Submitted:
02 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Transformation of AMP in Liquid Media
2.2. Monitoring of Laccase and Antibacterial Activities at Different Conditions
2.2.1. Effect of AMP Concentration
2.2.2. Effect of the Age of the Culture on AMP Removal
2.2.3. Reusability of the Same Culture
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Microorganisms
4.3. Experimental Procedures
4.3.1. Follow-Up of AMP Concentration Time-Course in the Culture Medium
4.3.2. In Vitro Analysis of Residual AMP
4.4. Analytical Procedures
4.4.1. HPLC analysis of Ampicillin in C. gallica Culture Filtrate
4.4.2. Antibacterial Activity Assay
4.4.3. Laccase Activity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Imai, S.; Shiraishi, A.; Gamo, K.; Watanabe, I.; Okuhata, H.; Miyasaka, H.; Ikeda, K.; Bamba, T.; Hirata, K. Removal of Phenolic Endocrine Disruptors by Portulaca Oleracea. J. Biosci. Bioeng. 2007, 103, 420–426. [Google Scholar] [CrossRef]
- Tan, B.L.L.; Hawker, D.W.; Müller, J.F.; Tremblay, L.A.; Chapman, H.F. Stir Bar Sorptive Extraction and Trace Analysis of Selected Endocrine Disruptors in Water, Biosolids and Sludge Samples by Thermal Desorption with Gas Chromatography-Mass Spectrometry. Water Res. 2008, 42, 404–412. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A Review of Emerging Organic Contaminants (EOCs), Antibiotic Resistant Bacteria (ARB), and Antibiotic Resistance Genes (ARGs) in the Environment: Increasing Removal with Wetlands and Reducing Environmental Impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of Antibiotic-Resistant Bacteria and Their Resistance Genes in Wastewater, Surface Water, and Drinking Water Biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef]
- Mai, Z.; Xiong, X.; Hu, H.; Jia, J.; Wu, C.; Wang, G. Occurrence, Distribution, and Ecological Risks of Antibiotics in Honghu Lake and Surrounding Aquaculture Ponds, China. Environ. Sci. Pollut. Res. 2023, 30, 50732–50742. [Google Scholar] [CrossRef]
