This Viewpoint is based on findings by Wieloch et al. (2024).
Introduction
Tree rings are natural archives containing encoded information about plant metabolic processes, their environmental dependences, and the climate of the past. This information is (to a large extent) inaccessible to manipulation and monitoring experiments, and dendrochronologists strive to decipher it to contribute to a better understanding of the climate system, plant functioning, and biogeochemical cycles. Stable carbon isotope (12C, 13C) analysis across tree-ring series is among the most advanced dendrochronological tools available today. This tool has (inter alia) been used to reconstruct leaf intrinsic water-use efficiency (CO2 uptake relative to H2O loss, iWUE), air temperature, solar radiation, relative humidity, precipitation, and drought over past centuries at numerous locations worldwide (Cernusak & Ubierna, 2022; Gagen et al., 2022).
Seventy years ago, tree-ring 13C/12C ratios were measured for the first time (Craig, 1953, 1954). While early studies analysed whole-wood samples, most recent studies analyse cellulose, a glucose polymer extracted from tree rings to preclude error due to variation in wood composition (Helle et al., 2022). Note, arguments given below apply to glucose and cellulose but not necessarily to wood. Tree-ring cellulose 13C/12C data are commonly expressed in terms of 13C discrimination, Δtrc, denoting carbon isotope changes caused by physiological processes (Farquhar & Richards, 1984). Current data interpretation invokes a simplified mechanistic model of 13C discrimination accounting for two processes: CO2 diffusion from ambient air into leaf intercellular air spaces and carbon assimilation by rubisco (Farquhar et al., 1982; McCarroll & Loader, 2004; Cernusak & Ubierna, 2022), combinedly termed diffusion-rubisco (DR) discrimination (Wieloch et al., 2018).
Variation in DR discrimination depends on the ratio of intercellular-to-ambient CO2 concentration (Farquhar et al., 1982; Evans et al., 1986; Voelker et al., 2016). Intercellular CO2 concentration, in turn, varies with the rate of CO2 supply through leaf stomata and the rate of CO2 assimilatory demand. Since stomata respond to moisture conditions, Δtrc correlations with humidity parameters are generally assumed to derive from CO2-supply-side effects on DR discrimination (Gagen et al., 2022). By contrast, CO2 assimilation responds to temperature and solar radiation, and corresponding Δtrc correlations are generally assumed to derive from CO2-demand-side effects on DR discrimination (Gagen et al., 2022). Moreover, there is a mechanistic relationship between DR discrimination and iWUE (Farquhar et al., 1982; Farquhar & Richards, 1984) which forms the basis of iWUE reconstructions from Δtrc (Cernusak & Ubierna, 2022; Saurer & Voelker, 2022). Nota bene, all current Δtrc interpretations assume DR discrimination governs Δtrc variation (Gagen et al., 2022). Discrimination downstream of rubisco, denoted post-rubisco (PR) discrimination (Wieloch et al., 2018), is considered constant for any given species (Gessler et al., 2014; Cernusak & Ubierna, 2022).
