Submitted:
04 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Yoga as a Treatment for Depression
1.2. The Therapeutic Potential of Biomarkers in Yoga Research for Depression
- Are specific types of YBI better than others in the treatment of depression?
- How often / for how long should YBI be used to achieve a meaningful response?
- Are there any adverse effects associated with the use of YBIs in depression?
- How do YBIs synergize with “standard” treatments for depression?
- What are the barriers and challenges in accessing YBIs for patients in diverse settings?
- A better understanding of the biological correlates of symptom improvement in depression
- A more evidence-based approach to YBI, facilitating a rapprochement between yoga practitioners and practitioners of modern medicine, thereby avoiding the extremes of skepticism and unrealistic claims [25]
- A personalized approach to the use of YBI in depression, through the identification of those patients who would respond best to them
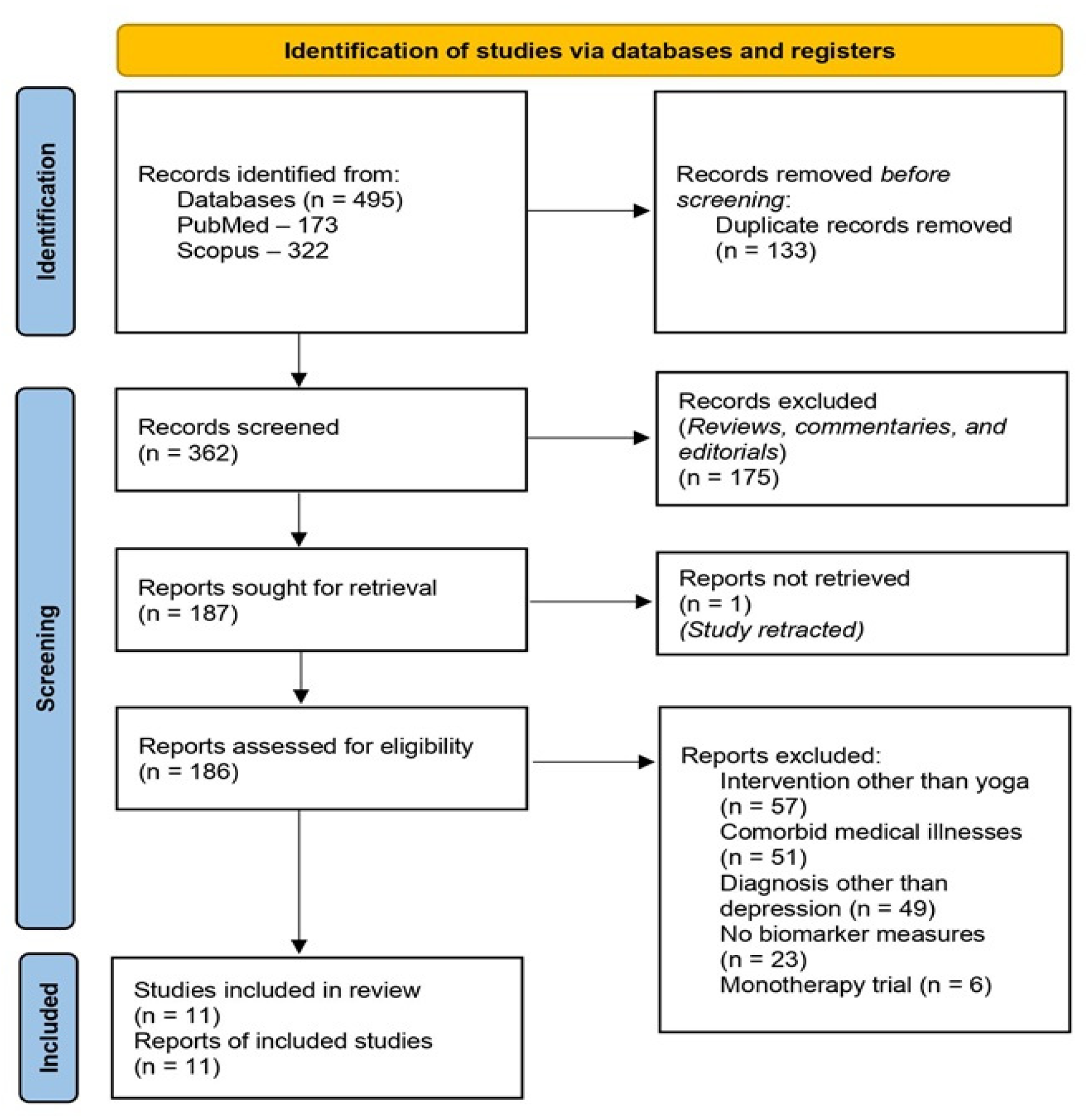
2. Review Process
- Clinical trials in patients with depression, defined as major depressive disorder or dysthymia
- No primary medical comorbidity, such as diabetes mellitus, cancer, or cardiovascular disease
- Use of a yoga-based intervention as an adjunct or add-on to standard treatment or “treatment as usual”
- Measurement of one or more biomarkers at baseline and/or during treatment
- Papers published in English
- Study sample characteristics: country of origin, setting, sample size
- Clinical diagnosis, including any comorbidities if documented
- Treatment(s) received in addition to YBI, if specified
- YBI characteristics: type of YBI, number of sessions
- Biomarkers measures
- Study results, both positive and negative, in terms of changes in biomarkers and/or associations between biomarkers and treatment response.