- Zinner, S.H. Overview of Antibiotic Use and Resistance: Setting the Stage for Tigecycline. Clin. Infect. Dis. 2005, 41, S289–S292. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Li, S. Transformation and Toxicity Evaluation of Tetracycline in Humic Acid Solution by Laccase Coupled with 1-Hydroxybenzotriazole. J. Hazard. Mater. 2017, 331, 182–188. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the Aquatic Environment--a Review--Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Erbay, A.; Bodur, H.; Akıncı, E.; Çolpan, A. Evaluation of Antibiotic Use in Intensive Care Units of a Tertiary Care Hospital in Turkey. J. Hosp. Infect. 2005, 59, 53–61. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Carlet, J.; Rambaud, C.; Pulcini, C. Alliance contre les bactéries multirésistantes: sauvons les antibiotiques ! Ann. Fr. Anesth. Réanimation 2012, 31, 704–708. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Y.; Riaz, L.; Yang, Q.; Du, B.; Wang, R. Multiple Antibiotic Resistance and DNA Methylation in Enterobacteriaceae Isolates from Different Environments. J. Hazard. Mater. 2021, 402, 123822. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Wang, Y.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z.; Yang, Y. Occurrence of Antibiotics and Antibiotic Resistance Genes and Their Correlations in Lower Yangtze River, China. Environ. Pollut. 2020, 257, 113365. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling Antibiotic Resistance: The Environmental Framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of Microplastics and Antibiotic Resistance Genes and Their Effects on the Aquaculture Environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Chen, Y.; Xu, J.; Yang, B.; Jiang, J. Ecological Response to Antibiotics Re-Entering the Aquaculture Environment with Possible Long-Term Antibiotics Selection Based on Enzyme Activity in Sediment. Environ. Sci. Pollut. Res. 2022, 29, 19033–19044. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Hossain, A.; Al Mamun, M.H.; Nagano, I.; Kitazawa, D.; Masunaga, S.; Matsuda, H. Antibiotics, Antibiotic-Resistant Bacteria, and Resistance Genes in Aquaculture: Risks, Current Concern, and Future Thinking. Environ. Sci. Pollut. Res. 2022, 29. [Google Scholar] [CrossRef]