Recently, nuclear magnetic resonance spectroscopy was used (for the first time in dendrochronology) to measure intramolecular
13C discrimination,
Δi’, in glucose extracted across a series of tree rings from
Pinus nigra Arnold (
i denotes glucose carbon position C-1 to C-6; Supporting Information Notes S1) (Wieloch
et al., 2018). Data of
Δ1’,
Δ2’, and
Δ3’ pertaining to 1961 to 1980 (
early period) and 1983 to 1995 (
late period) were analysed separately since these series exhibit a change point in 1980 (Wieloch
et al., 2024). Proposedly, the trees had access to groundwater during the early but not the late period (Wieloch
et al., 2022a) causing metabolism affecting
Δ1’ to
Δ3’ to move from a homeostatic to a climate-responsive state (Wieloch
et al., 2024). By contrast, no change point was detected in
Δ4’,
Δ5’, and
Δ6’. Based (
inter alia) on multiple regression modelling, the dataset contains several
13C signals (
Tables 1 and S1,
Figure S1). First, vapour pressure deficit (
VPD) affects both
Δ1’ and
Δ3’ during the late period (Wieloch
et al., 2024). This relationship is thought to derive from DR discrimination. Additional leaf-level
13C discrimination by phosphoglucose isomerase and/or glucose-6-phosphate dehydrogenase is thought to account for the stronger effect of
VPD on
Δ1’ compared to
Δ3’. Second, during the late period,
Δ1’ and
Δ2’ are related to
εmet denoting hydrogen isotope fractionation by metabolic processes at glucose H
1 and H
2, and
εmet can be substituted by precipitation (
PRE) without losing much of the models’ explanatory power (Wieloch
et al., 2022a, 2024). These relationships are thought to derive from
13C discrimination by phosphoglucose isomerase and glucose-6-phosphate dehydrogenase in tree stems (Wieloch
et al., 2024). Note, the described
Δ1’ to
Δ3’ models do not work for the early period (Wieloch
et al., 2024). Third, global radiation (
RAD) and temperature (
TMP) affect
Δ4’ to
Δ6’ over the entire study period (Wieloch
et al., 2024). These relationships are thought to derive from leaf-level
13C discrimination by glyceraldehyde-3-phosphate dehydrogenases affecting
Δ4’ and enzymes modifying the carbon-carbon double bond of phospho
enolpyruvate affecting
Δ5’ and
Δ6’ (Wieloch
et al., 2021, 2022b).
