Submitted:
05 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
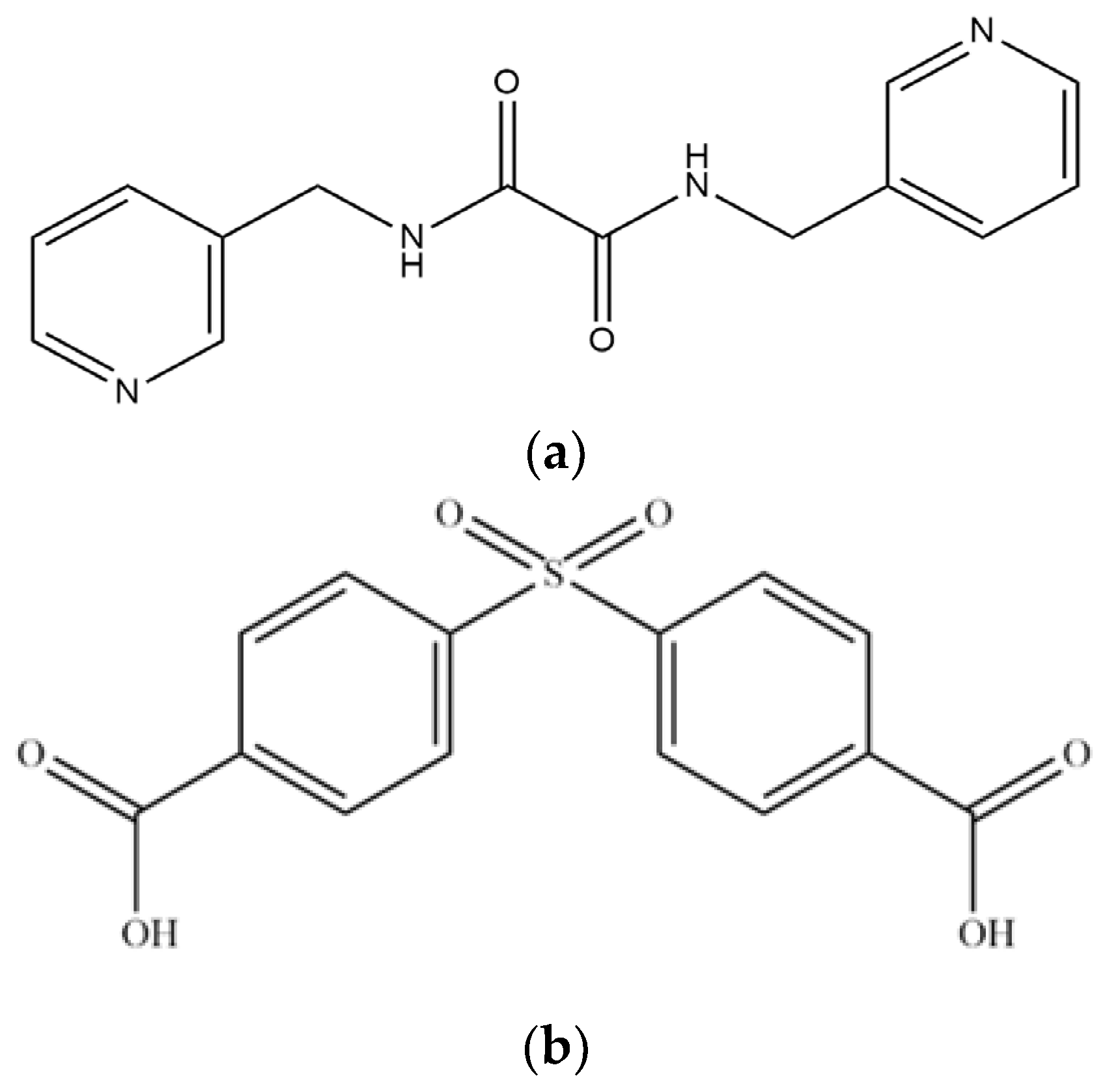
