1. Introduction
Psoriatic disease (PsD) is a term that has been first proposed in 2006 by Scarpa et al. [
1] and describes a chronic, heterogeneous inflammatory disease that is composed of a wide variety of pathologies that share common immunological pathways: such as psoriasis (PsO), psoriatic arthritis (PsA), inflammatory bowel disease (IBD) and uveitis. Skin, nails, entheses, axial and peripheral joints an less frequently also the gastrointestinal tract and the eye are frequently affected. In addition to these more "classical" clinical domains, it has recently been shown that patients with IBD often have an elevated cardiovascular risk profile, characterized by a higher prevalence of comorbidities such as diabetes, dyslipidaemia, hypertension, obesity, metabolic syndrome and cardiovascular diseases (heart attacks, strokes, thromboembolic events). [
2]
1.1. Psoriasis
Psoriasis is a disease that affects up to 3% of the world's population with an increasing incidence over the years, has no clear gender predilection and has a significant psychological impact on patients. A study by Rapp et al. [
3] shows that PsO, compared to a number of chronic diseases, such as cancer, myocardial infarction and congestive heart failure, is one of the most physically and psychologically debilitating diseases, considerably worsening the results of quality of life questionnaires. Only depression and chronic lung disease impaired psychological quality of life more than psoriasis. [
3]
There are five types of PsO:
- -
Plaque PsO, also known as psoriasis vulgaris. Is the most common (approximately 90% of cases) and is characterised by well-defined, erythematous, scaly plaque lesions, usually concentrated on the extensor surfaces of the limbs, the periumbilical, perianal and retroauricular region and the scalp. Nail psoriasis results from involvement of the nail bed or matrix and is quite common in patients with PsO (40-45%) and significantly more prevalent in patients with PsA (80-90%), and is particularly associated with involvement of the distal interphalangeal joints (DIP). The clinical manifestations are variable, and alterations such as nail pitting, onycholysis, subungual hyperkeratosis, changes in nail plate coloring and onychodystrophy can be observed. This functional and aesthetic alteration of the nails also entails an added psychological burden on the patient, and is currently a therapeutic challenge due to the low response rate to both topical and systemic treatments. [
4,
5]
- -
Pustular PsO is characterised by white, coalescing, sterile pustules about 2-3 mm in size. Clinically it is distinguished in two variants.
- -
Generalised pustular PsO may develop in people with no history of PsO or occur in people with previous PsO vulgaris. It is characterised by dark, scattered erythematous plaques with sterile pustules, which coalesce to form purulent aggregates. Skin lesions may progress rapidly and the disease is life-threatening. The skin lesions are associated with symptoms of systemic involvement such as high fever and malaise, together with elevated acute phase reactants and neutrophilic leukocytosis.
- -
Localized forms of pustular PsO include palmoplantar pustular PsO and acrodermatitis continua suppurativa. Interestingly, tumour necrosis factor inhibitors (anti-TNF) which are effective therapies for treating PsO have also been associated with the development of localised forms of pustular PsO. [
6,
7]
- -
Guttate PsO typically presents with an abrupt appearance of numerous erythematous papules about 2-6 mm in diameter, scaly and pruritic, in the form of a teardrop or droplet. It usually appears on the trunk and proximal extremities, while the palms and soles are generally unaffected. It is usually the most typical form of PsO in childhood. Sometimes, as a background, there may have been a previous streptococcal infection, mainly of the upper respiratory tract. [
6]
- -
Inverse PsO, also known as flexural or intertriginous, is a variety of plaque PsO that affects the body folds, most commonly the axillary, anogenital and inframammary folds. It may occur alone or, more frequently, may be accompanied by plaque PsO in other locations. Bacterial or fungal superinfections (mainly Candida species) are common, as persistently moist skin provides an ideal environment for the growth of micro-organisms. [
6]
- -
Erythrodermic PsO is a rare but very severe complication of psoriasis, occurring in 1-2% of patients. Clinically it is characterised by the appearance of exuberant scaling erythema affecting at least 80-90% of the body surface. Due to extensive skin involvement, patients may present with systemic symptoms (fever with chills, intense pruritus, signs of dehydration, arthralgias, asthenia and lymphadenopathy). Several factors have been described as possible triggers such as infections, systemic glucocorticoids, changes in medication, emotional stress, etc... [
6].
1.2. Psoriatic Arthritis
PsA is a chronic inflammatory disease with a heterogeneous presentation involving multiple tissues and clinical domains (arthritis, spondylitis, enthesitis and dactylitis).
Approximately 30% of patients with PsO may develop PsA, especially those with severe PsO or nail or scalp involvement. The estimated prevalence of PsA is between 30 and 100 cases per 10,000 people.
PsA usually precedes the onset of arthritis by an average of 10 years, although in up to 15% of cases arthritis may overlap with the onset of skin disease or even appear earlier. [
8] Historically, 5 patterns of PsA have been described, which may change and overlap during the course of the disease: polyarticular, oligoarticular, distal, arthritis mutilans and axial or spondyloarthritis. The current trend is to differentiate three clinical forms: peripheral, axial and mixed. [
9]
Peripheral manifestations:
Peripheral manifestations include peripheral arthritis, dactylitis and enthesitis.
The distal subtype affects the interphalangeal joints of the hands and feet. Onychopathy is especially frequent in this subtype of PsA. The oligoarticular subtype affects 4 or fewer joints and usually presents with an asymmetric distribution. The polyarticular subtype affects 5 or more joints, the involvement of which may be symmetrical and resembles rheumatoid arthritis (RA). Mutilating arthritis, a deforming and destructive subtype, involves marked bone destruction and manifests with telescoping and unstable fingers, is the most severe and least common type of PsA, occurring in less than 5% of patients and causing significant deformity and disability. Radiographically, peripheral joints affected by PsA show eccentric erosions and narrowing of the joint interlining together with bony proliferation and periostitis, all occurring in the same joint, giving the typical "pencil-in-cup" FIGURE. [
10]
Peripheral manifestations of PsA also include enthesitis and dactylitis.
An enthesis is a place where the tendon, ligament or joint capsule inserts into the bone to facilitate joint movement.
Enthesitis is inflammation of one of these structures. It can be found in up to 50% of patients and most commonly affects the plantar fascia and Achilles tendon, but can also affect the patellar tendon, iliac crest, epicondyles and supraspinatus insertions [
11].
Dactylitis, often referred to as "sausage toe", is characterised by diffuse swelling of an entire finger or toe. It uniformly affects the soft tissues between the metacarpophalangeal and interphalangeal joints and is seen in 40-50% of patients. It is often seen in the context of polyarthritis and in up to 2/3 of patients it manifests only in the feet. Dactylitis can be acute (swelling, reddening of the skin and pain) or chronic (swelling without inflammation) and is often associated with a more severe disease course. [
12]
Axial manifestations:
PsA shares genetic and clinical features with other forms of spondylarthritis (SpA) and is included in this group of diseases. The axial subtype is characterised by inflammation and postinflammatory structural changes of the spine and/or sacroiliac joints. Axial involvement occurs in 25-70% of patients with PsA, and only 2% to 5% of patients with PsA have exclusively axial involvement; most patients with axial disease as a manifestation of PsA also have peripheral arthritis.
A common feature of axial involvement is inflammatory rickets or low back pain characterised by insidious onset, morning stiffness > 30 minutes, improvement with exercise and worsening with rest, and nocturnal pain, especially in the second half of the night. [
13]
Risk factors for developing axial involvement in PsA are HLA-B27 positivity, the presence of radiological peripheral joint damage and increased erythrocyte sedimentation rate (ESR). [
14]
Although axial PsA is usually less severe than ankylosing spondylitis (AS), it significantly worsens the impact of the disease on patients' quality of life.
The presence of the HLA-B27 allelic variant is associated with more severe PsA, and these variants are found more frequently in patients with axial involvement. [
15]
In some cases, axial involvement in PsA may be clinically asymptomatic despite inflammation in the axial skeleton and the condition is detectable only by radiographic or radiological examination. Radiographic sacroiliitis is a common feature of axial PsA, occurs in 25% to 50% of patients and is often asymmetric. Typical structural changes in the spine include thick, asymmetrical non-marginal syndesmophytes and, in some cases, early involvement of the cervical spine, including fusion of the interfacial joints, which may occur in the absence of sacroiliitis or relevant joint involvement in the rest of the spine.