- Kong, K.-F.; Schneper, L.; Mathee, K. Beta-Lactam Antibiotics: From Antibiosis to Resistance and Bacteriology. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2010, 118, 1–36. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent Developments and Applications of Immobilized Laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Foulds, G. Pharmacokinetics of Sulbactam/Ampicillin in Humans: A Review. Rev. Infect. Dis. 1986, 8, S503–S511. [Google Scholar] [CrossRef]
- Campagnolo, E.R.; Johnson, K.R.; Karpati, A.; Rubin, C.S.; Kolpin, D.W.; Meyer, M.T.; Esteban, J.E.; Currier, R.W.; Smith, K.; Thu, K.M.; et al. Antimicrobial Residues in Animal Waste and Water Resources Proximal to Large-Scale Swine and Poultry Feeding Operations. Sci. Total Environ. 2002, 299, 89–95. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Jojoa-Sierra, S.D.; Berrio-Perlaza, K.E.; Ferraro, F.; Torres-Palma, R.A. Structure-Reactivity Relationship in the Degradation of Three Representative Fluoroquinolone Antibiotics in Water by Electrogenerated Active Chlorine. Chem. Eng. J. 2017, 315, 552–561. [Google Scholar] [CrossRef]
- Harrabi, M.; Varela Della Giustina, S.; Aloulou, F.; Rodriguez-Mozaz, S.; Barceló, D.; Elleuch, B. Analysis of Multiclass Antibiotic Residues in Urban Wastewater in Tunisia. Environ. Nanotechnol. Monit. Manag. 2018, 10, 163–170. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent Advancement in Remediation of Synthetic Organic Antibiotics from Environmental Matrices: Challenges and Perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Daramola, O.B.; Adetunji, A.T.; Ore, O.T.; Ayantunji, Y.J.; Omole, R.K.; Ajagbe, D.; Adekoya, S.O. Efficient Removal of Antibiotics from Water Resources Is a Public Health Priority: A Critical Assessment of the Efficacy of Some Remediation Strategies for Antibiotics in Water. Environ. Sci. Pollut. Res. 2022, 29, 56948–57020. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar Photo-Fenton Oxidation for the Removal of Ampicillin, Total Cultivable and Resistant E. Coli and Ecotoxicity from Secondary-Treated Wastewater Effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Al Abri, R.; Al Marzouqi, F.; Kuvarega, A.T.; Meetani, M.A.; Al Kindy, S.M.Z.; Karthikeyan, S.; Kim, Y.; Selvaraj, R. Nanostructured Cerium-Doped ZnO for Photocatalytic Degradation of Pharmaceuticals in Aqueous Solution. J. Photochem. Photobiol. Chem. 2019, 384, 112065. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Z.; Ohore, O.E.; Yang, J.; Peng, K.; Li, S.; Li, X. Impact of Antibiotics on Microbial Community in Aquatic Environment and Biodegradation Mechanism: A Review and Bibliometric Analysis. Environ. Sci. Pollut. Res. 2023, 30, 66431–66444. [Google Scholar] [CrossRef]