Here, the relative contributions of these intramolecular 13C signals to whole-glucose 13C discrimination (Δglu) were estimated by variance component analysis (Notes S2). Since glucose extracted from tree rings largely derives from cellulose, the results can be expected to also apply to tree-ring cellulose (Δtrc). They are used for a critical assessment of the classical concepts and practices of carbon-isotope dendrochronology. Subsequently, the potential value of intramolecular 13C analysis for constraining impacts of tropospheric ozone on forest metabolism and productivity is discussed. Lastly, it is tested whether intramolecular 13C signals can also be extracted from whole-molecule (Δglu) data.
Components of Δglu Variation and Implications for Reconstructions of Leaf Intrinsic Water-Use Efficiency
Leaf iWUE is regarded as an important functional property of plant ecosystems and a key determinant in the response of biogeochemical cycles to climate change (Beer et al., 2009). Retrospective assessment of iWUE relies on Δtrc analysis which, in turn, relies on the assumption that DR discrimination governs Δtrc variability (Ma et al., 2021; Cernusak & Ubierna, 2022; Saurer & Voelker, 2022). Here, this assumption is critically examined.
-
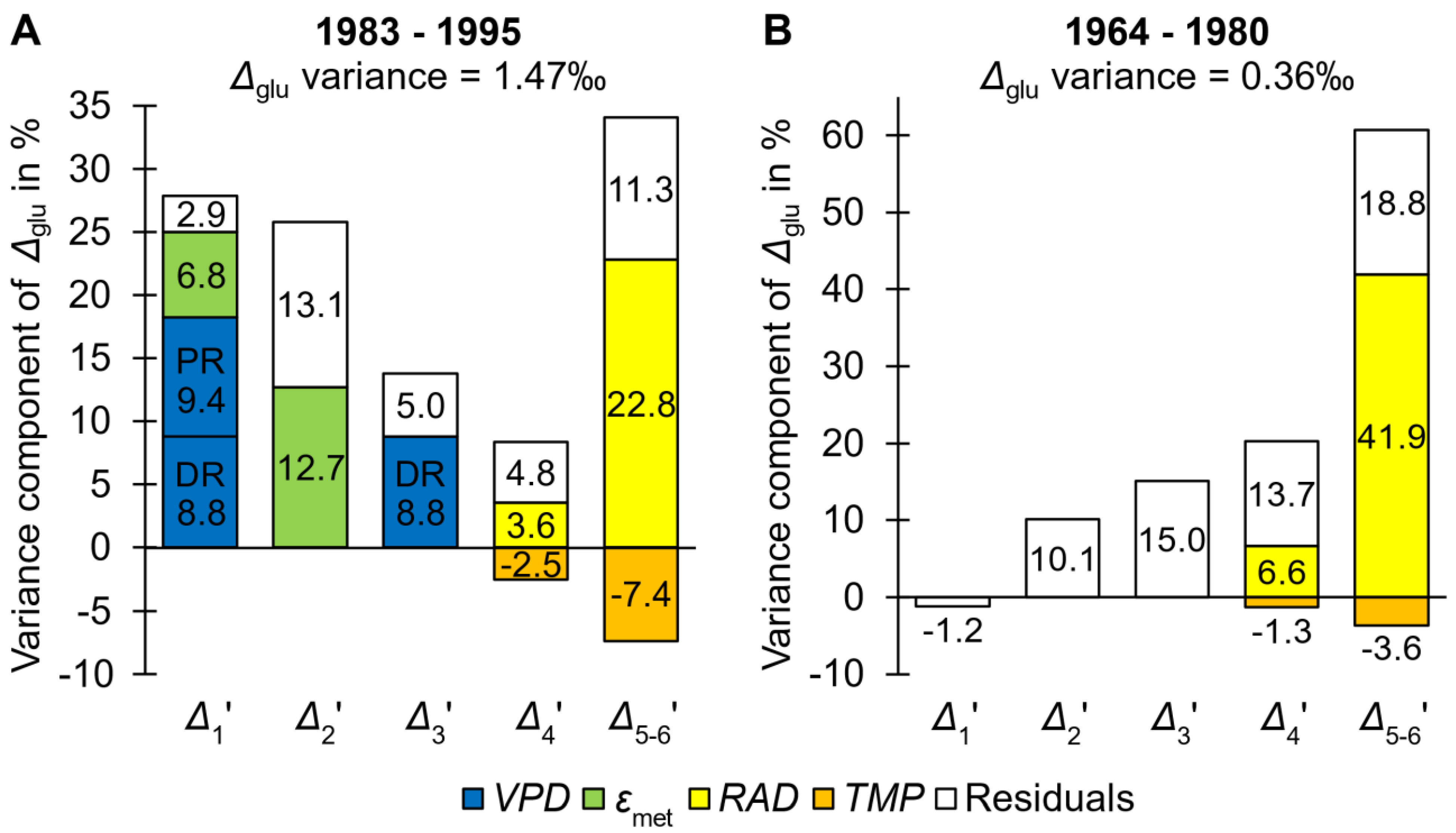
Figure 1A shows percent contributions of intramolecular isotope signals found by modelling and model residuals to
Δglu variation for the more dynamic late period (
Δglu variance = 1.47‰) (Wieloch
et al., 2024). Leaf
13C discrimination accounts for
c. 43.5% of the total
Δglu variance while stem
13C discrimination (related to
εmet) accounts for
c. 19.5%. The rest is residual variance (Notes S3). PR discrimination at the leaf- and stem-level (
c. 25.9% and 19.5%, respectively) each exceed the contribution of DR discrimination (
c. 8.8%×2 = 17.6%).
- Similarly,
Figure 1B shows percent contributions of intramolecular isotope signals found by modelling and model residuals to
Δglu variation for the less dynamic early period (
Δglu variance = 0.36‰). Evidently, the contribution of
Δ1’ to
Δglu is negligible. Moreover, measurement error can account for the entire variation in
Δ2’ (Notes S3). Hence,
Δ1’ and
Δ2’ are not considered further. However,
c. 50% of the total
Δ3’ variance may be systematic unmodelled variance (Notes S3). If we assume this variation results from DR discrimination, then DR discrimination accounts for
c. 7.5% of the total
Δglu variance (
c. 0.5×15) while PR discrimination accounts for
c. 43.6%.