Magnetic resonance imaging can help detect those inflammatory changes present in the early stages of the disease not identifiable on plain radiographic study, such as bone and soft tissue oedema in the sacroiliac joints and vertebral bodies. [
15]
1.3. Extra Musculoskeletal and Extracutaneous Manifestations
PsD is associated similarly to other SpA with a variety of immune-mediated extra-musculoskeletal manifestations, such as uveitis and IBD. The risk of developing these manifestations is higher in patients with PsA compared to those with psoriasis alone or in the general population. [
16]
IBD (Chron's disease and ulcerative colitis) occurs in 11% of patients with axial PsA and is significantly more common in patients with axial involvement than in those with peripheral-only PsA (2%). [
13]
Symptoms that should lead to a preferential referral to a gastroenterologist are chronic diarrhoea with bloody stools, chronic abdominal pain, symptoms that do not respect night rest, perianal fistula or abscess. In addition, other general signs and symptoms associated with these manifestations are oral thrush, anaemia, fever and weight loss. However, it should be noted that Epso patients may often have subclinical forms, detectable only through high faecal calprotectin values and endoscopic study. [
17]
Ophthalmic manifestations are estimated to occur in 10% of patients with PsD and 31% of patients with PsA, with uveitis being the most frequent ocular involvement.
Anterior uveitis (AU) is the most frequent clinical phenotype of uveitis in PsD. The prevalence ranges from 2 to 25 % of cases and is most often seen in patients with axial or HLA-B27 positive disease. Typical symptoms are usually intense photophobia with ocular pain, lacrimation, marked ocular redness and, in more severe cases, blurred vision due to the abundant inflammatory precipitate in the anterior chamber. Although recurrent AU is the most common type, there may be synchronous involvement of both eyes, which is rare in AU in the context of SpA. [
17,
18,
19]
The physician should consider these two common extra-articular manifestations when choosing the most appropriate treatment for patients.
1.4. Cardiovascular Comorbidities
More recently it has been shown that patients with PsA have a higher prevalence and incidence of cardiovascular disease (CVD) than the general population. This increased risk has been attributed not only to a higher prevalence of traditional risk factors (hypertension, obesity, diabetes and hyperlipidaemia), but also as a result of chronic systemic inflammation. Increased carotid atheromatous plaques have been found in these patients, suggesting that exposure to chronic inflammation may influence the excess cardiovascular risk. [
20]
Observational studies have shown an increased risk of morbidity from myocardial infarction, cerebrovascular disease and heart failure compared to the general population. [
21]
Among cardiovascular risk factors, hypertension has recently been found to be the most prevalent comorbidity in patients with PsA (39% of patients), followed by hyperlipidaemia, diabetes and obesity [
22]. For obesity, studies suggest that weight gain may be a consequence of the systemic inflammatory state, or that obesity itself may lead to more weight on the joints, altering joint mechanics and causing repetitive microtrauma, which could trigger enthesitis and synovitis [
23] and hinder response to treatment. [
17]
1.5. Other Comorbidities
In addition to the above, an increased incidence of hepatic steatosis, metabolic syndrome, depression, anxiety, fibromyalgia and osteoporosis has been reported in patients with PsD [
17,
24].
1.6. Assessment of Disease Activity
Assessing the degree of disease activity, both for the musculoskeletal domain and the severity and extent of psoriasis, is essential to choose the most appropriate treatment and to be able to apply modern "treat to target" treatment strategies.
To assess the degree of skin involvement there are several tools more or less used in daily clinical practice as well as in clinical trials. The Psoriasis Area Severity Index (PASI) score is the most widely used tool to assess the severity of psoriasis, and the PASI75 (75% or more reduction in PASI scores from baseline) represents one of the most commonly used targets in psoriasis trials. It is a combination of the severity of skin involvement with the extent of the affected area in a single score ranging from 0 (no disease) to 72 (maximum disease). The body is divided into four areas (head, upper limbs, trunk and lower limbs) and for each area the percentage (0% = 0 points; 100% = 6 points) of affected skin and the severity of erythema, induration and scaling (0-4 points) is estimated. Each of these areas has a different weight in the final calculation and scores on its own; once the degree of involvement has been calculated for each of these, they are combined to obtain the final result. [
25,
26]
When joint involvement is present, clinical trials usually measure the degree of activity using rheumatoid arthritis-derived tools such as the American College of Rheumatology (ACR) 20, ACR 50 and ACR 70 response rate (indicating reductions in the number of painful and swollen joints) of at least 20%, 50% and 70% from baseline, together with an improvement in at least three of the following five items: global assessment of arthritis by the patient and the responsible physician measured on a visual analogue scale (VAS), degree of pain by the patient measured on a VAS, degree of disability measured by the HAQ-DI, and changes in C-reactive protein (CRP) levels. [
27]
There are several composite scores that measure disease activity taking into consideration various domains, such as axial and peripheral skin and joint involvement. [
28]; among them, one of the most widely used in clinical practice is the Disease Activity Index for Psoriatic Arthritis (DAPSA) and is based on the sum of five variables: tender and swollen joint count, global assessment of the patient's disease on a 10 cm VAS, as well as global assessment of the patient's pain on the same scale and CRP as an acute phase parameter. [
29] It has been validated for use in PsA, where it has been shown to correlate well with disease activity and also correlates well with ultrasound-assessed synovitis. [
30]
1.7. Aetiopathogenesis
PsD shows a complex and not yet fully elucidated pathophysiology. It is widely known that the major histocompatibility complex is a key susceptibility locus for PsA and PsO, as demonstrated by the fact that approximately 25% of patients with PsA are human leukocyte antigen (HLA)-B27 positive. [
31]
The key feature of PsO is the uncontrolled proliferation of keratinocytes secondary to proinflammatory stimuli sustained by the activation of multiple cellular pathways. Activation of plasmacytoid dendritic cells by stimuli derived from damaged keratinocytes and interferon-α production activates myeloid dendritic cells which in turn promote the activation and differentiation of T helper type 1 (Th1) and T helper type 17 (Th17) cells in the lymph nodes. These lymphocytes return to the dermis where they release interleukin-12, 17 and 22 and tumour necrosis factor α (TNF-α), along with a variety of chemokines and other cytokines. [
32] The IL-23/Th17 axis has been found to play a central role in the development and maintenance of inflammatory processes. This axis, in addition to promoting keratinocyte proliferation and other features typical of psoriasis, has been found to be closely related to the pathogenesis of musculoskeletal manifestations. Thus, in entheses, the release of IL-23 in response to biomechanical stress or microtrauma activates TH17 and the production of IL-22 and TNF-α with consequent inflammation, bone erosion and pathological bone formation.
In response to these cytokines, mesenchymal cells differentiate into osteoblasts, forming enthesophytes and syndesmophytes in the spine. Increased expression of NF-κB receptor activator ligand (RANKL) by synoviocytes, together with the pro-inflammatory cytochemical environment, drives the differentiation of precursors into osteoclasts, with subsequent production of synovitis and bone resorption. [
10]
Many of these aberrantly produced proinflammatory cytokines and growth factors act as ligands for receptors attached to intracytoplasmic tyrosine kinases called JAKs. There are 4 types of JAK: JAK 1, JAK 2, JAK 3 and TYK 2. These tyrosine kinases are able to phosphorylate tyrosine residues of other adjacent molecules called STAT. The latter are a family of transcription factors that act by downstream signalling to JAKs and consist of 7 members (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT 5b and STAT6). [
33]
Thus, JAKs activate STAT proteins, which move to the nucleus, thereby modulating the synthesis of proinflammatory nuclear factors. The JAK/STAT pathway is an evolutionarily conserved pathway that mediates the effect of many different molecules (interleukins, interferons, colony-stimulating factors, growth factors and hormones). Among all these cytokines, IL-23 and type I interferons, which play a key role in the pathogenesis of PsD, bind to their transmembrane receptor which transduce the signal through JAK-type kinases.
2. JAKinhibs in Psoriasic Disease
The increasing understanding of the aetiopathogenic mechanisms of these immune-mediated diseases and of the proinflammatory intracellular signalling pathways involved in Epso has facilitated the identification and development of new small molecules capable of inhibiting JAK proteins, emerging as effective treatment options for rheumatic and dermatological manifestations. [
2]
While biological disease-modifying antirheumatic drugs (DMARDs) (DMARDb) are monoclonal antibodies directed against one or a small subset of cytokines, such as inhibitors of IL-17, IL-23, IL-12/23 or TNF-α, JAK inhibitors (JAKinhibs) simultaneously suppress the activation and production of multiple cytokines involved in the Th1, Th2, Th17 and Th22 immune pathways.