3. Review Findings
3.1. Characteristics of the Included Studies
3.2. Methodological Concerns
3.3. Biomarker and Treatment Characteristics
3.3. Changes in Biomarkers related to Yoga-Based Intervention
3.3.1. Immune-Inflammatory Markers
3.3.2. Autonomic Markers
3.3.3. Other Biomarkers
3.4. Relationship between Changes in Biomarkers and Clinical Improvement
4. Critical Analysis of the Review Findings
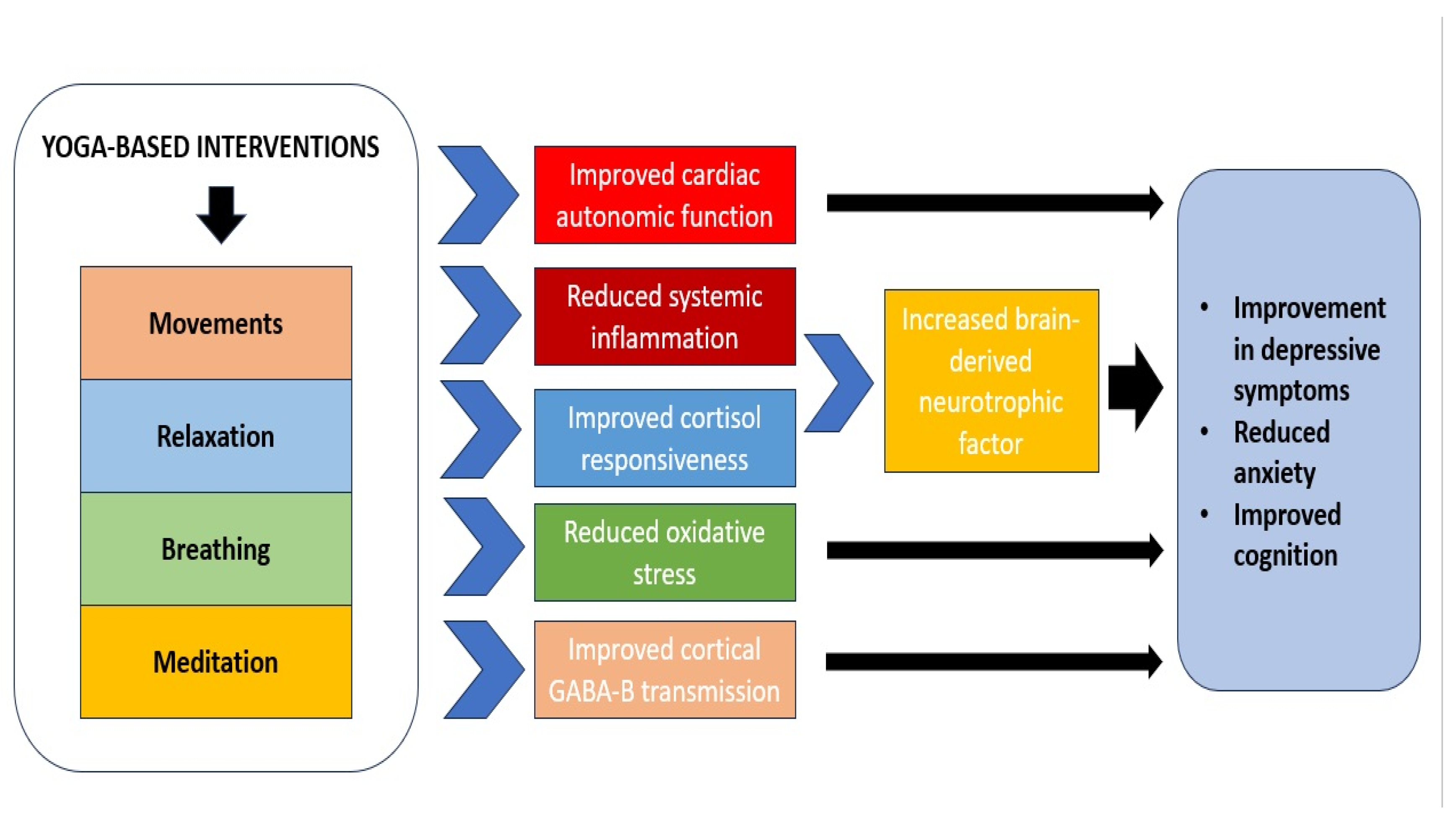
4.1. Pathophysiological Significance of Biomarker Results in Studies of YBI for Depression
4.2. Correlation or Causation?
4.3. Specificity of Biomarkers of Response to YBI in Depression
4.4. Comparison with Studies of Yoga Monotherapy for Depression
4.5. Methodological Concerns and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R. C. The costs of depression. Psychiatr Clin North Am 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Uher, R.; Payne, J. L.; Pavlova, B.; Perlis, R. H. (2014). Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety 2014, 31, 459–471. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.; McLean, L.; Fritz, K.; Lampe, L.; Malhi, G. S. Getting depression clinical practice guidelines right: time for change?. Acta Psychiatr Scand Suppl 2013, 444, 24–30. [Google Scholar] [CrossRef] [PubMed]
- de Silva, V. A.; Hanwella, R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: a meta-analysis of published studies. Int Clin Psychopharmacol 2012, 27, 8–16. [Google Scholar] [CrossRef]
- Rush, A. J., Trivedi, M., Fava, M., Thase, M., Wisniewski, S. The STAR*D Data Remain Strong: Reply to Pigott et al. Am J Psychiatry 2023, 180, 919–920. [CrossRef]
- Sakurai, H.; Noma, H.; Watanabe, K.; Uchida, H.; Furukawa, T. A. Cumulative remission rate after sequential treatments in depression: reappraisal of the STAR*D trial data. World Psychiatry 2024, 23, 156–157. [Google Scholar] [CrossRef]
- Pigott, H. E.; Kim, T.; Xu, C.; Kirsch, I.; Amsterdam, J. What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study's patient-level data with fidelity to the original research protocol. BMJ open 2023, 13, e063095. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S. N. Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord 2008, 10, 957–968. [Google Scholar] [CrossRef]