Hence, during both periods, DR discrimination is a comparably small contribution to total Δglu variation which argues against using Δtrc for reconstructions of interannual iWUE variation. Since the iWUE signal is better resolved at the intramolecular level, Δi’ analysis is expected to yield better estimates of iWUE.
Physiological Interpretation of Climate Signals in Δtrc
Currently, all reported
Δtrc-climate relationships are interpreted with respect to DR discrimination (Battipaglia & Cherubini, 2022; Churakova
et al., 2022; Gagen
et al., 2022; van der Sleen
et al., 2022). Thus, consideration is given only to two initial steps in the biosynthesis of tree-ring cellulose whereas
13C discrimination by the numerous reactions downstream of rubisco (PR discrimination) is assumed to be constant (
Figure S1). However, recent reports of multiple intramolecular isotope signals in tree-ring glucose (
Table 1) call for a critical reassessment of this practice.
At the site discussed here, DR discrimination responds to
VPD (for information about the site, see Notes S1 in Wieloch
et al., 2024). However, while DR discrimination accounts for
c. 17.6% of the total variation of
Δglu during the late period,
VPD-dependent PR discrimination accounts for an additional
c. 9.4% (
Figure 1A). Hence, both DR and PR discrimination contribute to the
VPD signal in
Δglu and their combined contribution accounts for
c. 27% of the total
Δglu variance. Interestingly, simple linear regression between
Δglu and
VPD falsely suggests that
VPD accounts for
c. 54% of the total
Δglu variance (
Figure S2). This twofold overestimation of the actual
VPD signal likely results from intercorrelation of
VPD with other climate parameters that also affect
Δglu. For instance,
RAD affects tree-ring glucose C-5 and C-6 (
Figure 1A), and there is intercorrelation between
RAD and
VPD (
r = 0.6,
p < 0.05,
n = 13) which will result in overestimation of the
VPD signal in
VPD-
Δglu simple linear regression.
More importantly, relationships of
Δglu with
RAD and
TMP derive from leaf-level PR discrimination (Wieloch
et al., 2021, 2022b, 2024), and
RAD-dependent PR discrimination alone exceeds the contribution of DR discrimination to
Δglu variation (
Figure 1, early period:
c. 48.5% versus
c. 7.5%, late period:
c. 26.4% versus
c. 17.6%). Similarly, relationships of
Δglu with
εmet and
PRE derive from stem-level PR discrimination (Wieloch
et al., 2024), and
εmet-dependent PR discrimination contributes similarly to
Δglu variation as DR discrimination (
Figure 1A;
c. 19.5% and 17.6%, respectively).
Hence, RAD-, TMP-, PRE-, and a fraction of the VPD-dependent Δglu variation is not caused by DR discrimination and associated physiological processes. Instead, most of the climate information in Δglu derives from PR discrimination and associated physiological processes.
Conclusions and Outlook
The picture emerging here is inconsistent with the classical (DR-discrimination-centred) concepts and practices of carbon-isotope dendrochronology. Evidently, processes downstream of rubisco in leaves and stems introduced most of the isotope signals and variation in the tree-ring series examined. Hence, most of the ecophysiological and climate information in this record relates to PR processes. This opens new and exciting research avenues. First, the isotope signal reflecting iWUE is better resolved at the intramolecular than at the whole-molecule level. Careful separation of this signal from other signals in Δi’ or Δtrc is expected to yield more accurate estimates of iWUE. Second, an isotope signal at tree-ring glucose C-5 and C-6 reports metabolic changes in response to tropospheric ozone. Ozone is known for its severe adverse effects on forest productivity, global carbon cycling, and climate change. Analysing the signal at C-5 and C-6 may help to constrain these effects in natural forest ecosystems. Third, Δi’ analysis gives access to deconvoluted information about multiple climate parameters and is therefore expected to enable distinctly more comprehensive paleoclimate reconstructions than Δtrc analysis, providing an improved baseline for climate predictions (Wieloch et al., 2024). Fourth, recent and future insights into plant 13C discrimination from Δi’ analysis may enable extraction of information about multiple ecophysiological processes from existing Δtrc datasets.
