ data pertaining to 1961 to 1980 (early period) and 1983 to 1995 (late period) separately, because the
Δ1-2’ and
Δ1-3’ series (denoting arithmetic averages of
Δ1’ and
Δ2’, and
Δ1’ to
Δ3’, respectively) exhibit a change point in 1980 [
16]. This change point was also found in
Δglu, and the variance of
Δglu is four-fold higher during the late compared to the early period (1.47‰ versus 0.3‰, 16). Proposedly, the trees had access to groundwater during the early but not during the late period [
17] causing metabolism affecting
Δ1’ to
Δ3’ to move from a largely homeostatic state into a climate-responsive state [
16]. By contrast, no change point was detected in
Δ4’,
Δ5’, and
Δ6’ or average series thereof [
16]. Therefore, splitting these series was not required.
We (
inter alia) used multiple linear regression modelling to find environmental and physiological covariates of
Δi’ (
SI Appendix, Table S1) and proposed several ecophysiological mechanisms introducing the corresponding
Δi’ signals (
SI Appendix, Figure S1) [
16]. First, air vapour pressure deficit (
VPD) was found to affect both
Δ1’ and
Δ3’ during the late period (
Table 1, 16). This relationship is thought to derive from DR discrimination (in leaves). Additional
13C discrimination by phosphoglucose isomerase (PGI) and/or glucose-6-phosphate dehydrogenase (G6PD) in leaves is thought to account for the stronger effect of
VPD on
Δ1’ compared to
Δ3’. Second, during the late period,
Δ1’ and
Δ2’ are related to
εmet denoting hydrogen isotope fractionation at glucose H
1 and H
2, and
εmet can be substituted by precipitation without losing much of the models’ explanatory power [
16,
17]. These relationships are thought to derive from
13C discrimination by PGI and G6PD in tree stems [
16]. Note, the described
Δ1’ to
Δ3’ models do not work for the early period [
16]. Third, global radiation (
RAD, data available from 1964) and air temperature (
TMP) were found to affect
Δ4’ to
Δ6’ over the entire study period [
16]. These relationships are thought to derive from
13C discrimination in leaves by glyceraldehyde-3-phosphate dehydrogenases (GAPDH) affecting
Δ4’ and enzymes modifying the carbon double bond of phospho
enolpyruvate affecting
Δ5’ and
Δ6’ [
13,