- Yabalak, E. An Approach to Apply Eco-Friendly Subcritical Water Oxidation Method in the Mineralization of the Antibiotic Ampicillin. J. Environ. Chem. Eng. 2018, 6, 7132–7137. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, D.M.; Serna-Galvis, E.A.; Ferraro, F.; Torres-Palma, R.A. Degradation of the Emerging Concern Pollutant Ampicillin in Aqueous Media by Sonochemical Advanced Oxidation Processes - Parameters Effect, Removal of Antimicrobial Activity and Pollutant Treatment in Hydrolyzed Urine. J. Environ. Manage. 2020, 261, 110224. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.-T.; Teng, Y.; Zhao, J. Activated Carbon Supported Nanoscale Zero Valent Iron for Cooperative Adsorption and Persulfate-Driven Oxidation of Ampicillin. Environ. Technol. Innov. 2020, 19, 100956. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A. Adsorptive Removal of Antibiotics from Water over Natural and Modified Adsorbents. Environ. Sci. Pollut. Res. 2019, 26, 34775–34788. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Q.; Zhu, Y.; Zhang, M.; Zhu, Y.; Farooq, U.; Lu, T.; Qi, Z.; Chen, W. Adsorption of Fluoroquinolone Antibiotics onto Ferrihydrite under Different Anionic Surfactants and Solution PH. Environ. Sci. Pollut. Res. 2023, 30, 78229–78242. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation – A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use – Present Knowledge and Future Challenges. J. Environ. Manage. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and Removal Methods of Antibiotics from Aqueous Matrices – A Review. J. Environ. Manage. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Mohamadisorkali, H.; Hossaini, H.; Hossini, H.; Makhdoumi, P. The Hybrid System Successfully to Consisting of Activated Sludge and Biofilter Process from Hospital Wastewater: Ecotoxicological Study. J. Environ. Manage. 2020, 276, 111098. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly Laccases Treatment to Challenge Micropollutants Issue in Municipal Wastewaters. Environ. Pollut. 2020, 257, 113579. [Google Scholar] [CrossRef]