In addition, JAKinhibs are a class of drugs that act rapidly, within a few days, with a striking beneficial class effect on pain.
The oral formulation they share makes them a well-tolerated class of drugs, with a clear advantage over FAMEb in terms of convenience of administration, improving therapeutic compliance.
Another aspect to highlight is that these small molecules do not suffer from immunogenicity problems.
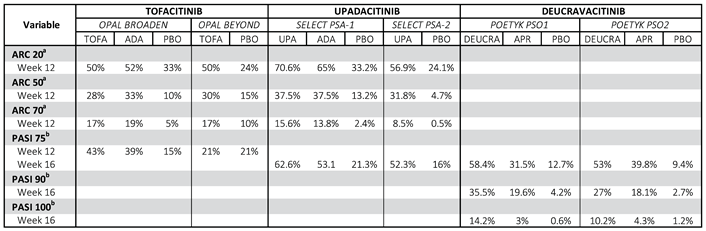
To date, the EMA/FDA has approved Tofacitinib and Upadacitinib for the treatment of PsA and Deucravacitinib for PsO.
2.1. Tofacitinib
Tofacitinib is a major inhibitor of JAK1 and JAK3 and minor inhibitors of JAK2 and TYK2 approved by the FDA and EMA for the treatment of active PsA in combination with MTX in patients with inadequate response or intolerance to a previous DMARD. The efficacy and safety of Tofacitinib was evaluated in 2 randomised, double-blind, placebo-controlled Phase III trials (OPAL BROADEN and OPAL BEYOND) [
34,
35], que se realizaron en pacientes adultos con PsA activa, definida como un recuento de articulaciones dolorosas e inflamadas igual o superior a 3 y con PsO en placas activa. Patients included in the OPAL BROADEN study were anti-TNF naïve and an active comparator (Adalimumab 40 mg/2 weeks) was also included in the study. For efficacy outcomes, ACR20 response and the Health Assessment Questionnaire Disability Index (HAQ-DI) at 3 months from the start of treatment were selected as primary endpoints. Secondary variables included among others: ACR50 and 75 response and PASI75.
Detailed results are shown in
Table 1.
In summary, treatment with Tofacitinib resulted in significantly higher ACR20 response rates compared to placebo at month 3 in both naïve and inadequate anti-TNF responders. In addition, significant differences were observed from week 2, demonstrating the rapidity of action of the drug. The HAQ-DI response rate at month 3 in both studies was statistically significantly higher in patients receiving Tofacitinib compared to those receiving placebo.
Results for secondary endpoints were also consistent (
Table 1).
Patients who participated in the pivotal trials were enrolled in an open-label extension study with long-term follow-up up to 48 months (OPAL BALANCE). [
36] This study included a total of 685 patients and found that improvements in joint and skin outcomes were sustained over time. It also provided important long-term safety data, reporting that the most common adverse events in patients treated with Tofacitinib were mild infections (nasopharyngitis, upper respiratory tract infection), headache and gastrointestinal symptoms (diarrhoea, nausea, vomiting) at a comparable rate to placebo and Adalimumab. The rate of treatment discontinuation due to adverse effects in the first 3 months was comparable (around 2%).
The incidence rate of serious infections (pneumonia most frequently) in patients treated with Tofacitinib was slightly higher than with Adalimumab, although this did not reach statistical significance.
The incidence of reactivation of Herpes Zoster (HZ) infection was higher in patients treated with Tofacitinib than in the other two groups, however, most cases of HZ resolved without complications.
Dose-dependent cytopenias (anaemia, leukopenia, lymphopenia), and elevation of liver cytolysis enzymes and lipid parameters (total cholesterol, LDL, HDL) were reported.
Overall, the safety profile observed in patients with PsA is consistent with the profile of rheumatoid arthritis patients, in terms of the type and frequency of adverse effects observed, except for major cardiovascular events, where long-term data demonstrated a lower incidence in the PsA patient cohorts compared to the results of rheumatoid arthritis studies. [
2]
2.2. Upadacitinib
The efficacy and safety of Upadacitinib, a selective inhibitor of JAK1 and, to a lesser extent, JAK2, has been evaluated in two pivotal 24-week double-blind phase III clinical trials (CTs) (SELECT-PsA-1 and SELECT-PsA-2) and is currently approved by EMA and FDA for the treatment of PsA. [
37,
38]
The SELECT-PsA-1 trial comparing Upadacitinib at different doses (15 mg or 30 mg once daily) with Adalimumab 40mg every 2 weeks subcutaneously and placebo (1:1:1:1) in approximately 1705 adult patients with active PsA and inadequate response to at least one synthetic DMARD (DMARD) found that ACR20 response rates (primary response endpoint) at week 12 were significantly lower than those of placebo: 1) in approximately 1705 adult patients with active PsA and inadequate response to at least one synthetic DMARD (DMARDs) found that the ACR20 (primary response variable) response rates at week 12 of the groups treated with the new molecule were higher than the rates of placebo patients and not lower than the rates of Adalimumab (
Table 1).
This study also demonstrated the efficacy of Upadacitinib on skin outcomes, as evidenced by good PASI75 response rates. [
37]
In SELECT-PsA-2, 642 patients with PsA and an inadequate response or intolerance to at least one DMARDb were randomized (2:2:1:1) to Upadacitinib 15 mg or 30 mg once daily, and two placebo groups. At week 12, ACR20 response rates of patients receiving Upadacitinib were significantly higher compared to placebo, as were skin outcomes.
Efficacy manifested rapidly in all responses, with the most pronounced ACR20 responses detected as early as week 2.
Secondary endpoints in both studies included, among others: ACR50 and ACR70 response rate at week 12 and week 16 (
Table 1). [
38]
In the trial extension periods of 260 weeks (5 years) in SELECT-PsA-1 and 156 weeks (3 years) in SELECT-PsA-2, patients in the placebo group were re-randomised at week 24 to receive Upadacitinib. For these subjects, the improvements achieved after Upadacitinib initiation were similar to those observed in subjects who started Upadacitinib on day 1, with comparable results at week 56.
In terms of safety, data from Phase III RCTs show an acceptable safety profile, which is also comparable to that of Adalimumab. The most common adverse reactions with Upadacitinib 15 mg were non-serious upper respiratory tract infections, nasopharyngitis, urinary tract infections, headaches, nausea and diarrhea, elevated creatine phosphokinase (CPK) and alanine aminotransferase blood transaminases and hypertension. Rates of serious infections (pneumonia most frequently), herpes zoster (HZ), anemia, neutropenia and lymphopenia remained numerically higher with Upadacitinib 15mg than with Adalimumab, but without statistically significant differences. Rates of malignancies (excluding non-melanoma cancer), major adverse cardiovascular events and venous thromboembolism were similar in all groups.
Higher rates of serious infections and elevated liver transaminases have also been observed in patients treated with Upadacitinib in combination with MTX compared to patients treated with Upadacitinib alone. Overall, the safety profile of Upadacitinib in PsA was consistent with previous experience in patients with rheumatoid arthritis.
2.3. Deucravacitinib
In PsO, Deucravacitinib, a highly selective, allosteric oral TYK2 inhibitor, has recently been approved by the EMA and FDA for the treatment of moderate-to-severe plaque PsO based on data from its two phase III CTs POETYK PSO-1 and POETYK PSO-2. [
39,
40] (
Table 1)
This molecule is also being studied for PsA (NCT03881059) and recent results from a phase II randomised clinical trial (RCT) where 203 patients with PsA were randomised to Deucravacitinib at doses of 6 mg and 12 mg once daily and placebo have shown statistically significant differences in ACR 20 response rate at week 16 for both treatment groups. [
41]
Phase III RCTs are currently under development.
JAK kinase inhibition appears to be a promising treatment for PsD and several RCTs are ongoing for other JAK inhibitors.
It is recognised that the clinical characteristics of patients included in RCTs may differ from those in daily clinical practice and it is essential to conduct observational studies to obtain real-world evidence needed to improve the decision strategy and to verify evidence from the literature.
3. Objectives
Therefore, the aim of our study is to evaluate the efficacy and safety of Tofacitinib and Upadacitinib (the only JAKinhibs currently approved for the treatment of PsA) in both the musculoskeletal and cutaneous domains of PsD in daily clinical practice, analysing data from a cohort of patients seen in a shared multidisciplinary Dermatology-Rheumatology practice.
