Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
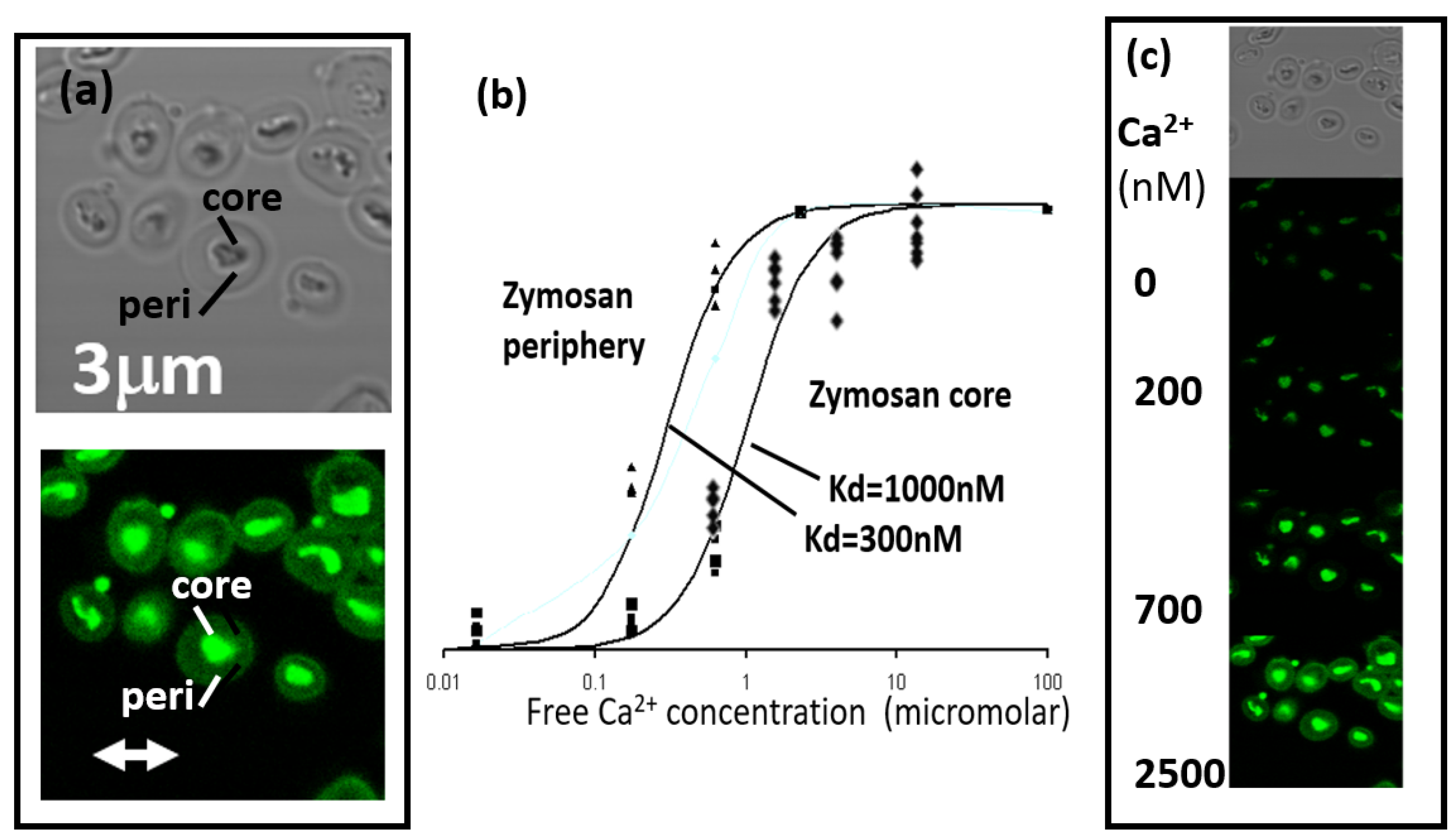
2.1. Fluo4-Zymosan Targets Report Ca2+ Concentration
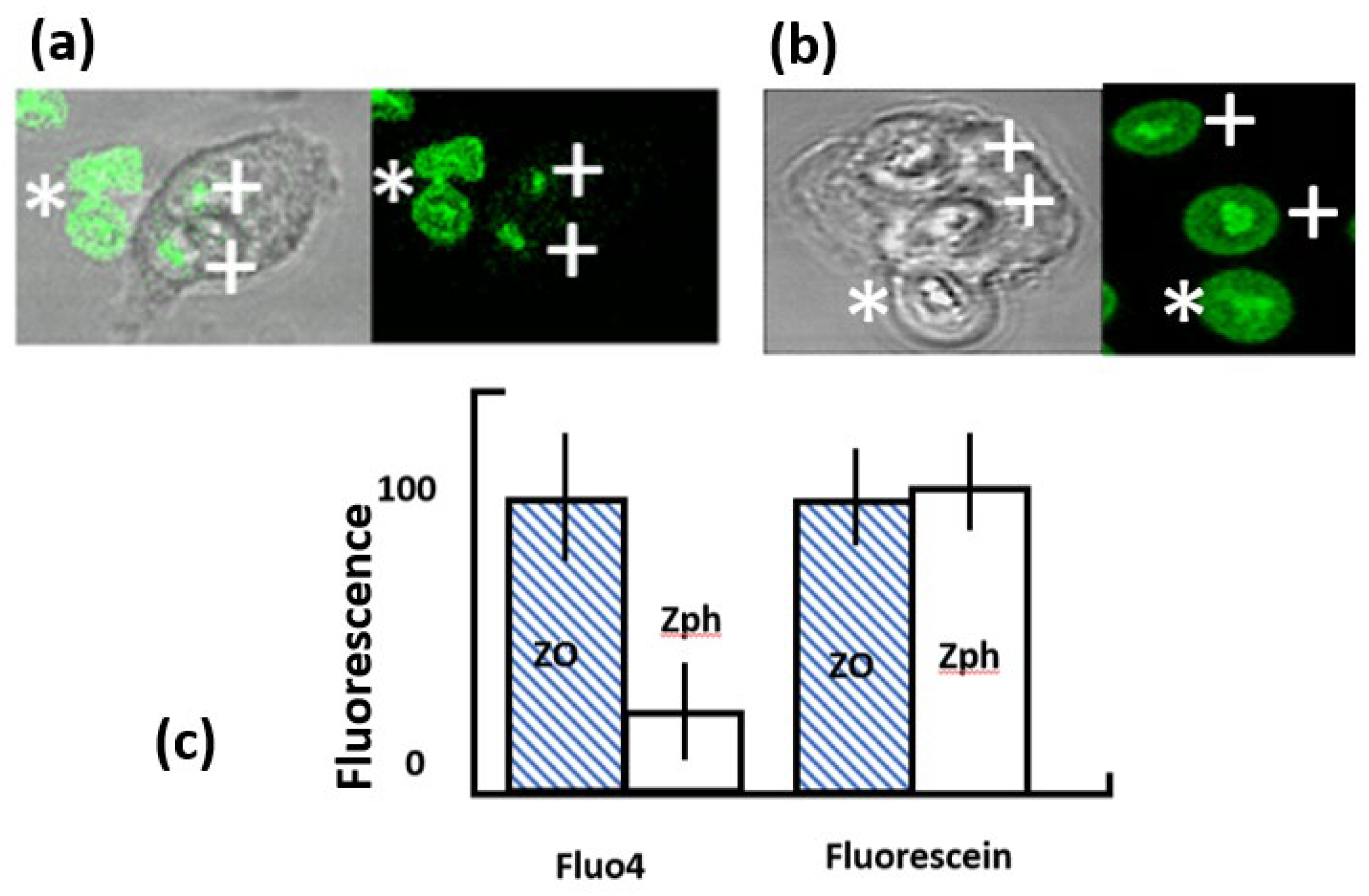
2.2. Phagosomal fluo4 Signal is Decreased
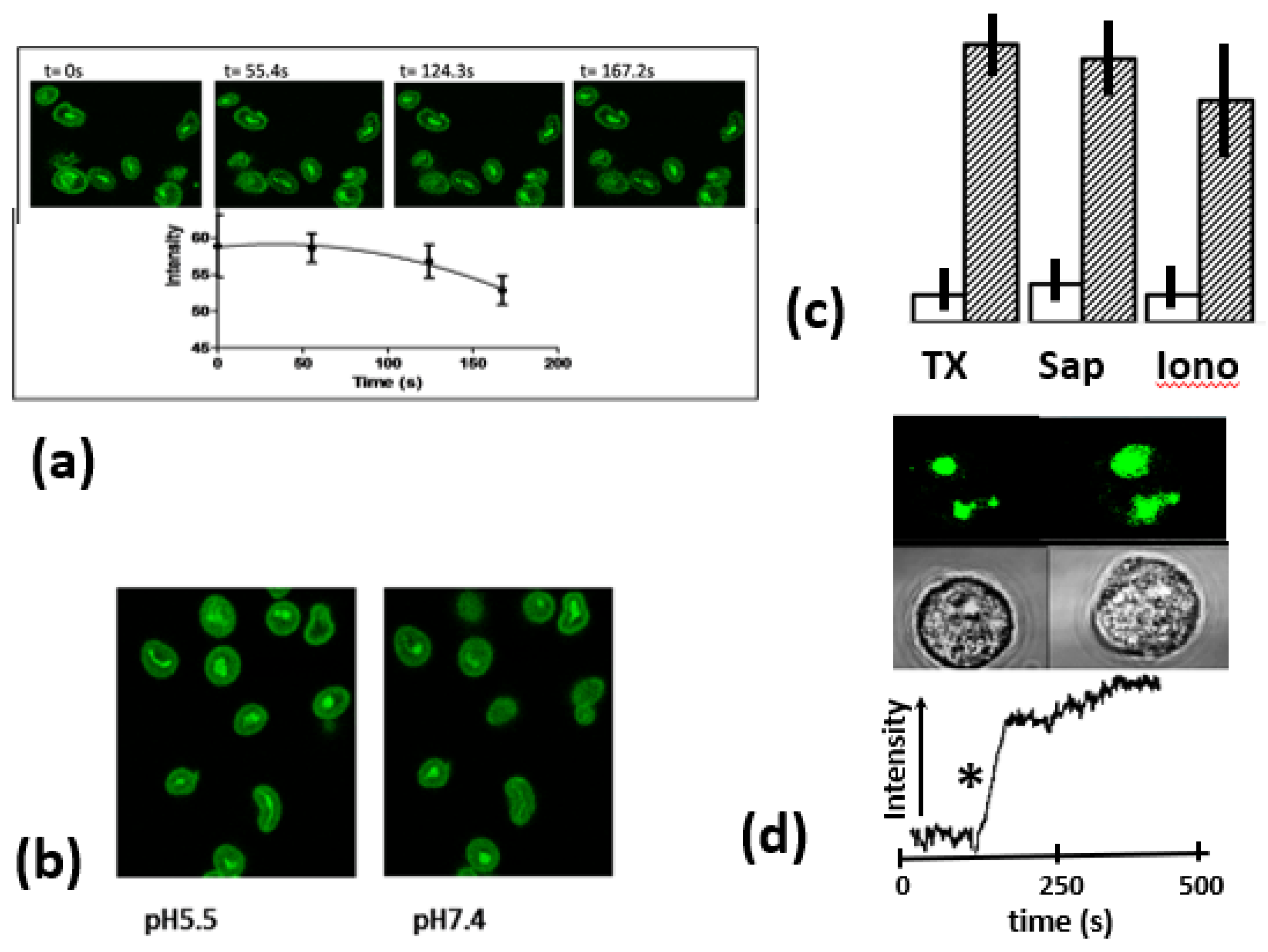
2.3. Phagosomal fluo4 Signal Decrease not due to Oxidants
2.4. Phagosomal fluo4 Zymosan REMAINED Responsive to Ca2+ Changes
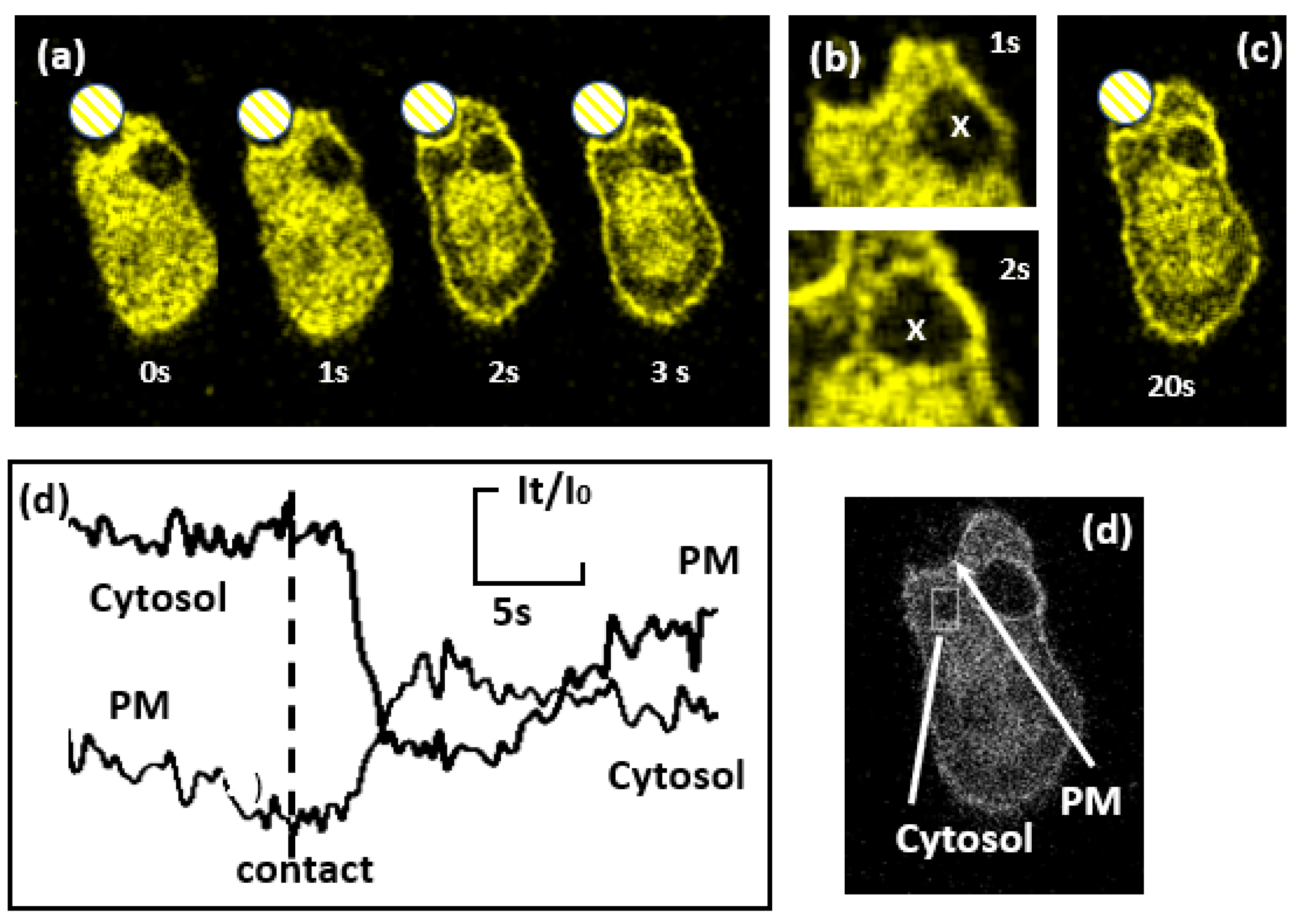
2.5. Kinetics of INTRA-phagosomal Ca2+ Decrease