- Russell, J.N.; Yost, C.K. Alternative, Environmentally Conscious Approaches for Removing Antibiotics from Wastewater Treatment Systems. Chemosphere 2021, 263, 128177. [Google Scholar] [CrossRef]
- Lucas, D.; Badia-Fabregat, M.; Vicent, T.; Caminal, G.; Rodríguez-Mozaz, S.; Balcázar, J.L.; Barceló, D. Fungal Treatment for the Removal of Antibiotics and Antibiotic Resistance Genes in Veterinary Hospital Wastewater. Chemosphere 2016, 152, 301–308. [Google Scholar] [CrossRef]
- Copete-Pertuz, L.S.; Plácido, J.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Mora, A. Elimination of Isoxazolyl-Penicillins Antibiotics in Waters by the Ligninolytic Native Colombian Strain Leptosphaerulina Sp. Considerations on Biodegradation Process and Antimicrobial Activity Removal. Sci. Total Environ. 2018, 630, 1195–1204. [Google Scholar] [CrossRef]
- Copete-Pertuz, L.S.; Plácido, J.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Mora, A. Elimination of Isoxazolyl-Penicillins Antibiotics in Waters by the Ligninolytic Native Colombian Strain Leptosphaerulina Sp. Considerations on Biodegradation Process and Antimicrobial Activity Removal. Sci. Total Environ. 2018, 630, 1195–1204. [Google Scholar] [CrossRef]
- Dhawan, S.; Lal, R.; Hanspal, M.; Kuhad, R.C. Effect of Antibiotics on Growth and Laccase Production from Cyathus Bulleri and Pycnoporus Cinnabarinus. Bioresour. Technol. 2005, 96, 1415–1418. [Google Scholar] [CrossRef]
- Praveen, K.; Reddy, B.R. Effect of antibiotics on ligninolytic enzymes production from stereum ostrea and phanerochaete chrysosporium under submerged fermentation. 2012. [Google Scholar]
- Zouari-Mechichi, H.; Mechichi, T.; Dhouib, A.; Sayadi, S.; Martínez, A.T.; Martínez, M.J. Laccase Purification and Characterization from Trametes Trogii Isolated in Tunisia: Decolorization of Textile Dyes by the Purified Enzyme. Enzyme Microb. Technol. 2006, 39, 141–148. [Google Scholar] [CrossRef]
- Sandhu, D.K.; Arora, D.S. Laccase Production ByPolyporus Sanguineus under Different Nutritional and Environmental Conditions. Experientia 1985, 41, 355–356. [Google Scholar] [CrossRef]