- Chen, C.; Shan, W. Pharmacological and non-pharmacological treatments for major depressive disorder in adults: A systematic review and network meta-analysis. Psychiatry Res 2019, 281, 112595. [Google Scholar] [CrossRef]
- Levy, L. B..; O'Hara, M. W. Psychotherapeutic interventions for depressed, low-income women: a review of the literature. Clin Psychol Rev 2010, 30, 934–950. [Google Scholar] [CrossRef]
- Ghaemi, S. N.; Vöhringer, P. A.; Vergne, D. E. (2012). The varieties of depressive experience: diagnosing mood disorders. The Psychiatr Clin North Am, 35, 73–86. [CrossRef]
- Furukawa, T. A.; Shinohara, K.; Sahker, E.; Karyotaki, E.; Miguel, C.; Ciharova, M.; Bockting, C. L. H.; Breedvelt, J. J. F.; Tajika, A.; Imai, H.; Ostinelli, E. G.; Sakata, M.; Toyomoto, R.;…; Cuijpers, P. Initial treatment choices to achieve sustained response in major depression: a systematic review and network meta-analysis. World Psychiatry 2021, 20, 387–396. [CrossRef]
- Warnick, S. J.; Mehdi, L.; Kowalkowski, J. Wait-there's evidence for that? Integrative medicine treatments for major depressive disorder. Int J Psychiatry Med 2021, 56, 334–343. [Google Scholar] [CrossRef]
- Asher, G. N.; Gartlehner, G.; Gaynes, B. N.; Amick, H. R.; Forneris, C.; Morgan, L. C.; Coker-Schwimmer, E; Boland, E.; Lux, L. J.; Gaylord, S.; Bann, C.; Pierl, C. B.; Lohr, K. N. Comparative Benefits and Harms of Complementary and Alternative Medicine Therapies for Initial Treatment of Major Depressive Disorder: Systematic Review and Meta-Analysis. J Altern Complement Med 2017, 23, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Ng, J. Y.; Nazir, Z.; Nault, H. Complementary and alternative medicine recommendations for depression: a systematic review and assessment of clinical practice guidelines. BMC Complement Med Ther 2020, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J. B. R.; Pane, O.; Panesar, S. K.; Updike, W.; Moore, T. R. Treatment of Mood and Depressive Disorders With Complementary and Alternative Medicine: Efficacy Review. J Midwifery Womens Health 2023, 68, 421–429. [Google Scholar] [CrossRef]
- Introduction to Yoga – Vikaspedia. Available online: https://vikaspedia.in/health/ayush/yoga-1/introduction-to-yoga (accessed on 28 February 2024).