4. Methods
Observational, retrospective, single-centre, single-centre study in which data were collected on patients diagnosed with PsA seen in the last 2 years (from December 2021 to December 2023) in the shared Dermatology-Rheumatology consultation of a second-level hospital in the province of Granada (Spain).
Only data from patients over 18 years of age who had been diagnosed with PsA according to the criteria of the Classification Criteria for Psoriatic Arthritis (CASPAR) group were included [
42], who started treatment with Tofacitinib or Upadacitinib due to failure of one or more DMARDb and who had no major contraindications to starting treatment with that class of drugs (>65 years, personal history of cardiovascular events, multiple cardiovascular risk factors, personal history of malignancy, high risk of thromboembolic disease, pregnancy and/or breastfeeding, active infection or personal history of M. tuberculosis, hepatitis B or C virus infection). All patients were refractory to at least 1 DMARDb. Tofacitinib was used at a dose of 5mg orally twice daily or at a dose of 11mg once daily and Upadacitinib at a dose of 15mg once daily.
Follow-up at our clinic was carried out with quarterly appointments and patients attended once a month for control and monitoring tests at their health centre, where they were seen by a primary care physician.
All continuous variables were tested for normality and results were expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR), as appropriate.
Variables collected at the baseline visit included: age, sex, concomitant diseases, time of disease progression, presence of radiographic erosions, number of previous biologic treatments, concomitant treatment with any DMARDs (Methotrexate, Leflunomide or Sulphasalazine), chronic glucocorticoid treatment and dose.
As for the outcome variables, at the baseline appointment and at each subsequent appointment the number of painful joints (NAD), number of swollen or inflamed joints (NAT), patient's global assessment of arthritis activity measured through a VAS ranging from 0 to 100mm, patient's global assessment of arthritis activity measured through a VAS ranging from 0 to 100mm, patient's global assessment of pain secondary to arthritis activity measured through a VAS ranging from 0 to 100mm, patient's global assessment of pain secondary to arthritis activity measured through a VAS ranging from 0 to 100mm, patient's global assessment of pain secondary to arthritis measured by VAS ranging from 0-100mm, assessor's global assessment of arthritis measured by VAS ranging from 0-100mm, CRP (mg/l), erythrocyte sedimentation rate (ESR), PASI and DAPSA.
At 3 months after initiation of treatment, ACR 20/50/70 response and PASI75 response rates were calculated for each patient. Outcome variables were assessed and compared between baseline and 3 months.
This study was not funded by any pharmaceutical company. It was the result of an independent initiative by the investigators and has been approved by the Provincial Ethics Committee of Hospital Services of Granada with the code HUSC_DER_REU_2004_001.
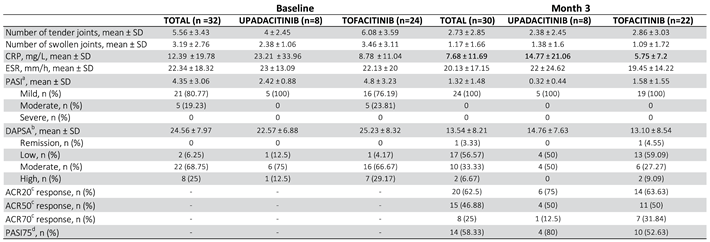
5. Results
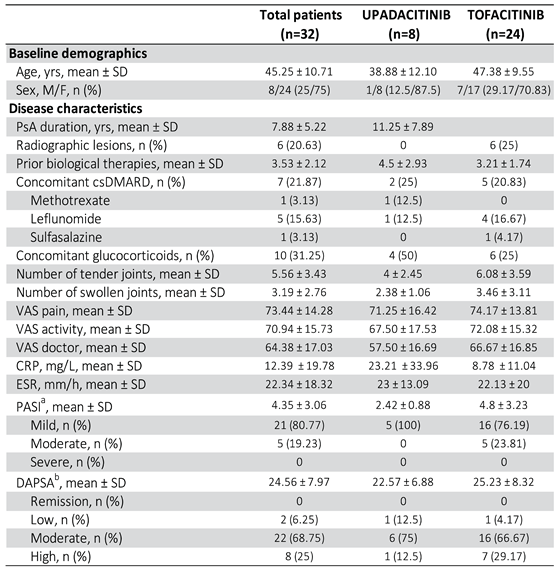
5.1. Baseline Demographic and Clinical Characteristics
Data from 32 patients (24 women and 8 men) were included, of whom 24 (75%) started treatment with Tofacitinib and 8 (25%) with Upadacitinib (
Table 2).
The mean age of the total patients was 45.25 ± 10.71 years, with some differences between the Tofacitinib (47.38 ± 9.55) and Upadacitinib (38.88 ± 12.10) groups.
All patients fulfilled the CASPAR criteria for the diagnosis of PsA. The joint involvement pattern of PsA was peripheral (n = 26), mixed (n = 6) and no pure axial PsA. The mean time of disease progression from diagnosis of PsA to initiation of treatment with one of the two JAKinhibs was 6.5 years ± 5.22 years, slightly longer in patients treated with Upadacitinib than in those who started Tofacitinib (
Table 2).
In terms of clinical characteristics, of the total patients, 26 (81.25%) had had or had cutaneous psoriasis at the time of JAKinhib initiation.
In terms of radiographic features, 6 patients (18.75%) had objectionable erosions on hand radiographs at the time of treatment initiation.
Prior to the start of JAKinhib, all patients had received at least one DMARDb, with a mean of 3.53 ± 2.12 previous treatments received per patient.
At the time of initiation of Tofacitinib or Upadacitinib: 7 patients (21.87%) were on concomitant treatment with a DMARD, of which 4 with methotrexate, one with sulphasalazine and one with leflunomide. In the remaining 25 (78.13%) patients, Jakinihb was used as monotherapy. The number of subjects treated with a concomitant corticosteroid regimen at the start of JAKinhib was 10 patients (31.25%), all on Prednisone 5mg/day or lower.
Regarding analytical data, mean CRP levels were 12.39 ± 19.78 and mean ESR 22.34 ± 18.32 before starting Tofacitinib or Upadacitinib.
As for baseline disease activity data: mean number of painful and swollen joints was 5.56 ± 3.43 and 3.19 ± 2.76 respectively; mean VAS of pain and activity according to the patient was 73.44 ± 14.28 and 70.94 ± 15.73 respectively; mean VAS from the physician's point of view was 64.38 ± 17.03.
As for the composite activity indices: the mean baseline DAPSA was 24.56 ± 7.97, with 2 patients (6.25%) classified as low activity, 22 (68.75%) as moderate activity and 8 (25%) as high activity; the baseline PASI of the 26 patients (81.25%) with psoriasis at baseline was 4.35 ± 3.06, with 21 patients (80.77%) with mild involvement, 5 (19.23%) with moderate involvement and none with severe involvement according to the established thresholds. All 5 patients who received Upadacitinib and who had psoriasis at baseline had mild involvement based on PASI categories.
5.2. Efficacy Results
Overall, after the first 3 months of therapy with Tofacitinib and Upadacitinib, most patients experienced rapid joint improvement as well as good results in skin clearance. Selected endpoints (NAD, NAT, CRP, ESR, ESR, PASI, DAPSA, ACR20, ACR50, ACR70 and PASI75) showed significant improvement in the first quarter of therapy (
Table 3).