- Rogalski, J.; Lundell, T.K.; Leonowicz, A.; Hatakka, A.I. Influence of Aromatic Compounds and Lignin on Production of Ligninolytic Enzymes by Phlebia Radiata. Phytochemistry 1991, 30, 2869–2872. [Google Scholar] [CrossRef]
- Ben Ayed, A.; Akrout, I.; Albert, Q.; Greff, S.; Simmler, C.; Armengaud, J.; Kielbasa, M.; Turbé-Doan, A.; Chaduli, D.; Navarro, D.; et al. Biotransformation of the Fluoroquinolone, Levofloxacin, by the White-Rot Fungus Coriolopsis Gallica. J. Fungi 2022, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of Tetracycline by Immobilized Laccase and the Proposed Transformation Pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; You, S.; Zhang, J.; Qi, W.; Su, R.; He, Z. An Effective In-Situ Method for Laccase Immobilization: Excellent Activity, Effective Antibiotic Removal Rate and Low Potential Ecological Risk for Degradation Products. Bioresour. Technol. 2020, 308, 123271. [Google Scholar] [CrossRef] [PubMed]
- Daâssi, D.; Zouari-Mechichi, H.; Belbahri, L.; Barriuso, J.; Martínez, M.J.; Nasri, M.; Mechichi, T. Phylogenetic and Metabolic Diversity of Tunisian Forest Wood-Degrading Fungi: A Wealth of Novelties and Opportunities for Biotechnology. 3 Biotech 2016, 6, 46. [Google Scholar] [CrossRef]
- Meenupriya, J.; Thangaraj, M. Isolation and Molecular Characterization of Bioactive Secondary Metabolites from Callyspongia Spp. Associated Fungi. Asian Pac. J. Trop. Med. 2010, 3, 738–740. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Skorobogat’ko, O.V.; Vartanov, S.S.; Varfolomeyev, S.D. Laccase. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
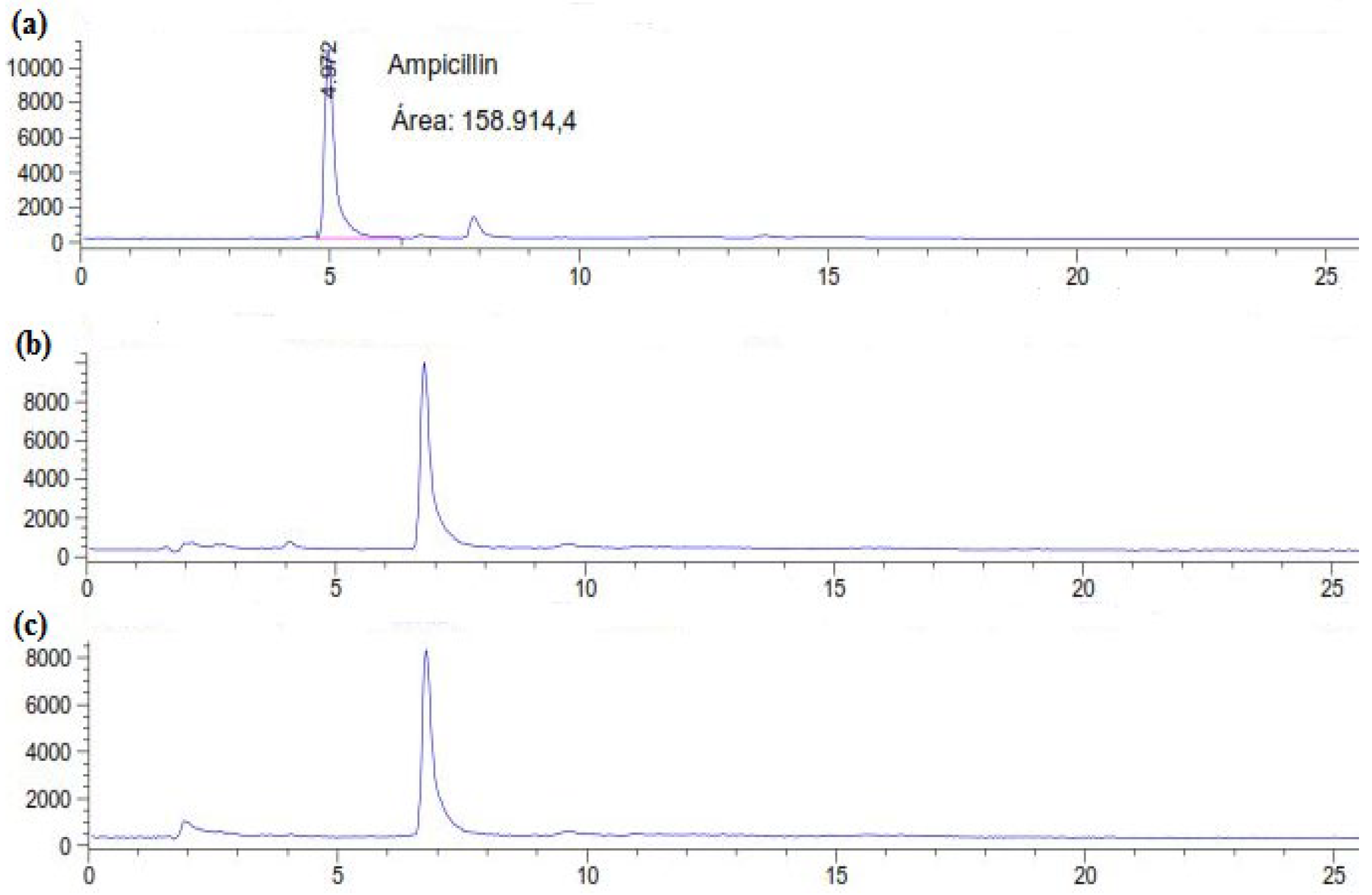
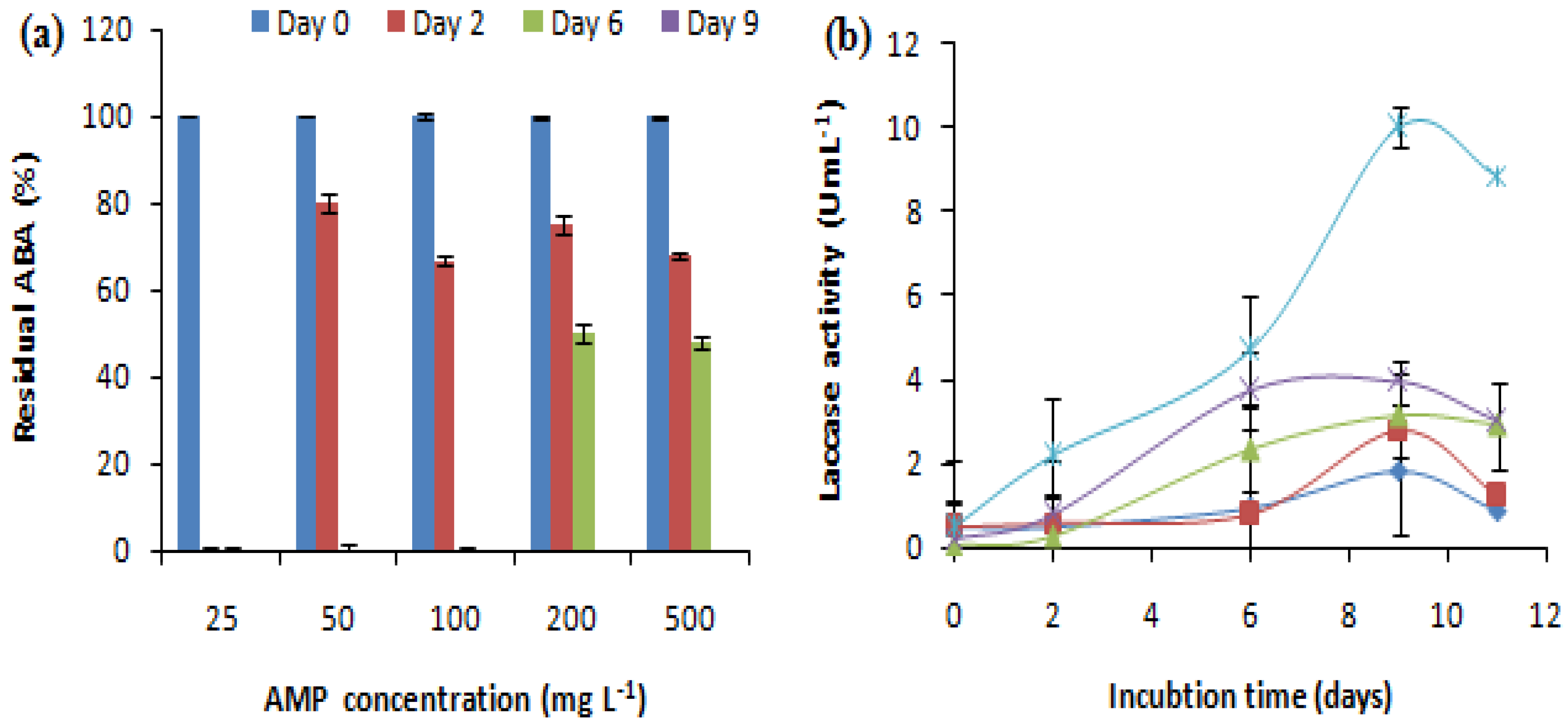
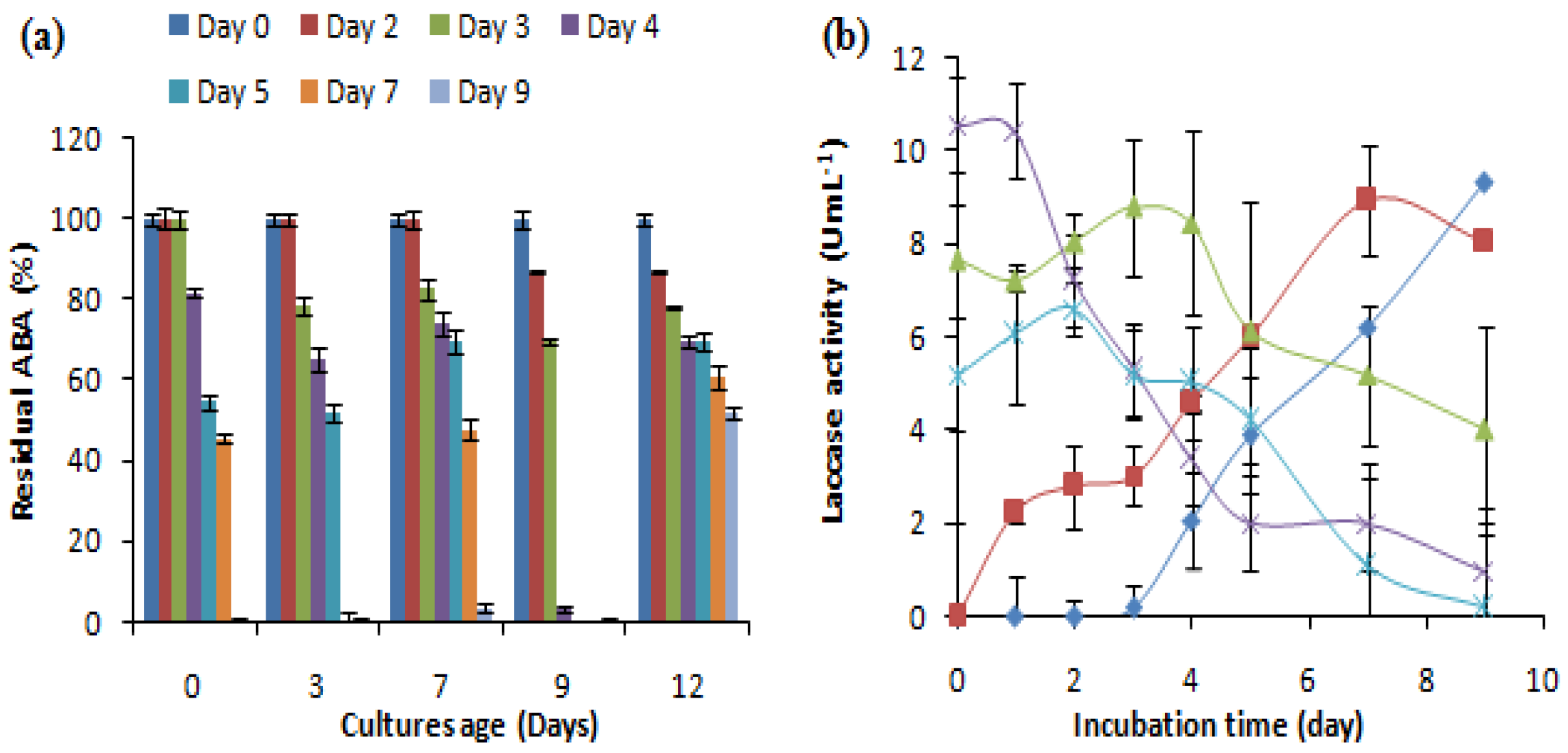
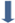 : AMP
injection in the culture medium. Each datapoint (mean ± standard deviation) is
the result of triplicate experiments.
: AMP
injection in the culture medium. Each datapoint (mean ± standard deviation) is
the result of triplicate experiments.
 : AMP
injection in the culture medium. Each datapoint (mean ± standard deviation) is
the result of triplicate experiments.