- Srinivasan, T. Is yoga an intervention? Int J Yoga 2012, 5, 1–2. [Google Scholar] [CrossRef]
- Bhavanani, A. B. Yoga is not an intervention but may be yogopathy is. Int J Yoga 2012, 5, 157–158. [Google Scholar] [CrossRef]
- Naga Venkatesha Murthy, P. J.; Janakiramaiah, N.; Gangadhar, B. N.; Subbakrishna, D. K. P300 amplitude and antidepressant response to Sudarshan Kriya Yoga (SKY). J Affect Disord 1998, 50, 45–48. [Google Scholar] [CrossRef]
- Janakiramaiah, N.; Gangadhar, B. N.; Naga Venkatesha Murthy, P. J.; Harish, M. G.; Subbakrishna, D. K.; Vedamurthachar, A. Antidepressant efficacy of Sudarshan Kriya Yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord 2000, 57, 255–259. [Google Scholar] [CrossRef]
- Cramer, H.; Anheyer, D.; Lauche, R.; Dobos, G. A systematic review of yoga for major depressive disorder. J Affect Disord 2017, 213, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A. V.; Balneaves, L. G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R. L.; Ravindran, L.; Yatham, L. N.; Kennedy, S. H.; Lam, R. W.; MacQueen, G. M.; Milev, R. V.; Parikh, S. V.; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V. N.; Grover, S. Clinical Practice Guidelines for the management of Depression. Indian J Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, D.; Yang, J. Effectiveness of yoga for major depressive disorder: A systematic review and meta-analysis. Front Psychiatry 2023, 14, 1138205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Subramaniam, E.; Bhavanani, A. B.; Sarkar, S.; Balasundaram, S. Effect of adjunct yoga therapy in depressive disorders: Findings from a randomized controlled study. Indian J Psychiatry 2019, 61, 592–597. [Google Scholar] [CrossRef]
- Srivastava, A.; Kuppili, P. P.; Gupta, T.; Nebhinani, N.; Chandani, A. Kriya Yoga in Patients with Depressive Disorders: A Pilot Study. J Neurosci Rural Pract 2021, 12, 362–367. [Google Scholar] [CrossRef]
- Bieber, M.; Görgülü, E.; Schmidt, D.; Zabel, K.; Etyemez, S.; Friedrichs, B.; Prvulovic, D.; Reif, A.; Oertel, V. Effects of body-oriented yoga: a RCT study for patients with major depressive disorder. Eur Arch Psychiatry Clin Neurosci 2021, 271, 1217–1229. [Google Scholar] [CrossRef]
- Vollbehr, N. K.; Stant, A. D.; Hoenders, H. J. R.; Bartels-Velthuis, A. A.; Nauta, M. H.; Castelein, S.; Schroevers, M. J.; de Jong, P. J.; Ostafin, B. D. Cost-effectiveness of a mindful yoga intervention added to treatment as usual for young women with major depressive disorder versus treatment as usual only: Cost-effectiveness of yoga for young women with depression. Psychiatry Res 2024, 333, 115692. [Google Scholar] [CrossRef]
- Nauphal, M.; Mischoulon, D.; Uebelacker, L.; Streeter, C.; Nyer, M. Yoga for the treatment of depression: Five questions to move the evidence-base forward. Complement Ther Med 2019, 46, 153–157. [Google Scholar] [CrossRef]
- Bressington, D.; Mui, J.; Yu, C.; Leung, S. F.; Cheung, K.; Wu, C. S. T.; Bollard, M.; Chien, W. T. Feasibility of a group-based laughter yoga intervention as an adjunctive treatment for residual symptoms of depression, anxiety and stress in people with depression. J Affect Disord 2019, 248, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vollbehr, N. K.; Hoenders, H. J. R.; Bartels-Velthuis, A. A.; Nauta, M. H.; Castelein, S.; Schroevers, M. J.; Stant, A. D.; Albers, C. J.; de Jong, P. J.; Ostafin, B. D. Mindful yoga intervention as add-on to treatment as usual for young women with major depressive disorder: Results from a randomized controlled trial. J Consult Clin Psychol 2022, 90, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Naveen, G.H.; Rao, M.G.; Vishal, V.; Thirthalli, J.; Varambally, S.; Gangadhar, B.N. Development and feasibility of yoga therapy module for out-patients with depression in India. Indian J Psychiatry 2013, 55, S350–S356. [Google Scholar] [CrossRef] [PubMed]
- Ivanets, N. N.; Svistunov, A. A.; Chubarev, V. N.; Kinkulkina, M. A.; Tikhonova, Y. G.; Syzrantsev, N. S.; Sologova, S. S.; Ignatyeva, N. V.; Mutig, K.; Tarasov, V. V. Can Molecular Biology Propose Reliable Biomarkers for Diagnosing Major Depression?. Curr Pharm Des 2021, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Douglass, L. How did we get here? A history of yoga in America, 1800-1970. Int J Yoga Ther 2007, 17, 35–42. [Google Scholar] [CrossRef]
- Kinser, P. A.; Goehler, L. E.; Taylor, A. G. How might yoga help depression? A neurobiological perspective. Explore 2012, 8, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M. R.; Gautam, S.; Sagar, R.; Kumar, M.; Dada, R. Yoga in major depressive disorder: molecular mechanisms and clinical utility. Front Biosci 2021, 13, 56–81. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); Bethesda (MD): National Institutes of Health (US); 2016. Available online at: https://www.ncbi.nlm.nih. 3267.