Taken together, Δi’ analysis has significant disruptive potentials regarding the scientific development of the field of carbon-isotope dendrochronology. Unfortunately, measuring Δi’ by nuclear magnetic resonance spectroscopy is labour-intensive and requires technology and know-how inaccessible to most dendrochronological laboratories. However, protocols enabling Δi’ measurements by Orbitrap mass spectrometry are currently under development and may soon make Δi’ data broadly accessible (Neubauer et al., 2023; Dion-Kirschner et al., 2023; Gessler et al., 2024). Moving from whole-molecule to intramolecular tree-ring isotope analysis is comparable to using a more powerful microscope and promises novel information about metabolism and climate across space and time (Wieloch et al., 2018, 2021, 2022b,a, 2024; Gessler et al., 2024).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Data Availability
The author declares that the data supporting the findings of this study are available within the paper and its supporting information.
Acknowledgements
This work was carried out with funding from “Formas - a Swedish Research Council for Sustainable Development” (2022-02833, Grant recipient: TW). I am grateful to Preprints.org (MDPI AG, Basel, Switzerland) for publishing preprints of this paper (
https://doi.org/10.20944/preprints202403.0014.v1).
Competing Interests
None declared.
References
-
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD. 2012. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annual Review of Plant Biology 63: 637–661. [CrossRef]
-
Battipaglia G, Cherubini P. 2022. Stable isotopes in tree rings of mediterranean forests. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 605–629.
-
Beer C, Ciais P, Reichstein M, Baldocchi D, Law BE, Papale D, Soussana J-F, Ammann C, Buchmann N, Frank D, et al. 2009. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Global Biogeochemical Cycles 23: GB2018. [CrossRef]
-
Betz GA, Gerstner E, Stich S, Winkler B, Welzl G, Kremmer E, Langebartels C, Heller W, Sandermann H, Ernst D. 2009. Ozone affects shikimate pathway genes and secondary metabolites in saplings of European beech (Fagus sylvatica L.) grown under greenhouse conditions. Trees 23: 539–553. [CrossRef]
-
Cernusak LA, Ubierna N. 2022. Carbon isotope effects in relation to CO2 assimilation by tree canopies. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 291–310.
-
Churakova OV, Porter TJ, Kirdyanov AV, Myglan VS, Fonti MV, Vaganov EA. 2022. Stable isotopes in tree rings of boreal forests. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 581–603.
-
Craig H. 1953. The geochemistry of the stable carbon isotopes. Geochimica Et Cosmochimica Acta 3: 53–92. [CrossRef]
-
Craig H. 1954. Carbon-13 variations in Sequoia rings and the atmosphere. Science 119: 141–143. [CrossRef]
-
Dion-Kirschner H, KongJohnson C, Sharp K, Dalleska NF, Eiler JM, Sessions AL. 2023. Position-specific carbon isotope analysis of tree ring cellulose via Orbitrap mass spectrometry. AGU Fall Meeting Abstracts: H51H-07. https://agu.confex.com/agu/fm23/meetingapp.cgi/Paper/1409820.
-
Dizengremel P. 2001. Effects of ozone on the carbon metabolism of forest trees. Plant Physiology and Biochemistry 39: 729–742. [CrossRef]
-
Emberson L. 2020. Effects of ozone on agriculture, forests and grasslands. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 378: 20190327.
-
Evans JR, Farquhar GD, Sharkey TD, Berry JA. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Australian Journal of Plant Physiology 13: 281–292.
-
Farquhar GD, O’Leary MH, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology 9: 121–137. [CrossRef]
-
Farquhar GD, Richards RA. 1984. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11: 539–552. [CrossRef]
-
Gagen M, Battipaglia G, Daux V, Duffy J, Dorado-Liñán I, Hayles LA, Martínez-Sancho E, McCarroll D, Shestakova TA, Treydte K. 2022. Climate signals in stable isotope tree-ring records. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 537–579.