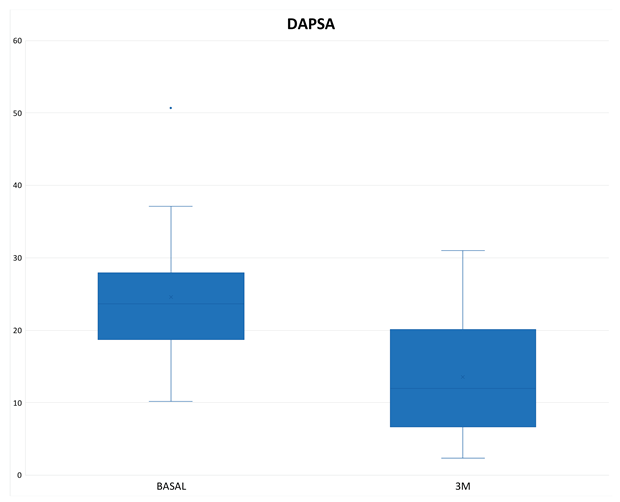
The mean DAPSA at 3 months was 13.54 ± 8.21 with a reduction of 11.02 points from baseline (
Figure 1), achieving remission in 1 patient (3.33%), low disease activity in 17 patients (56.66%), with 10 patients (33.3%) remaining at moderate activity and 2 (6.66%) at high (
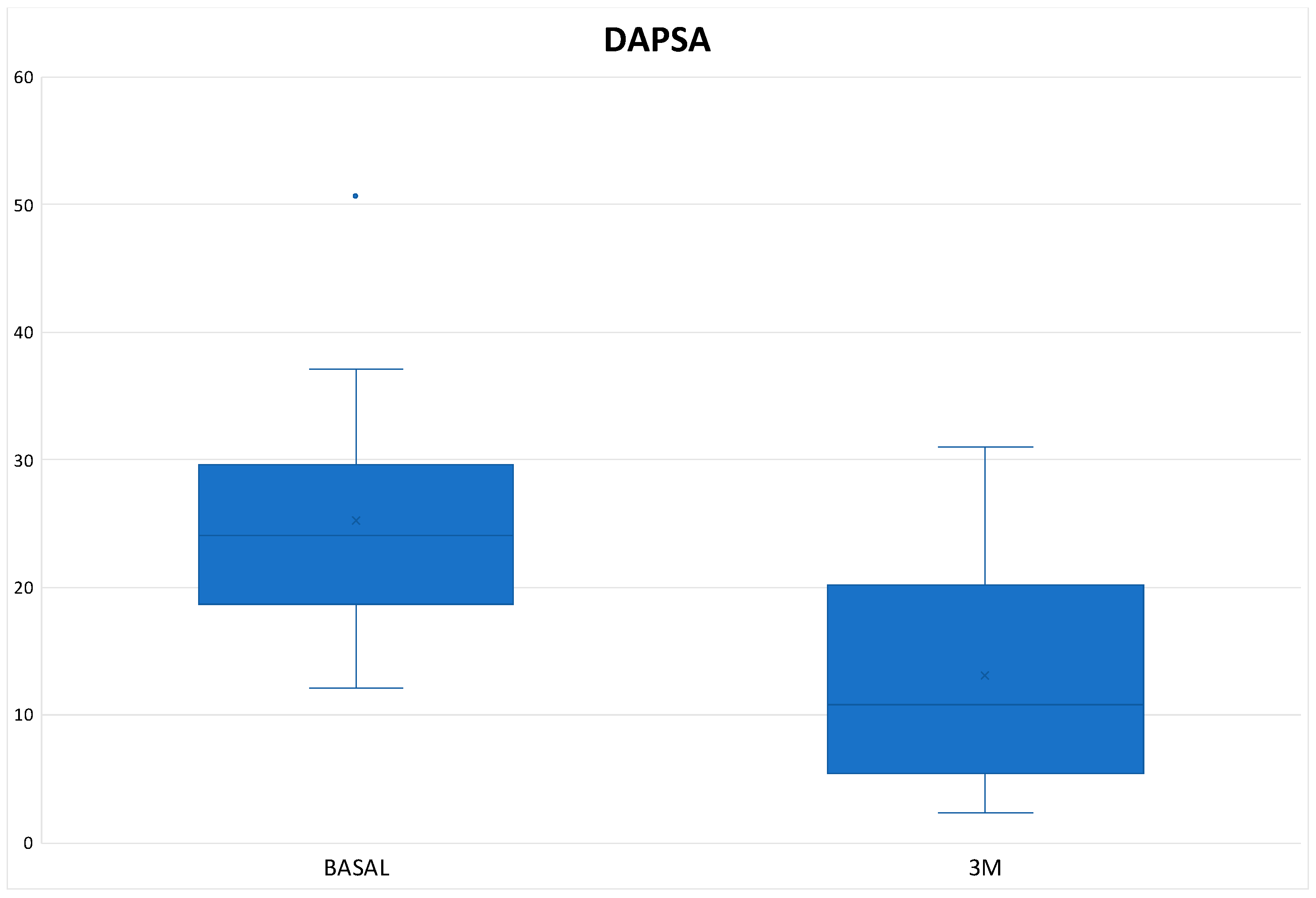
Table 3). In the Tofacitinib group the mean DAPSA was 13.10 ± 8.54 and of the 22 patients who remained on treatment at 3 months (initial total of 24 patients, of whom 2 stopped treatment before 3 months), 14 patients (63.64%) achieved remission or low disease activity [1 patient (4.54%) achieved remission and 13 (59.10%) achieved low disease activity] (
Figure 2 and