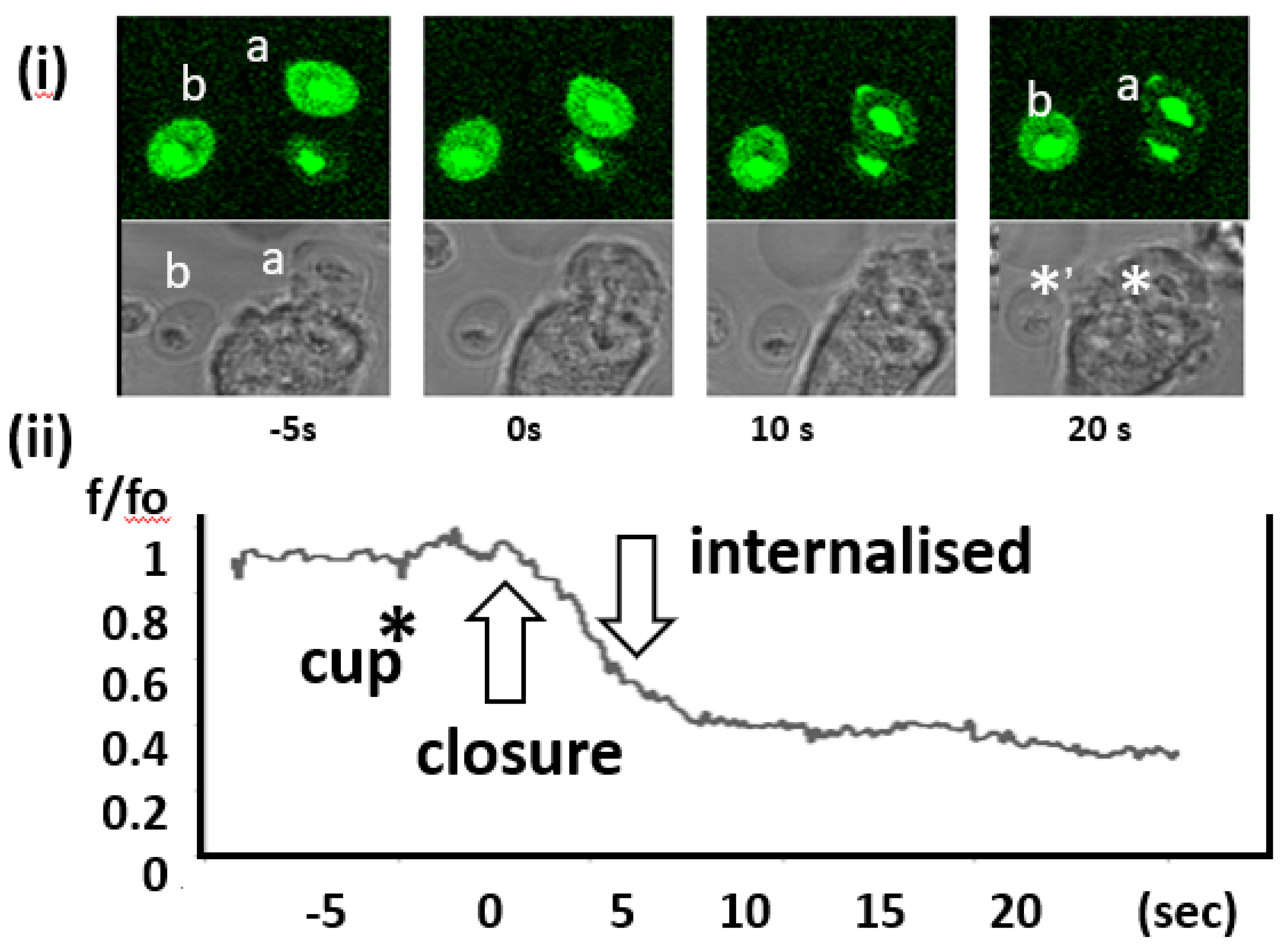
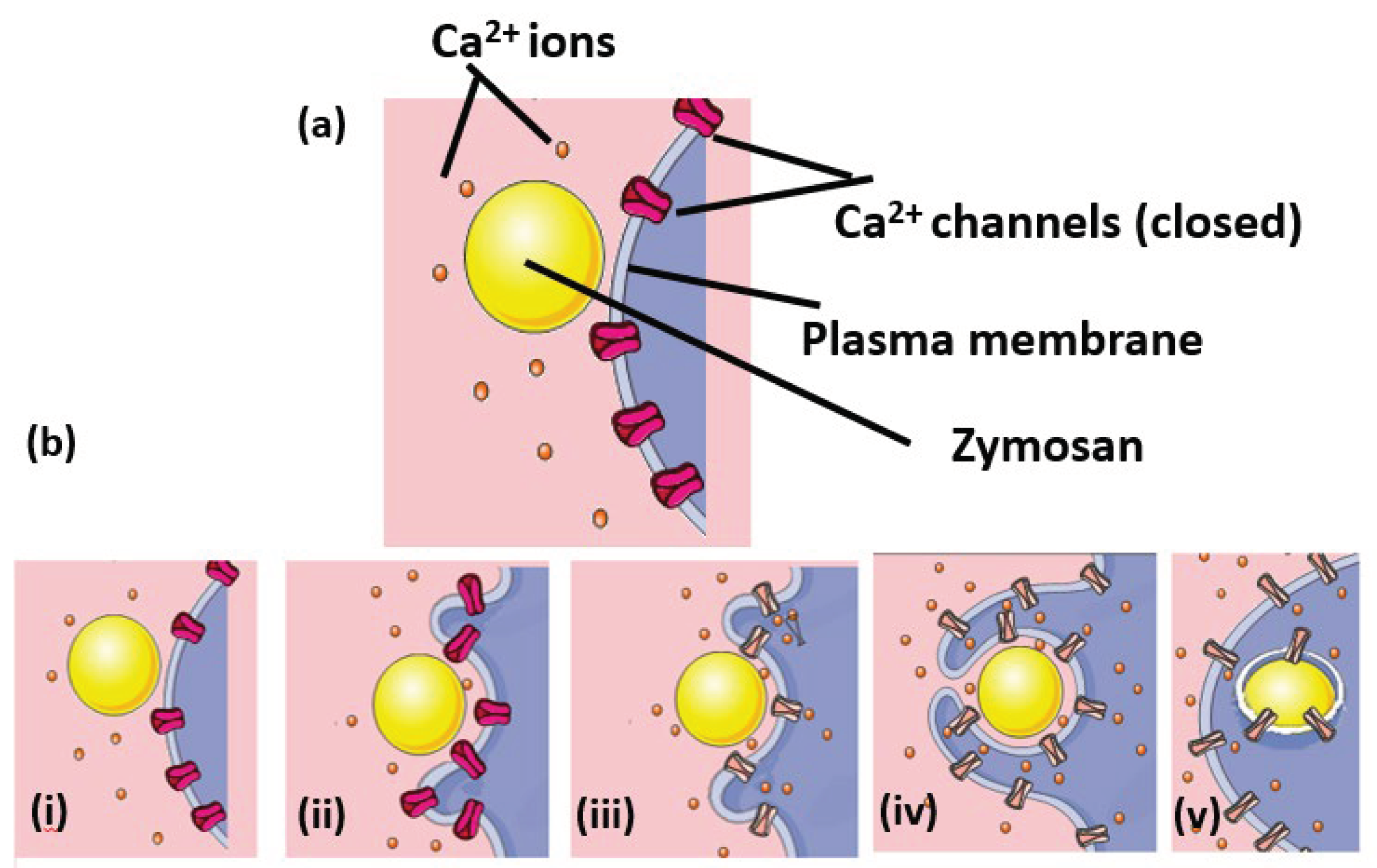
2.6. Location of Open Ca2+ Channels
3. Discussion
4. Materials and Methods
4.1. Cell Preparation
4.2. Properties of the Phagocytic Target
4.3. Imaging and Intra-Phagosomal Ca2+ Monitoring
4.4. Estimation of Intraphagosomal Ca2+ Concentration
4.5. Raw 264.7 Cell Transfection
Acknowledgments
Appendix A
References
- Dewitt, S.; Hallett, M.B. (2002) Cytosolic free Ca2+ changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J. Cell Biol. 159, 181-189.