-
Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK. 2014. Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiology 34: 796–818. [CrossRef]
-
Gessler A, Wieloch T, Saurer M, Lehmann MM, Werner RA, Kammerer B. 2024. The marriage between stable isotope ecology and plant metabolomics – new perspectives for metabolic flux analysis and the interpretation of ecological archives. New Phytologist 244: 21–31. [CrossRef]
-
Helle G, Pauly M, Heinrich I, Schollän K, Balanzategui D, Schürheck L. 2022. Stable isotope signatures of wood, its constituents and methods of cellulose extraction. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 135–190.
-
Janzik I, Preiskowski S, Kneifel H. 2005. Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco (Nicotiana tabacum L. cv. Bel W3). Planta 223: 20–27. [CrossRef]
-
Ma WT, Tcherkez G, Wang XM, Schäufele R, Schnyder H, Yang Y, Gong XY. 2021. Accounting for mesophyll conductance substantially improves 13C-based estimates of intrinsic water-use efficiency. New Phytologist 229: 1326–1338.
-
McCarroll D, Loader NJ. 2004. Stable isotopes in tree rings. Quaternary Science Reviews 23: 771–801.
-
Neubauer C, Kantnerová K, Lamothe A, Savarino J, Hilkert A, Juchelka D, Hinrichs K-U, Elvert M, Heuer V, Elsner M, et al. 2023. Discovering nature’s fingerprints: Isotope ratio analysis on bioanalytical mass spectrometers. Journal of the American Society for Mass Spectrometry 34: 525–537. [CrossRef]
-
Saurer M, Maurer S, Matyssek R, Landolt W, Günthardt-Goerg MS, Siegenthaler U. 1995. The influence of ozone and nutrition on δ13C in Betula pendula. Oecologia 103: 397–406. [CrossRef]
-
Saurer M, Voelker S. 2022. Intrinsic water-use efficiency derived from stable carbon isotopes of tree-rings. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 481–498.
-
van der Sleen P, Zuidema PA, Pons TL. 2022. Stable isotopes in tree rings of tropical forests. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M, eds. Stable isotopes in tree rings: Inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, 631–649.
-
Voelker SL, Brooks JR, Meinzer FC, Anderson R, Bader MK-F, Battipaglia G, Becklin KM, Beerling D, Bert D, Betancourt JL, et al. 2016. A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Global Change Biology 22: 889–902.
-
Wieloch T, Ehlers I, Yu J, Frank D, Grabner M, Gessler A, Schleucher J. 2018. Intramolecular 13C analysis of tree rings provides multiple plant ecophysiology signals covering decades. Scientific Reports 8: 5048.
-
Wieloch T, Grabner M, Augusti A, Serk H, Ehlers I, Yu J, Schleucher J. 2022a. Metabolism is a major driver of hydrogen isotope fractionation recorded in tree-ring glucose of Pinus nigra. New Phytologist 234: 449–461. [CrossRef]
-
Wieloch T, Holloway-Phillips M, Yu J, Niittylä T. 2024. New insights into the mechanisms of plant isotope fractionation from combined analysis of intramolecular 13C and deuterium abundances in Pinus nigra tree-ring glucose. New Phytologist. [CrossRef]
-
Wieloch T, Sharkey TD, Werner RA, Schleucher J. 2022b. Intramolecular carbon isotope signals reflect metabolite allocation in plants. Journal of Experimental Botany 73: 2558–2575. [CrossRef]
-
Wieloch T, Werner RA, Schleucher J. 2021. Carbon flux around leaf-cytosolic glyceraldehyde-3-phosphate dehydrogenase introduces a 13C signal in plant glucose. Journal of Experimental Botany 72: 7136–7144. [CrossRef]
-
Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. 2009. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology 15: 396–424. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).