- Califf, R.M. Biomarker definitions and their applications. Exp Biol Med 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Tricco, A. C.; Lillie, E.; Zarin, W.; O'Brien, K. K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M. D. J.; Horsley, T.; Weeks, L.; Hempel, S.; Akl, E. A.; Chang, C.; McGowan, J.; Stewart, L.; Hartling, L.; Aldcroft, A.; Wilson, M. G.; Garritty, C.; Lewin, S.; … Straus, S. E. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Wirsching, J.; Graßmann, S.; Eichelmann, F.; Harms, L. M.; Schenk, M.; Barth, E.; Berndzen, A.; Olalekan, M.; Sarmini, L.; Zuberer, H.; Aleksandrova, K. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS). BMC Med Res Methodol 2018, 18, 122. [Google Scholar] [CrossRef]
- Rajkumar, R. P. Immune-inflammatory markers of response to repetitive transcranial magnetic stimulation in depression: A scoping review. Asian J Psychiatry 2024, 91, 103852. [Google Scholar] [CrossRef]
- Sharma, V.K.; Das, S.; Mondal, S.; Goswami, U. Comparative effect of Sahaj Yoga on EEG in patients of major depression and healthy subjects. Biomedicine 2007, 27, 95–99. [Google Scholar]
- Sharma, V.K.; Das, S.; Mondal, S.; Goswami, U. Effect of Sahaj Yoga on autonomic functions in healthy subjects and patients of major depression. Biomedicine 2008, 28, 139–141. [Google Scholar]
- Naveen, G.H.; Thirthalli, J.; Rao, M.G.; Varambally, S.; Christopher, R.; Gangadhar, B.N. Positive therapeutic and neurotropic effects of yoga in depression: a comparative study. Indian J Psychiatry 2013, 55, 400–404. [Google Scholar] [CrossRef]
- Sarubin, N.; Nothdurfter, C.; Schule, C.; Lieb, M.; Uhr, M.; Born, C.; Zimmermann, R.; Buhner, M.; Konopka, K.; Ruppercht, R.; Baghai, T.C. The influence of Hatha yoga as add-on treatment in major depression on hypothalamic-pituitary-adrenal axis activity: a randomized trial. J Psychiatr Res 2014, 53, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Naveen, G.H.; Varambally, S.; Thirthalli, J.; Rao, M.; Christopher, R.; Gangadhar, B.N. Serum cortisol and BDNF in patients with major depression – effect of yoga. Int Rev Psychiatry 2016, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Toschi-Dias, E.; Tobaldini, E.; Solbiati, M.; Costantino, G.; Sanlorenzo, R.; Doria, S.; Irtelli, F.; Mencacci, C.; Montano, N. Sudarshan Kriya Yoga improves cardiac autonomic control in patients with anxiety-depression disorders. J Affect Disord 2017, 214, 74–80. [Google Scholar] [CrossRef]
- Tolahunase, M.R.; Sagar, R.; Faiq, M.; Dada, R. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: a randomized controlled trial. Restor Neurol Neurosci 2018, 36, 423–442. [Google Scholar] [CrossRef]
- Bhargav, P.H.; Reddy, P.V.; Govindaraj, R.; Gulati, K.; Ravindran, A.; Gayathri, D.; Karmani, S.J.; Udupa, K.; Venkatasubramanian, G.; Philip, M.; Debnath, M.; Bharath, R.D.; Sathyaprabha, T.N.; Gangadhar, B.N.; Muralidharan, K. Impact of a course of add-on supervised yoga on cortical inhibition in major depressive disorder: a randomized controlled trial. Can J Psychiatry 2021, 66, 179–181. [Google Scholar] [CrossRef]
- Gulati, K.; Bhargav, P.H.; Reddy, P.V.; Govindaraj, R.; Ravindran, A.; Gayathri, D.; Karmani, S.J.; Udupa, K.; Philip, M.; Debnath, M.; Bharath, R.D.; Sathyaprabha, T.N.; Venkatasubramanian, G.; Muralidharan, K. Adjunct yoga therapy: influence on heart rate variability in major depressive disorder – a randomized controlled trial. Asian J Psychiatry 2021, 65, 102832. [Google Scholar] [CrossRef]
- Nugent, N.R.; Brick, L.; Armey, M.F.; Tyrka, A.R.; Ridout, K.K.; Uebelacker, L.A. Benefits of yoga on IL-6: findings from a randomized controlled trial of yoga for depression. Behav Med 2021, 47, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Subbanna, M.; Talukdar, P.M.; Abdul, F.; Debnath, M.; Reddy, P.V.; Arasappa, R.; Venkatasubramanian, G.; Muralidharan, K.; Gangadhar, B.N.; Bhargav, P.H.; Karmani, S. Long-term add-on Yoga therapy offers clinical benefits in major depressive disorder by modulating the complement pathway: a randomized controlled trial. Asian J Psychiatry 2021, 66, 102876. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumor necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, Z.; Gullett, N.; Walsh, A.; Zonca, V.; Pedersen, G.A.; Souza, L.; Kieling, C.; Fisher, H.L.; Kohrt, B.A.; Mondelli, V. Cortisol and development of depression in adolescence and young adulthood – a systematic review and meta-analysis. Psychoneuroendocrinology 2022, 136, 105625. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J Psychiatry 2016, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Nielsen, M.G.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; Gennarelli, M. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 2010, 11, 763–773. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A meta-analysis of oxidative stress markers in depression. PloS One 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Yang, J.; Li, R.; Shi, Y.; Jiang, S.; Liu, J. Is serum complement C1q related to major depressive disorder? Indian J Psychiatry 2020, 62, 659–663. [Google Scholar] [CrossRef]
- Tang, W.; Liu, H.; Chen, L.; Zhao, K.; Zhang, Y.; Zheng, K.; Zhu, C.; Zheng, T.; Liu, J.; Wang, D.; Yu, L.; Fang, X.; Zhang, C.; Su, K.P. Inflammatory cytokines, complement factor H and anhedonia in drug-naïve major depressive disorder. Brain Behav Immun 2021, 95, 238–244. [Google Scholar] [CrossRef]
- Romeo, B.; Choucha, W.; Fossati, P.; Rotge, J.Y. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J Psychiatr Neurosci 2018, 43, 58–66. [Google Scholar] [CrossRef]
- Kinjo, M.; Wada, M.; Nakajima, S.; Tsugawa, S.; Nakahara, T.; Blumberger, D.M.; Mimura, M.; Noda, Y. Transcranial magnetic stimulation neurophysiology of patients with major depressive disorder: a systematic review and meta-analysis. Psychol Med 2021, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Y.; Zhang, Y.; Chen, L.; Zou, Y.; Xiao, J.; Min, W.; Yuan, C.; Ye, Y.; Li, M.; Tu, M.; Hu, J.; Zou, Z. Heart rate variability in generalized anxiety disorder, major depressive disorder and panic disorder: a network meta-analysis and systematic review. J Affect Disord 2023, 330, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Estevao, C. The role of yoga in inflammatory markers. Brain Behav Immun Health 2022, 20, 100421. [Google Scholar] [CrossRef]
- Shobana, R.; Maheshkumar, K.; Venkateswaran, S.T.; Geetha, M.B.; Padmavathi, R. Effects of long-term yoga training on autonomic function among the healthy adults. J Family Med Prim Care 2023, 11, 3471–3475. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Bauer, I.E. A systematic review of randomized control trials on the effects of yoga on stress measures and mood. J Psychiatr Res 2015, 68, 270–2872. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhardwaj, S.; Gupta, A.; Katoch, V.M.; Sharma, K.K.; Gupta, R. Influence of 24-week yoga intervention on cardiovascular risk factors and inflammatory markers in type 2 diabetes. Int J Yoga 2023, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M.; Sagar, R.; Dada, R. Impact of yoga and meditation on cellular aging in apparently healthy individuals: a prospective, open-label single-arm exploratory study. Oxid Med Cell Longev 2017, 2017, 7928981. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, F.; Guan, X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-κB signaling pathway in olfactory bulbectomized rats. Phytother Res 2019, 33, 1480–1489. [Google Scholar] [CrossRef]
- Calabrese, F., Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci 2014, 8, 430. [CrossRef] [PubMed]
- Shi, Y.; Luan, D.; Song, R.; Zhang, Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol 2020, 41, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Gould, T.D.; Prueitt, W.L.; Nanavati, J.; Grunebaum, M.F.; Farber, N.B.; Singh, B.; Selvaraj, S.; Machado-Vieira, R.; Achtyes, E.D.; Parikh, S.V.; Frye, M.A.; Zarate, C.A.; Goes, F.S. Blood-based biomarkers of antidepressant response to ketamine and esketamine: a systematic review and meta-analysis. Mol Psychiatry 2022, 27, 3658–3669. [Google Scholar] [CrossRef] [PubMed]
- Claudino, F.C.A.; Goncalves, L.; Schuch, F.B.; Martins, H.R.S.; da Rocha, N.S. The effects of individual psychotherapy in BDNF levels of patients with mental disorders: a systematic review. Front Psychiatry 2020, 11, 445. [Google Scholar] [CrossRef]