: AMP
injection in the culture medium. Each datapoint (mean ± standard deviation) is
the result of triplicate experiments.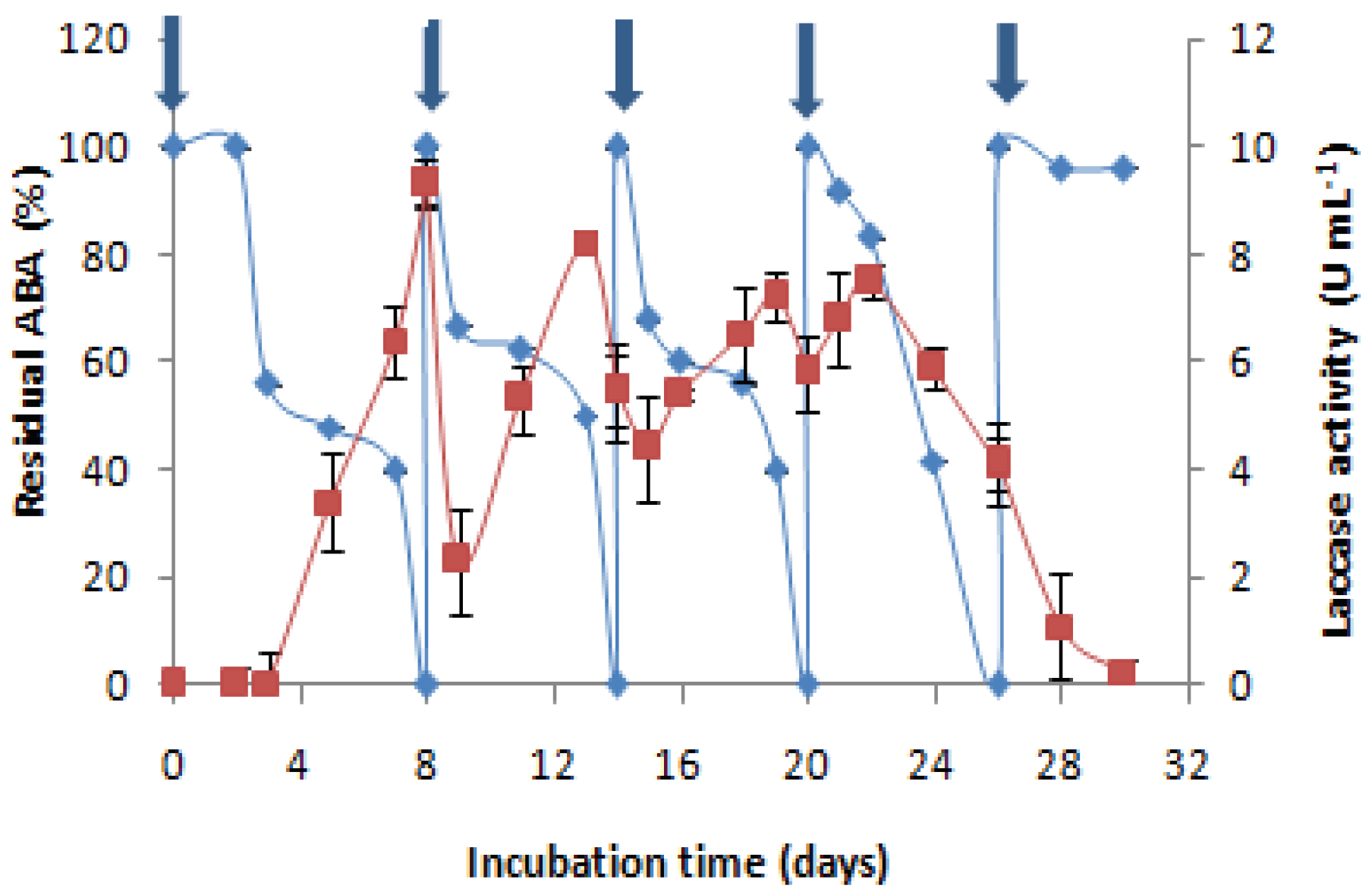
| Antibiotic | Class | λmax (nm) | Chemical structure |
|---|---|---|---|
| Ampicillin | β-lactam | 204 | 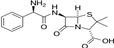 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