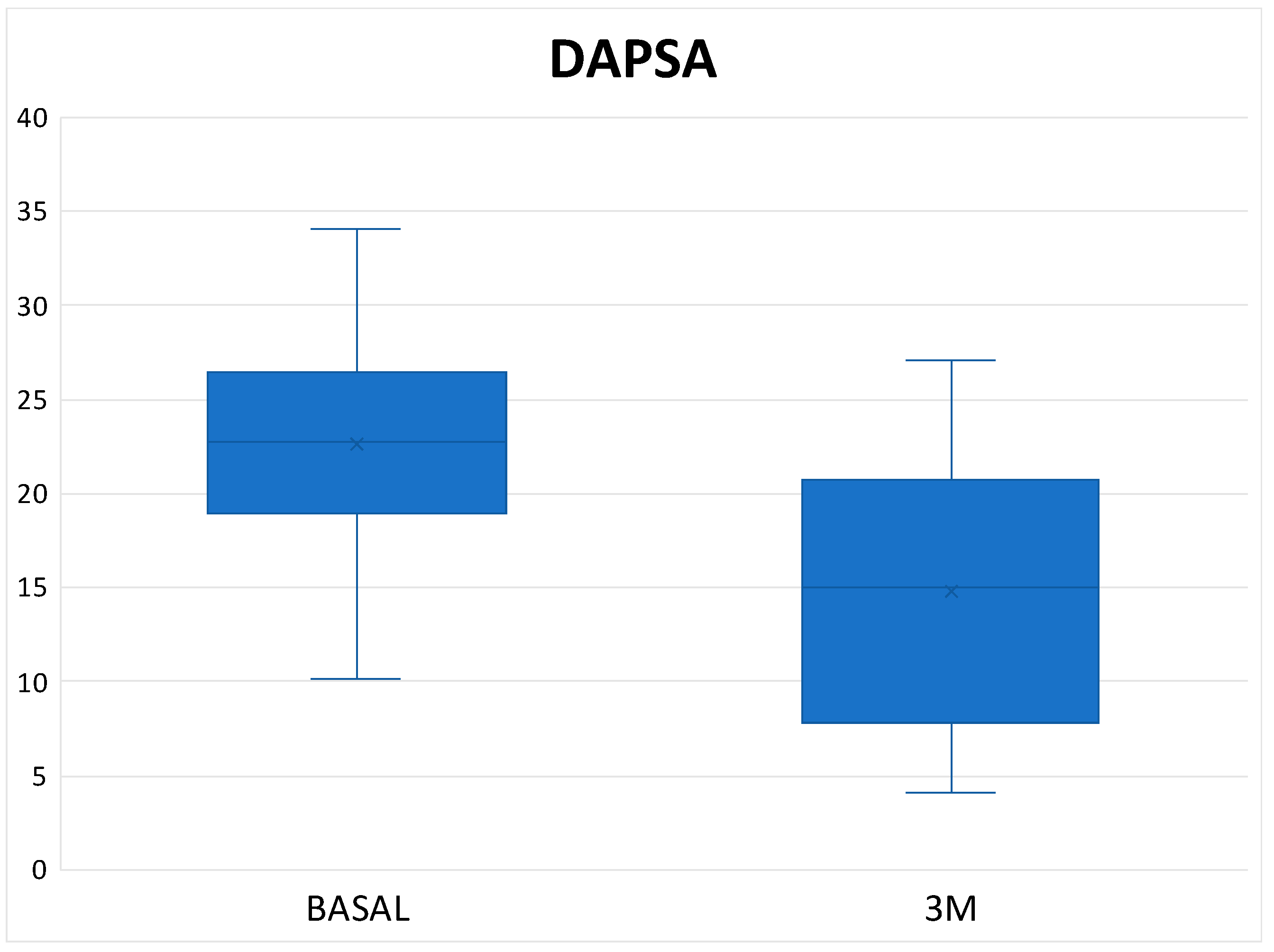
Table 3). In the 8 patients who were on treatment with Upadacitinib, the mean DAPSA at three months was 14.76 ± 7.63 and 50% of them achieved low disease activity; the other half remained at moderate activity (
Figure 3 and
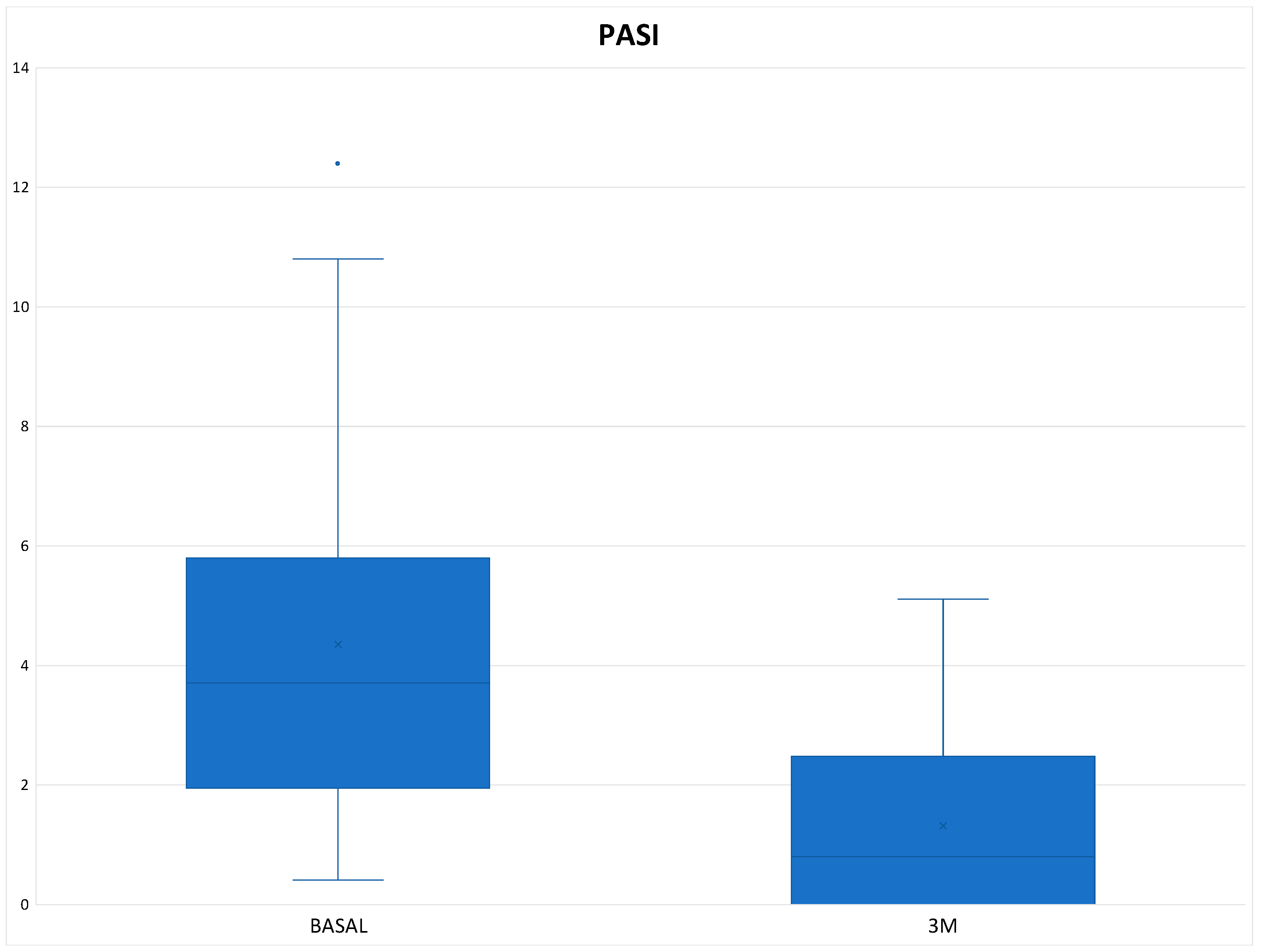
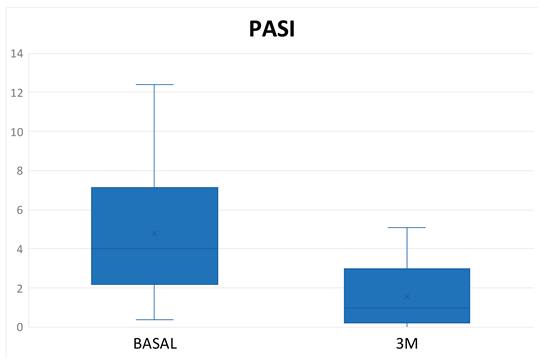
Table 3). In terms of PASI, there was a reduction of 3.03 points at 3 months of treatment (
Figure 4), with a total mean of 1.32 ± 1.48 and all patients were classified as "mildly affected" according to the established thresholds (
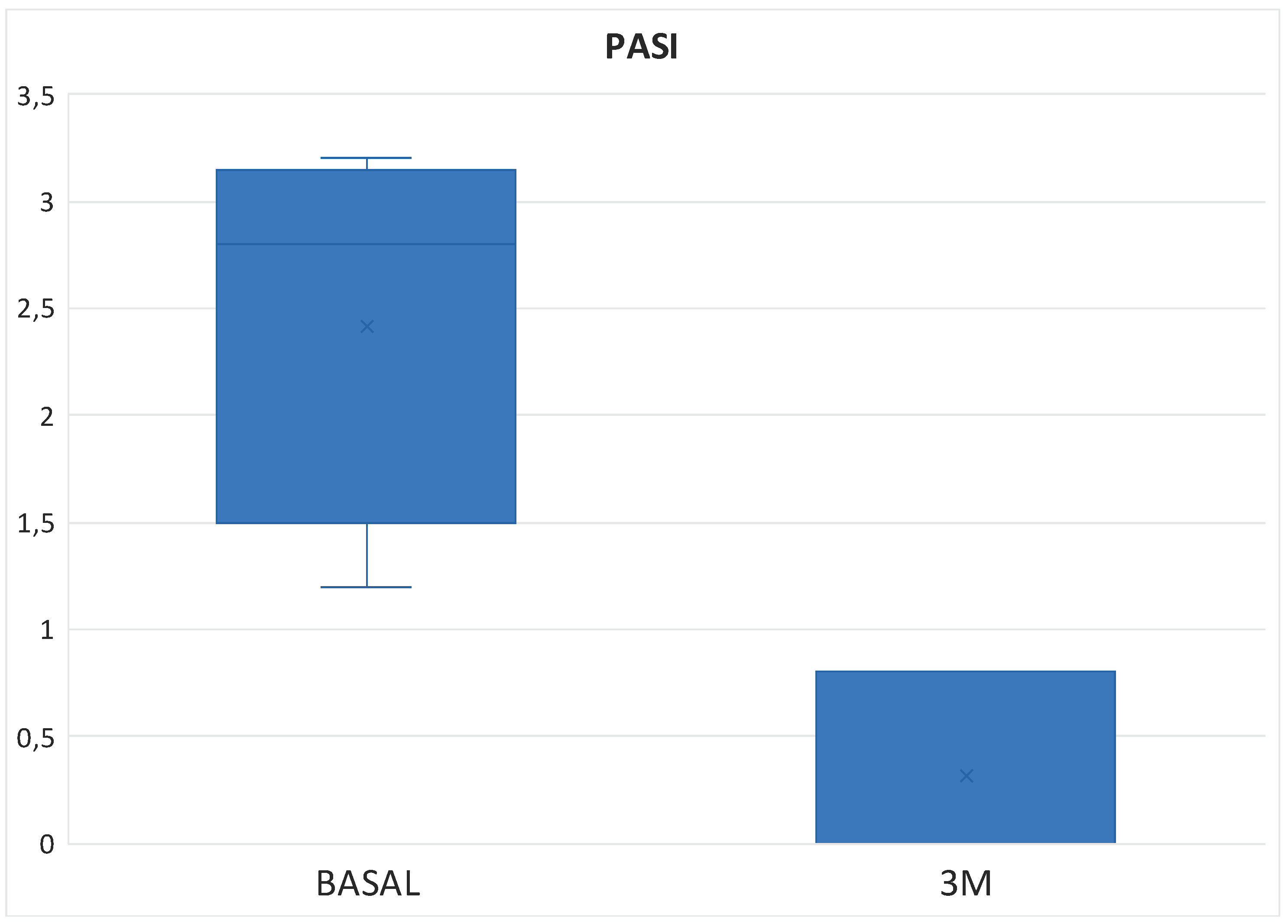
Table 3). In the Tofacitinb group the mean PASI was 1.58 ± 1.55 (
Figure 5) and in the Upadacitinib group it was 0.32 ± 0.44 (
Figure 6).