15]. For more comprehensive information about these mechanisms, the reader is referred to previous reports [
12,
13,
15,
16,
18].
Components of Δglu Variation and Implications for Reconstructions Of Intrinsic Water-Use Efficiency
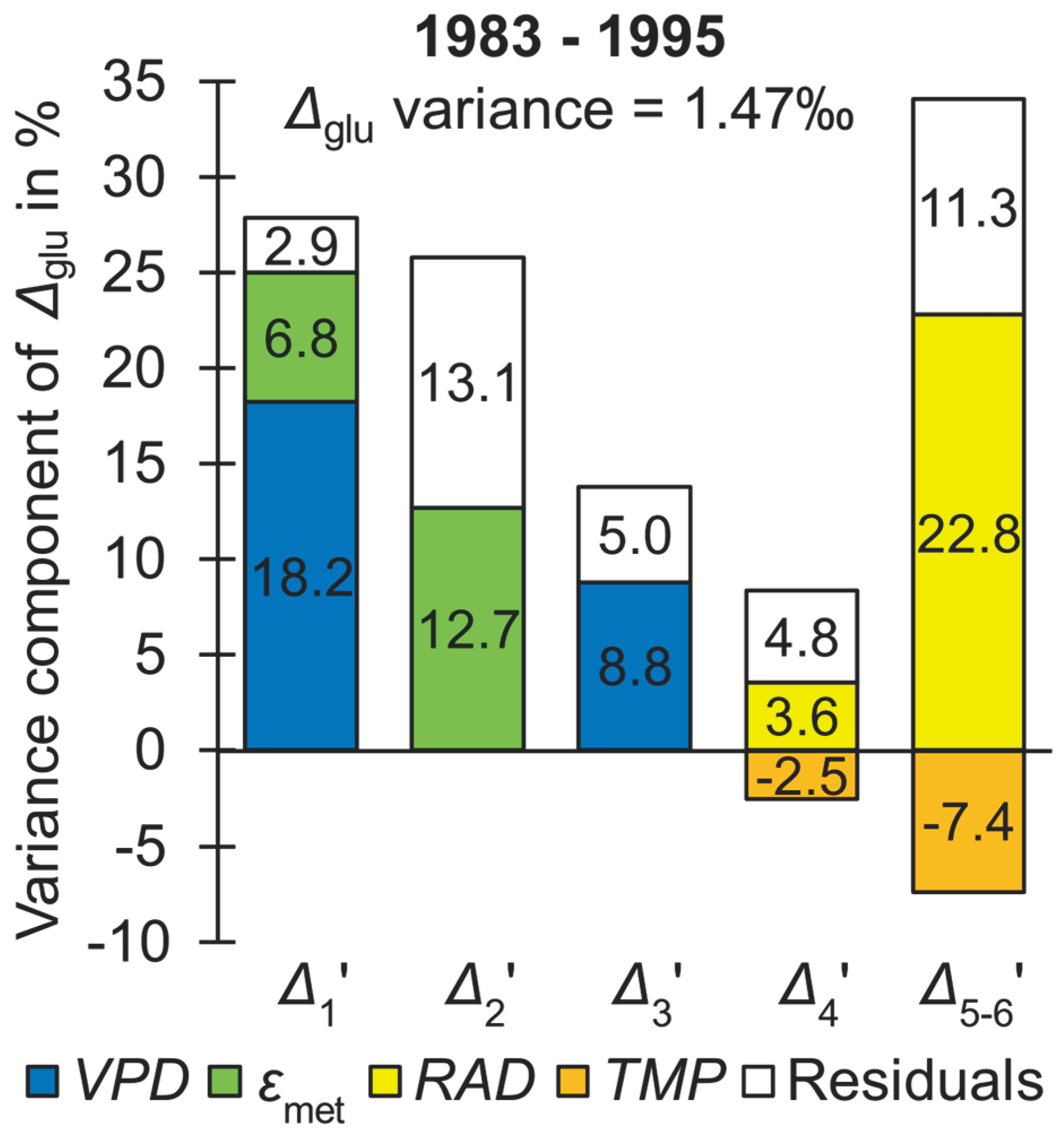
Figure 1 shows percent contributions of intramolecular isotope signals found by modelling (
Table 1 and
Table S1) and model residuals to
Δglu variation for the more dynamic late period (1983 to 1995,
n = 13,
Δglu variance = 1.47‰). Data of
Δi’ have significant measurement errors which largely account for the residuals of the
Δ1’,
Δ3’,
Δ4’, and
Δ5-6’ models (
SI Appendix, Supporting text T2). By contrast, measurement errors account for only ≈30% of the residual variance of the
Δ2’ model. Hence, this model may require extensions to capture the entire systematic variation of
Δ2’. That said, the systematic
Δ2’ variation not captured by modelling accounts for only ≈9% of the total
Δglu variation (13.1*0.7, 16). Disregarding this component, leaf
13C discrimination accounts for ≈43.5% of the total variance of
Δglu while stem
13C discrimination (related to
εmet) accounts for ≈19.5%. Furthermore, the contribution of leaf PR discrimination (≈25.9%) exceeds the contribution of leaf DR discrimination (≈8.8%*2 ≈ 17.6%). Hence, in contrast to its current practical treatment [
11], PR discrimination is neither constant in leaves nor in stems.
iWUE is regarded as an important functional property of plant ecosystems and a key determinant in the response of regional and global carbon, water, and energy cycles to climate change [
19,
20]. Retrospective assessment of
iWUE across large spatiotemporal scales relies on
Δtrc analysis [
1,
2,
3,
4,
5] which, in turn, relies on the assumption that DR discrimination governs
Δtrc variability [
6,
11,
21]. However, DR discrimination accounts for merely ≈17.6% of the total variance of
Δglu during the late period while PR discrimination accounts for ≈45.4% (
Figure 1). Hence, in the present case,
Δtrc should probably not be used as proxy of
iWUE since the
iWUE signal is strongly confounded by other signals. Since the
iWUE signal is better resolved at the intramolecular level,
Δi’ analysis is expected to provide more accurate estimates of
iWUE.