- Nunes, P.; Demaurex, N. (2010) The role of calcium signaling in phagocytosis. J. Leuk. Biol. 88, 57–68. [CrossRef]
- Francis, E.A., Heinrich, V., ( 2017) Single-cell investigation of the role of calcium bursts in human immune cells. Biophysical J. 112 , 400A-400A. [CrossRef]
- Jaumouillé, V., Grinstein, S. (2016 ) Molecular Mechanisms of Phagosome Formation Microbiology Spectrum 4. [CrossRef]
- Uribe-Querol, E., Rosales, C. ( 2020) Phagocytosis: Our current understanding of a universal biological process. Frontiers Immunol. 11. [CrossRef]
- Hallett, M.B. Davies, E.V. . Campbell A.K. (1990) Oxidase activation in individual neutrophils is dependent on the onset and magnitude of the Ca2+ signal. Cell Calcium, 11 , 655-663. [CrossRef]
- Bei. L., Hu, T., Qian, Z.M., Shen, X. (1998) Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim. Biophys. Acta – Mol. Cell Res. 1404, 475-483. [CrossRef]
- Westman, J., Grinstein, S., Maxson, M.E. (2019) Revisiting the role of calcium in phagosome formation and maturation. J Leuk, Biol, 106, 837-851. [CrossRef]
- Nunes, P., Cornut, D., Demaurex, N. (2012) STIM1 juxtaposes ER to phagosomes, generating Ca2+ hotspots that boost phagocytosis. Curr. Biol. 22 , 1990-1997. [CrossRef]
- Roberts, R.E., Vervliet, T., Bultynck, G., Parys, J.B., Hallett, M.B. (2020) EPIC3, a novel Ca2+ indicator located at the cell cortex and in microridges, detects high Ca2+ subdomains during Ca2+ influx and phagocytosis. Cell Calcium 92 Article Number:102291. [CrossRef]
- Zhang, H., Clemens, R,A, Lowell, C.A.(2014) STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense. Blood 123, 2238-2249. [CrossRef]
- Guido, D., Demaurex, N., Nunes, P. (2015) Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell Sci. 128, 4074-4082. [CrossRef]
- Bessis, M. (1973) Living Blood Cells and Their Ultrastructure. Springer, Berlin.