At 3 months, ACR20 response rates with both Tofacitinib and Upadacitinib were significant, with 20 patients (66.66%) of 30 total achieving this outcome [14 (63.63%) with Tofacitinib and 6 (75%) with Upadacitinib]. Similarly good results were also obtained with ACR50 and ACR70 response rates, respectively 50% and 26.66% (
Table 3).
At the cutaneous level, both JAKinhibs proved effective, achieving PASI75 response rates of 58.33% (52.63% in the Tofacitinib group and 80% in the Upadacitinib group).
None of the patients who had no skin involvement at the time of initiation of JAKinhib therapy had any psoriatic lesions at 3 months.
The remaining data on outcome variables are summarised in
Table 3.
5.3. Safety, Retention and Adverse Effects Data
The retention rate of JAKinhibs at 3 months was 93.75%, respectively 91.66% in the Tofacitinib group and 100% in the Upadacitinib group. Two patients discontinued treatment with Tofacitinib before 3 months due to mild adverse events: the first patient discontinued treatment due to gastro-oesophageal reflux resistant to proton pump inhibitor treatment that ceased when Tofacitinib was withdrawn and the second due to diarrhoea and a fixed drug exanthema skin reaction that was diagnosed by the dermatology department and subsided when treatment was discontinued. No serious adverse events were observed.
The patients had no mild or severe infections. There was no case of HZ reactivation.
No thrombotic or cardiovascular events were observed, no cytopenias or thrombopenias occurred, and mean haemoglobin, lipid and transaminase levels remained stable throughout follow-up.
No neoplasm appeared during the treatment period.
6. Discussion
JAKinhibs represent a novel and interesting class of drugs that have been shown to be safe, effective and rapid in the treatment of PsD by inhibiting the action of several inflammatory cytokines important for the disease process.
As mentioned above, both Tofacitinib and Upadacitinib have demonstrated efficacy in RCTs of PsA refractory to DMARDs (OPAL Broaden and SELECT-PsA-1) and TNF inhibitors (OPAL Beyond and SELECT-PsA-2). In the OPAL Beyond trial, which included patients refractory to at least one biologic therapy, as in our cohort, the 3-month ACR20 and 50 response rates with Tofacitinib at 5mg/12h were significantly higher compared to placebo, as were changes in HAQ-DI score. However, in terms of PASI75 response, the 5mg/12h dose failed to demonstrate superiority to placebo. In the post hoc OPAL Balance study presenting pooled data from the two phase III RCTs, a significantly higher proportion of patients treated with Tofacitinib achieved a PASI75 response at month 3 compared to placebo. [
36]
As for Upadacitinib, in SELECT-PsA-2 in patients who had previously failed a DMARDb, the ACR20, 50, 70 and PASI75 response rates were significantly higher than Placebo, and long-term persistence was demonstrated.
Comparing the baseline characteristics of the patients in our cohort with those included in the pivotal RCTs [
35,
38], we can state that, on average, our patients were younger (45,25 ± 10,71 vs 49.5 ± 12.3 [
35] y 53,0 ± 12,0 años [
38]), with a greater prevalence of women (75% vs 49% [
35] y 54% [
38]) and with a shorter duration of disease (7,8 ± 5,22 vs 9,6 ± 7,6 [
35] y 9,6 ± 8,4 [
38]).
The painful joint count (5,56 ± 3,43 vs 20,5 ± 13,0 [
35] y 24,9 ± 17,3 [
38]) and swollen (3,19 ± 2,76 vs 12,1 ± 10,6 [
35] y 11,3 ± 8,2 [
38]) and the PASI score (4,35 ± 3,06 vs 7,6 (0,6–32,2) [
35] y 10,1 ± 9,2 [
38]) were higher in patients in the trials.
In terms of treatment, in our cohort there was a higher proportion of patients on low-dose corticosteroids at the start of the JAKinhib (31,25% vs 28% [
35] y 10,4% [
38]) and had received a greater number of prior DMARDs, while the proportion of patients on concomitant treatment with any DMARDs was higher in patients in the trials.
In terms of the results obtained, as in the trials of both treatments, our patients achieved a rapid improvement in joint activity (DAPSA) and skin activity (PASI) indices, with ACR20/50/70 and PASI75 response rates even higher than those obtained in the pivotal studies.
It should be noted that, at the time treatment with JAKinhib was initiated, our patients on average had less joint and skin involvement compared to those in the trials, and were also refractory to more biologics and had a lower rate of concomitant treatment with DMARDs. Therefore, the results obtained in our cohort reinforce the idea that both Tofacitinib and Upadacitinib have a very good efficacy profile in patients with psoriatic arthritis.
In terms of tolerability and safety, these drugs have proven to be very well tolerated and safe, with only 6.25% of patients experiencing mild adverse events leading to discontinuation of treatment. Notably, we observed a lower frequency of adverse events in our study compared to ECRs. The fact that JAKinhibs were administered as monotherapy in more than ¾ of the patients in our series and that the mean age was approximately 45 years, being young patients with few comorbidities, may explain the lower frequency of adverse events in our clinical practice.
7. Limitations
Our study has several limitations. Firstly, those derived from the retrospective design of the study and the relatively small number of patients included, especially in the Upadacitinib treatment group, which limits the possibility of demonstrating the statistical significance of the data. In addition, the follow-up period was short, with data only available at 3 months. In addition, this is a purely descriptive study, with no arm for comparison with other treatments. Another limitation of this study was the lack of outcome variables studying responses in enthesitis and dactylitis. Furthermore, we are aware that retention rates may be artificially higher in patients with refractory disease who have fewer therapeutic options.
8. Conclusions
As in the pivotal trials, our patients experienced a rapid and sustained improvement in the number of painful/inflamed joints and joint activity indices. A significant improvement in PASI score was also observed. There were no major safety issues, as no patient experienced a serious adverse effect and only two patients discontinued treatment due to minor adverse events.
In conclusion, our data support that Tofacitinib and Upadacitinib are effective, rapid and safe in daily clinical practice for the treatment of PsD refractory to multiple biologic therapies, despite clinical differences with patients included in the pivotal trials.
Author Contributions
Conceptualization, Ricardo Ruiz-Villaverde, Pilar Morales-Garrido, Marta Cebolla-Verdugo, Mar Rodriguez-Troncoso and Enrique Raya-Alvarez; Data curation, Jose Carlos Ruiz Carrascosa and Marta Cebolla-Verdugo; Formal analysis, Pilar Morales-Garrido, Jose Carlos Ruiz Carrascosa, Alvaro Prados-Carmona and Mar Rodriguez-Troncoso; Investigation, Marta Cebolla-Verdugo and Alvaro Prados-Carmona; Methodology, Ricardo Ruiz-Villaverde, Pilar Morales-Garrido, Jose Carlos Ruiz Carrascosa, Mar Rodriguez-Troncoso and Enrique Raya-Alvarez; Resources, Ricardo Ruiz-Villaverde, Pilar Morales-Garrido, Alvaro Prados-Carmona and Mar Rodriguez-Troncoso; Supervision, Ricardo Ruiz-Villaverde and Enrique Raya-Alvarez; Validation, Ricardo Ruiz-Villaverde and Alvaro Prados-Carmona; Writing – original draft, Mar Rodriguez-Troncoso.