- Pelosof, R.; Dos Santos, L.A.; Farhat, L.C.; Gattaz, W.F.; Talib, L.; Brunoni, A.R. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: an updated systematic review and meta-analysis. World J Biol Psychiatry 2023, 43, 24–33. [Google Scholar] [CrossRef]
- Cakici, N.; Sutterland, A.L.; Penninx, B.W.J.H.; de Haan, L.; van Beveren, N.J.M. Changes in peripheral blood compounds following psychopharmacological treatment in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Psychol Med 2021, 51, 538–549. [Google Scholar] [CrossRef]
- Halappa, N.G.; Thirthalli, J.; Varambally, S.; Rao, M.; Christopher, R.; Nanjundaiah, G.B. Improvement in neurocognitive functions and serum brain-derived neurotrophic factor levels in patients with depression treated with antidepressants and yoga. Indian J Psychiatry 2018, 60, 32–37. [Google Scholar] [CrossRef]
- Murthy, P.J.; Gangadhar, B.N.; Janakiramaiah, N.; Subbakrishna, D.K. Normalization of P300 amplitude following treatment in dysthymia. Biol Psychiatry 1997, 42, 740–743. [Google Scholar] [CrossRef]
- Naga Venkatesha Murthy, P.J.; Janakiramaiah, N.; Gangadhar, B.N.; Subbakrishna, D.K. P300 amplitude and antidepressant response to Sudarshan Kriya Yoga (SKY). J Affect Disord 1998, 50, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Jain, F.A.; Cook, I.A.; Leuchter, A.F.; Hunter, A.M.; Davydov, D.M.; Ottaviani, C.; Tartter, M.; Crump, C.; Shapiro, D. Heart rate variability and treatment outcome in major depression: a pilot study. Int J Psychophysiol 2014, 93, 204–210. [Google Scholar] [CrossRef]
- Tolahunase, M.R.; Sagar, R.; Dada, R. 5-HTTLPR and MTHFR 677C>T polymorphisms and response to yoga-based lifestyle intervention in major depressive disorder: a randomized active-controlled trial. Indian J Psychiatry 2018, 60, 410–426. [Google Scholar] [CrossRef]
- Streeter, C.C.; Gerbarg, P.L.; Brown, R.P.; Scott, T.M.; Nielsen, G.H.; Owen, L.; Sakai, O.; Sneider, J.T.; Nyer, M.B.; Silveri, M.M. Thalamic gamma aminobutyric acid level changes in major depressive disorder after a 12-week Iyengar yoga and coherent breathing intervention. J Altern Complement Med 2020, 26, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Aditi Devi, N.; Philip, M.; Varambally, S.; Christopher, R.; Gangadhar, B.N. Yoga as a monotherapy alters proBDNF – mature BDNF ratio in patients with major depressive disorder. Asian J Psychiatry 2023, 81, 103429. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Taljaard, M.; Van den Heuvel, E.R.; Levine, M.A.H.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol 2017, 46, 746–755. [Google Scholar] [CrossRef]
| Study | Country of origin | Sample size and diagnosis | Yoga intervention | Concurrent treatment(s) | Biomarkers estimated | Results | BIO-CROSS score |
|---|---|---|---|---|---|---|---|
| Sharma et al., 2007 [44] | India | n = 30, MDD (DSM-IV criteria) | Sahaj yoga meditation (8 weeks) | Antidepressants as usual | EEG alpha activity | No significant effect of yoga on alpha activity. Correlation with clinical improvement not reported. | 11 |
| Sharma et al., 2008 [45] | India | n = 30, MDD (DSM-IV criteria) | Sahaj yoga meditation (8 weeks) | Antidepressants as usual | Autonomic parameters – PR, RR, GSR | No significant effect of yoga on autonomic parameters. Correlation with clinical improvement not reported. | 12 |
| Naveen et al., 2013 [46] | India | n = 35, MDD (DSM-IV criteria) | Depression-specific yoga module (12 weeks) |
Antidepressants as usual | Serum BDNF | Significant increase in BDNF both in yoga and control groups; increased BDNF correlated with reduced depressive symptoms only in yoga group | 17 |
| Sarubin et al., 2014 [47] | Germany | n = 60, MDD (DSM-IV criteria) | Hatha yoga (1 hour/week for 5 weeks) | Escitalopram (10 mg/day) or quetiapine (300 mg/day) | Cortisol response to serial DEX/CRH tests | No significant effect of yoga on cortisol responses. Changes in cortisol correlated with reduced depressive symptoms regardless of treatment. | 19 |