Physiological Interpretation of Δtrc-Climate Relationships
Depending on site characteristics (dry, moist, etc.), various climate parameters may govern Δtrc variability (‘Introduction’, 6). That said, all reported Δtrc-climate relationships are currently interpreted with respect to DR discrimination.
At the dry site discussed here, DR discrimination responds to
VPD [
16]. However, while DR discrimination accounts for ≈17.6% of the total
Δglu variance during the late period,
VPD-dependent PR discrimination accounts for an additional ≈9.4% (
Figure 1). Hence, both DR and PR discrimination contribute to the
VPD signal in
Δglu and their combined contribution accounts for ≈27% of the total
Δglu variance. Interestingly, linear regression between
Δglu and
VPD falsely suggests that
VPD accounts for ≈54% of the total
Δglu variance. This twofold overestimation of the
VPD signal can be expected to result from intercorrelation of
VPD with other climate parameters (e.g., Pearson correlation between
VPD and
RAD:
r = 0.6,
p < 0.05,
n = 13).
More importantly, relationships of
Δglu with
RAD and
TMP derive from leaf-level PR discrimination [
13,
15,
16]. Similarly, relationships of
Δglu with
εmet and
PRE derive from stem-level PR discrimination [
16].
RAD-dependent PR discrimination accounts for ≈48.5% and ≈26.4% of the total
Δglu variance during the early and late period, respectively, and exceeds the contribution of DR discrimination during both periods (
Figure 1;
SI Appendix, Supporting text T3). Hence, most of the climate information in
Δglu derives from PR discrimination and this can be expected to also apply to
Δtrc.
Detecting Intramolecular Isotope Signals at the Whole-Molecule Level
It is highly interesting to ask whether intramolecular isotope analysis outperforms whole-molecule analysis or, more specifically, whether isotope signals detected in
Δi’ can also be found in
Δglu. To test this,
Δglu data of the late period were modelled as function of
VPD,
εmet,
RAD, and
TMP by multiple linear regression. Initially, potential interaction among independent variables was not considered. In the resulting model,
Δglu is not significantly related with
VPD and
TMP (
Table 2, M1). Subsequent models considered interaction among independent variables, and the model with the highest explanatory power is shown in
Table 2 (M2). Again, in this model,
Δglu is not significantly related with
TMP. Hence, some isotope-climate relationships evident at the intramolecular level are invisible to whole-molecule analysis (
cf.
Table 1 and
Table S1, and
Figure 1). Additionally, whole-molecule analysis is blind to the intramolecular location of isotope signals and, therefore, offers no clues on the signals’ metabolic origins. Thus, intramolecular isotope analysis exceeds the capabilities of whole-molecule analysis.
Perspective
Here, recently reported results on the first dataset of intramolecular
13C discrimination in tree rings were synthesised. The picture emerging from this synthesis is not in line with the classical (DR-discrimination-centred) concepts and practices of carbon-isotope dendrochronology and indicates that intramolecular carbon isotope analysis has a significant disruptive potential with respect to the scientific development of the field. It is important to note, however, that all currently proposed physiological interpretations of intramolecular
13C signals require further testing and possibly revisions. Nevertheless, based on the large number of detected intramolecular
13C signals (
Figure 1), the transition from whole-molecule to intramolecular carbon isotope analysis is expected to substantially enhance the amount and quality of information we can retrieve from tree rings. Furthermore, it is expected to substantially change how we interpretate and use tree-ring carbon isotope data.
Since disentangling intramolecular isotope signals by whole-molecule analysis cannot be achieved with sufficient confidence (
Table 2), the field of carbon-isotope dendrochronology is strongly encouraged to enter the intramolecular level. Unfortunately, protocols using nuclear magnetic resonance spectroscopy to measure intramolecular
13C discrimination are labour-intensive and require technology and know-how that is inaccessible to most dendrochronological laboratories. However, protocols enabling such measurements by Orbitrap mass spectrometry are currently under development [
22,
23] and may soon facilitate the widespread use of intramolecular data in dendrochronology, paleoclimatology, biogeochemistry, and plant physiology. This exciting technological development will, in all probability, take our abilities to extract information archived in tree rings to the next level.