References
- Scarpa, R.; Ayala, F.; Caporaso, N.; Olivieri, I. Psoriasis, Psoriatic Arthritis, or Psoriatic Disease? J. Rheumatol. 2006, 33, 210–212. [Google Scholar] [PubMed]
- Megna, M.; Potestio, L.; Ruggiero, A.; Cacciapuoti, S.; Maione, F.; Tasso, M.; Caso, F.; Costa, L. JAK Inhibitors in Psoriatic Disease. Clin. Cosmet. Investig. Dermatol. 2023, Volume 16, 3129–3145. [Google Scholar] [CrossRef]
- Rapp, S.R.; Feldman, S.R.; Exum, M.L.; Fleischer, A.B.; Reboussin, D.M.; Carolina, N. Psoriasis Causes as Much Disability as Other Major Medical Diseases. J AM C ERMATOL 1999. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.T.; Chong, W.-S.; Tey, H.L. Nail Psoriasis: A Review. Am. J. Clin. Dermatol. 2012, 13, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Canal-García, E.; Bosch-Amate, X.; Belinchón, I.; Puig, L. Psoriasis ungueal. Actas Dermo-Sifiliográficas 2022, 113, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. The Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Hoegler, K.M.; John, A.M.; Handler, M.Z.; Schwartz, R.A. Generalized Pustular Psoriasis: A Review and Update on Treatment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1645–1651. [Google Scholar] [CrossRef]
- Karmacharya, P.; Chakradhar, R.; Ogdie, A. The Epidemiology of Psoriatic Arthritis: A Literature Review. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101692. [Google Scholar] [CrossRef]
- Gratacós Masmitjà, J.; González Fernández, C.M.; Gómez Castro, S.; Rebollo Laserna, F.J. Efficacy of Tofacitinib in the Treatment of Psoriatic Arthritis: A Systematic Review. Adv. Ther. 2021, 38, 868–884. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Kaeley, G.S.; Eder, L.; Aydin, S.Z.; Gutierrez, M.; Bakewell, C. Enthesitis: A Hallmark of Psoriatic Arthritis. Semin. Arthritis Rheum. 2018, 48, 35–43. [Google Scholar] [CrossRef]
- Kishimoto, M.; Deshpande, G.A.; Fukuoka, K.; Kawakami, T.; Ikegaya, N.; Kawashima, S.; Komagata, Y.; Kaname, S. Clinical Features of Psoriatic Arthritis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101670. [Google Scholar] [CrossRef]
- Jadon, D.R.; Sengupta, R.; Nightingale, A.; Lindsay, M.; Korendowych, E.; Robinson, G.; Jobling, A.; Shaddick, G.; Bi, J.; Winchester, R.; et al. Axial Disease in Psoriatic Arthritis Study: Defining the Clinical and Radiographic Phenotype of Psoriatic Spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 701–707. [Google Scholar] [CrossRef]
- Chandran, V.; Tolusso, D.C.; Cook, R.J.; Gladman, D.D. Risk Factors for Axial Inflammatory Arthritis in Patients with Psoriatic Arthritis. J. Rheumatol. 2010, 37, 809–815. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Jadon, D.R.; Van Den Bosch, F.; Mease, P.J.; Gladman, D.D. Axial Involvement in Psoriatic Arthritis: An Update for Rheumatologists. Semin. Arthritis Rheum. 2021, 51, 880–887. [Google Scholar] [CrossRef]
- Charlton, R.; Green, A.; Shaddick, G.; Snowball, J.; Nightingale, A.; Tillett, W.; Smith, C.H.; McHugh, N. Risk of Uveitis and Inflammatory Bowel Disease in People with Psoriatic Arthritis: A Population-Based Cohort Study. Ann. Rheum. Dis. 2018, 77, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Novelli, L.; Lubrano, E.; Venerito, V.; Perrotta, F.M.; Marando, F.; Curradi, G.; Iannone, F. Extra-Articular Manifestations and Comorbidities in Psoriatic Disease: A Journey Into the Immunologic Crosstalk. Front. Med. 2021, 8, 737079. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Nannini, C.; Cassara, E.; Kaloudi, O.; Niccoli, L. Uveitis in Spondyloarthritis: An Overview. J. Rheumatol. Suppl. 2015, 93, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Kemeny-Beke, A.; Szodoray, P. Ocular Manifestations of Rheumatic Diseases. Int. Ophthalmol. 2020, 40, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Thavaneswaran, A.; Chandran, V.; Cook, R.; Gladman, D.D. Increased Burden of Inflammation over Time Is Associated with the Extent of Atherosclerotic Plaques in Patients with Psoriatic Arthritis. Ann. Rheum. Dis. 2015, 74, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Polachek, A.; Touma, Z.; Anderson, M.; Eder, L. Risk of Cardiovascular Morbidity in Patients With Psoriatic Arthritis: A Meta-Analysis of Observational Studies. Arthritis Care Res. 2017, 69, 67–74. [Google Scholar] [CrossRef]
- Radner, H.; Lesperance, T.; Accortt, N.A.; Solomon, D.H. Incidence and Prevalence of Cardiovascular Risk Factors Among Patients With Rheumatoid Arthritis, Psoriasis, or Psoriatic Arthritis. Arthritis Care Res. 2017, 69, 1510–1518. [Google Scholar] [CrossRef]
- Kumthekar, A.; Ogdie, A. Obesity and Psoriatic Arthritis: A Narrative Review. Rheumatol. Ther. 2020, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Schwartzman, S.; Husni, M.E. Recognizing and Managing Comorbidities in Psoriatic Arthritis. Curr. Opin. Rheumatol. 2015, 27, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, T.; Pettersson, U. Severe Psoriasis--Oral Therapy with a New Retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Ellis, C.N. Evaluating Psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J. Am. Acad. Dermatol. 2004, 51, 563–569. [Google Scholar] [CrossRef]
- Felson, D.T.; Anderson, J.J.; Boers, M.; Bombardier, C.; Furst, D.; Goldsmith, C.; Katz, L.M.; Lightfoot, R.; Paulus, H.; Strand, V. American College of Rheumatology. Preliminary Definition of Improvement in Rheumatoid Arthritis. Arthritis Rheum. 1995, 38, 727–735. [Google Scholar] [CrossRef]
- Scarpa, R.; Caso, F. Spondyloarthritis: Which Composite Measures to Use in Psoriatic Arthritis? Nat. Rev. Rheumatol. 2018, 14, 125–126. [Google Scholar] [CrossRef]
- Nell-Duxneuner, V.P.; Stamm, T.A.; Machold, K.P.; Pflugbeil, S.; Aletaha, D.; Smolen, J.S. Evaluation of the Appropriateness of Composite Disease Activity Measures for Assessment of Psoriatic Arthritis. Ann. Rheum. Dis. 2010, 69, 546–549. [Google Scholar] [CrossRef]
- Husic, R.; Gretler, J.; Felber, A.; Graninger, W.B.; Duftner, C.; Hermann, J.; Dejaco, C. Disparity between Ultrasound and Clinical Findings in Psoriatic Arthritis. Ann. Rheum. Dis. 2014, 73, 1529–1536. [Google Scholar] [CrossRef]
- Eder, L.; Chandran, V.; Pellet, F.; Shanmugarajah, S.; Rosen, C.F.; Bull, S.B.; Gladman, D.D. Human Leucocyte Antigen Risk Alleles for Psoriatic Arthritis among Patients with Psoriasis. Ann. Rheum. Dis. 2012, 71, 50–55. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Samuel, C.; Cornman, H.; Kambala, A.; Kwatra, S.G. A Review on the Safety of Using JAK Inhibitors in Dermatology: Clinical and Laboratory Monitoring. Dermatol. Ther. 2023, 13, 729–749. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieślak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Coates, L.C.; Fleishaker, D.; Kivitz, A.J.; Mease, P.J.; Gladman, D.D.; FitzGerald, O.; Wang, C.; Wu, J.; Hsu, M.-A.; et al. Safety and Efficacy of Tofacitinib up to 48 Months in Patients with Active Psoriatic Arthritis: Final Analysis of the OPAL Balance Long-Term Extension Study. Lancet Rheumatol. 2021, 3, e270–e283. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for Psoriatic Arthritis Refractory to Biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaçi, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus Placebo and Apremilast in Moderate to Severe Plaque Psoriasis: Efficacy and Safety Results from the 52-Week, Randomized, Double-Blinded, Placebo-Controlled Phase 3 POETYK PSO-1 Trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Thaçi, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus Placebo and Apremilast in Moderate to Severe Plaque Psoriasis: Efficacy and Safety Results from the 52-Week, Randomized, Double-Blinded, Phase 3 Program fOr Evaluation of TYK2 Inhibitor Psoriasis Second Trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef]
- Mease, P.J.; Deodhar, A.A.; van der Heijde, D.; Behrens, F.; Kivitz, A.J.; Neal, J.; Kim, J.; Singhal, S.; Nowak, M.; Banerjee, S. Efficacy and Safety of Selective TYK2 Inhibitor, Deucravacitinib, in a Phase II Trial in Psoriatic Arthritis. Ann. Rheum. Dis. 2022, 81, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. ; CASPAR Study Group Classification Criteria for Psoriatic Arthritis: Development of New Criteria from a Large International Study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).