| Naveen et al., 2016 [48] | India | n = 35, MDD (DSM-IV criteria) | Depression-specific yoga module (12 weeks) |
Antidepressants as usual | Serum cortisol | Significant reduction in serum cortisol in yoga group. Correlation with clinical improvement not reported. | 15 |
| Toschi-Dias et al., 2017 [49] | Italy | n = 46, “depression or anxiety disorders” (DSM-IV criteria) | Sudarshan Kriya Yoga (10 sessions over 2 weeks) | Antidepressants as usual | Cardiac autonomic parameters recorded through ECG | Reduced sympathetic modulation and improved parasympathetic modulation and cardiorespiratory coupling in yoga group. Temporal but not direct correlation with improvement in depressive symptoms. | 13 |
| Tolahunase et al., 2018 [50] | India | n = 58, MDD (DSM-IV criteria) | Yoga and meditation lifestyle intervention (12 weeks) | Antidepressants as usual | Serum 8OH2dG, BDNF, cortisol, DHEAS, IL-6, oxidative stress markers (ROS, TAC), sirtuin-1, telomerase | Significant increase in BDNF, DHEAS, sirtuin-1 and telomerase and decrease in cortisol, IL-6, ROS, TAC and 8OH2dG in yoga group. Changes in BDNF correlated with reduction in depressive symptoms. | 14 |
| Bhargav et al., 2021 [51] | India | n = 70, MDD (DSM-IV criteria) | Depression-specific yoga module (12 weeks) |
Antidepressants as usual | TMS measures of cortical inhibition (CI, CSP, RMT, LICI, SICI) | Significant increase in CSP in yoga group. Correlation with clinical improvement not reported. | 13 |
| Gulati et al., 2021 [52] | India | n = 68, MDD (DSM-IV criteria) | Depression-specific yoga module (12 weeks) |
Antidepressants as usual | HRV parameters recorded through ECG | Trend towards greater decrease in HRF low-frequency/high-frequency ratio in yoga group; no significant group differences. Correlation with clinical improvement not reported. |
18 |
| Nugent et al., 2021 [53] | United States | n = 84, MDD (DSM-IV criteria) | Hatha yoga vs Healthy Living Workshop (10 weeks) |
Antidepressants as usual | Serum CRP, IL-6, TNF-α | Significant reduction in IL-6 in yoga group. Correlation with clinical improvement not reported. | 17 |
| Subbanna et al., 2021 [54] | India | n = 22, MDD (DSM-IV criteria) | Depression-specific yoga module (12 weeks) |
Antidepressants as usual | Plasma complement components C1q, C3, C3b/iC3b, C4, Factor B, Factor H, properdin | Significant reduction in C1q, Factor H and properdin in yoga group; not correlated with improvement in depression. Significant reduction in C4 in control group | 13 |
| Study | Sample Characteristics | Yoga-Based Intervention Characteristics | Biomarkers Assessed | Results |
|---|---|---|---|---|
| Murthy et al., 1997 [80] | Dysthymia, n = 15, India | Sudarshan Kriya yoga (12 weeks) |
Auditory P300 event-related potential | Significant increase in P300 amplitude after yoga; temporal correlation with symptomatic improvement |
| Naga Venkatesha Murthy et al., 1998 [81] | Depression, n = 30 (MDD, n = 15; dysthymia, n = 15), India | Sudarshan Kriya yoga (12 weeks) |
Baseline auditory P300 event-related potential | No association between baseline P300 amplitude and response to yoga |
| Jain et al., 2014 [82] | MDD, n = 16, United States | Iyengar yoga (20 sessions over 8 weeks) | Baseline HRV parameters measured using ECG | Lower HRV rVLF predicted response to yoga |
| Tolahunase et al., 2018 [83] | MDD, n = 89, India | Yoga-based lifestyle intervention (5 sessions/week over 12 weeks) | 5-HTTLPR and MTHFR functional polymorphisms | No significant association between either polymorphism and response to yoga |
| Streeter et al., 2020 [84] | MDD, n = 28, United States | Iyengar yoga (12 weeks, randomized to high- or low-dose) | Thalamic GABA measured using MRS | Increased thalamic GABA low-dose yoga group; negative correlation between thalamic GABA and depression severity in high-dose group |
| Aditi Devi et al., 2023 [85] | MDD, n = 13, India | Brief yoga module (10 sessions over 2 weeks) |
Serum BDNF, pro-BDNF, mature BDNF | Significant increase in total and mature BDNF; reduced pro-BDNF / mature BDNF ratio; temporal correlation with symptomatic improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