- Lundqvist-Gustafsson, H., Gustafsson,M., Dahlgren C. (2020) Dynamic Ca(2+)changes in neutrophil phagosomes. A source for intracellular Ca(2+)during phagolysosome formation? Cell Calcium 27, 353-362. [CrossRef]
- Becker P.L., Fay F.S. (1987) Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am. J. Physiol. 253: C613–C618. [CrossRef]
- Soto E.R, . Ostroff G.R. (2008) Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjugate Chem. 19, 840–848. [CrossRef]
- Gee K.R., Brown, K. A., Chen, W. N., Bishop-Stewart, J., Gray, D., Johnson I. (2000) Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes Cell Calcium 27, 97-106. [CrossRef]
- Dewitt, S., Darley, R.L., Hallett, M.B. (2009) Translocation or just location? Pseudopodia affect fluorescent signals. J. Cell Biol. 184, 197-203. [CrossRef]
- Meyer, R.A. (1979) Light-scattering from biological cells-dependence of backscatter radiation on membrane thickness and refractive index. Applied Optics 18, 585-588. [CrossRef]
- Mie, G. (1908) Articles on the optical characteristics of turbid tubes, especially colloidal metal solutions. Ann. Der Physik 25, 377-445.
- Mantegazza, A.R., Savina, A., Vermeulen, M., Pérez, L. et al (2008) NADPH oxidase controls phagosomal pH , Blood 112 : 4712–4722. [CrossRef]
- Segal, A.W. (2005) How neutrophils kill microbes. Ann. Rev. Immunol. 23 , 197-223. [CrossRef]
- Dewitt, S., Laffafian I., Hallett M.B. (2003) Phagosomal oxidative activity during β2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+signal: Ca2+ controls time not space. J. Cell Sci. 116, 2857–2865. [CrossRef]
- Jacob M.C., Favre, M., Bensa J.C. (1991) Membrane cell permeabilization with Saponin and multiparametric flow cytometry. Cytometry 12, 550-558. [CrossRef]
- Hallett MB, Al-Jumaa, Dewitt S. (2014) Optical methods for the measurement and manipulation of cytosolic calcium signals in neutrophils. Methods in Molecular Biology 1124, 107-120: Neutrophil Methods and Protocols (Ed Quinn MT, DeLeo FR), Humana Press.
- Francis, E.A. Heinrich. V. (2018) Mechanistic understanding of single-cell behavior is essential for transformative advances in biomedicine. Yale J Biol Med. 2018, 91, 279–289.
- Anke D., Kiya, T., Gong, H., Gao, X., Malik, A.B. (2017) Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages J. Cell Sci. 130, 735–744. [CrossRef]
- Starkus, J.G., Fleig A., Reinhold, P. (2010) The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular. J Physiol. 588. 1227–1240. [CrossRef]
- Oancea, E., Meyer, T. (1998). Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 95, 307-318. [CrossRef]
- Teruel,M.N., Meyer T. (2002). Parallel single-cell monitoring of receptor-triggered membrane translocation of a calcium-sensing protein module. Science 295 1910-1912. [CrossRef]
- Kilpatrick, B.S., Eden, E.R., Schapira, A.H., Futter, C.F., Patel, S. (2013) Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci.126, 60-66. [CrossRef]
- Sawyer, D.W.; Sullivan, J.A.; Mandell, G.L. (1985) Intracellular free calcium localization in neutrophils during phagocytosis. Science. 230, 663–666. [CrossRef]
- Roberts, R.E., Martin,M., Marion, S., Elumalai, G.L., Lewis, K., Hallett, M.B. (2020) Ca2+-activated cleavage of ezrin visualised dynamically in living myeloid cells during cell surface area expansion. J. Cell Sci. 133, art no jcs236968. [CrossRef]
- Morris, M. R., Dewitt, S., Laffafian, I. and Hallett, M. B.(2003). Phagocytosis by inflammatory phagocytes: experimental strategies for stimulation and quantification. In Inflammation Protocols. Methods in Molecular Biology, Vol.225, pp. 35-46. New Jersey: Humana Press.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