1. Introduction
2. Results and Discussion
2.1. Preparations for Complexes 1 – 4
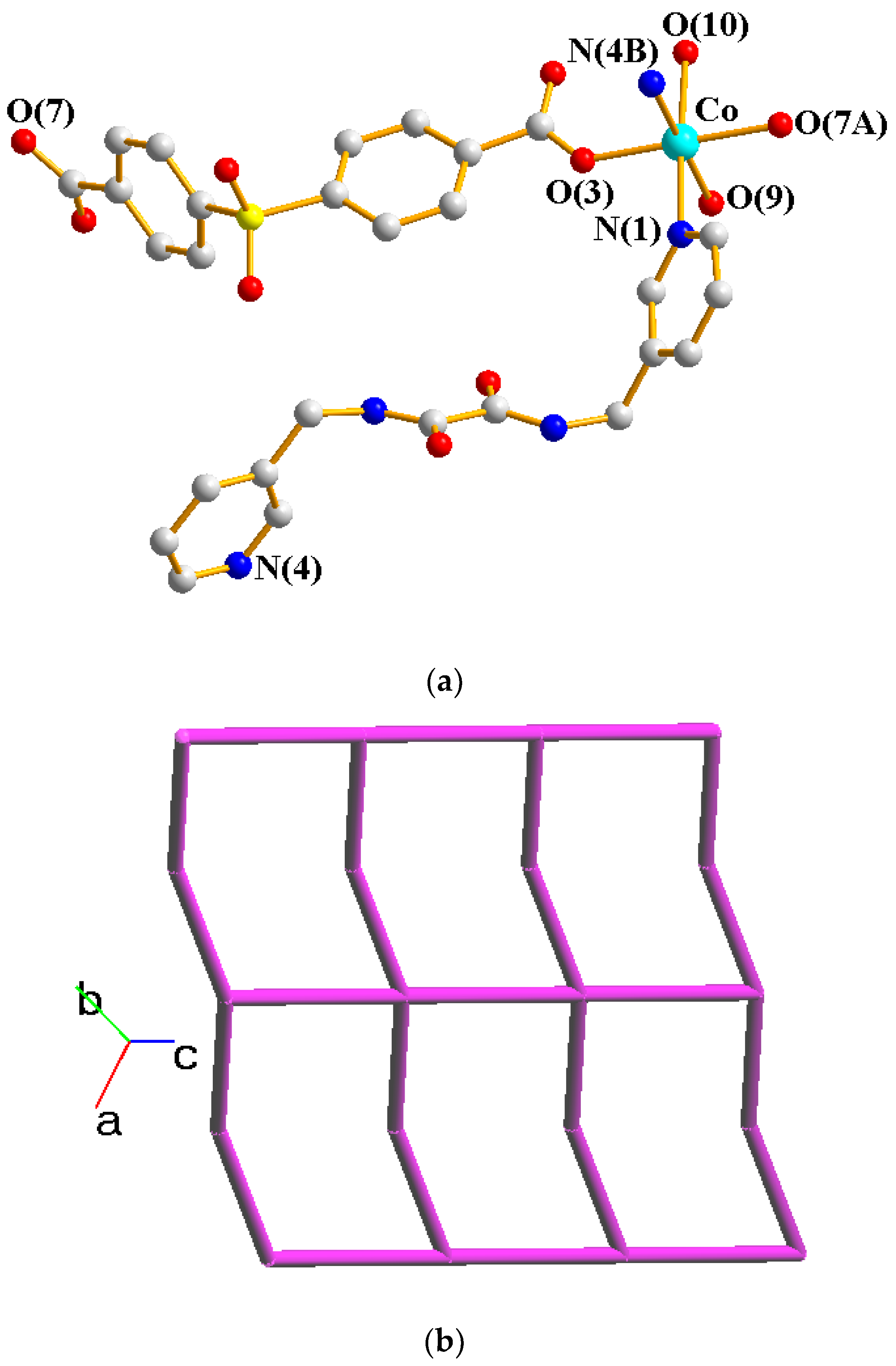
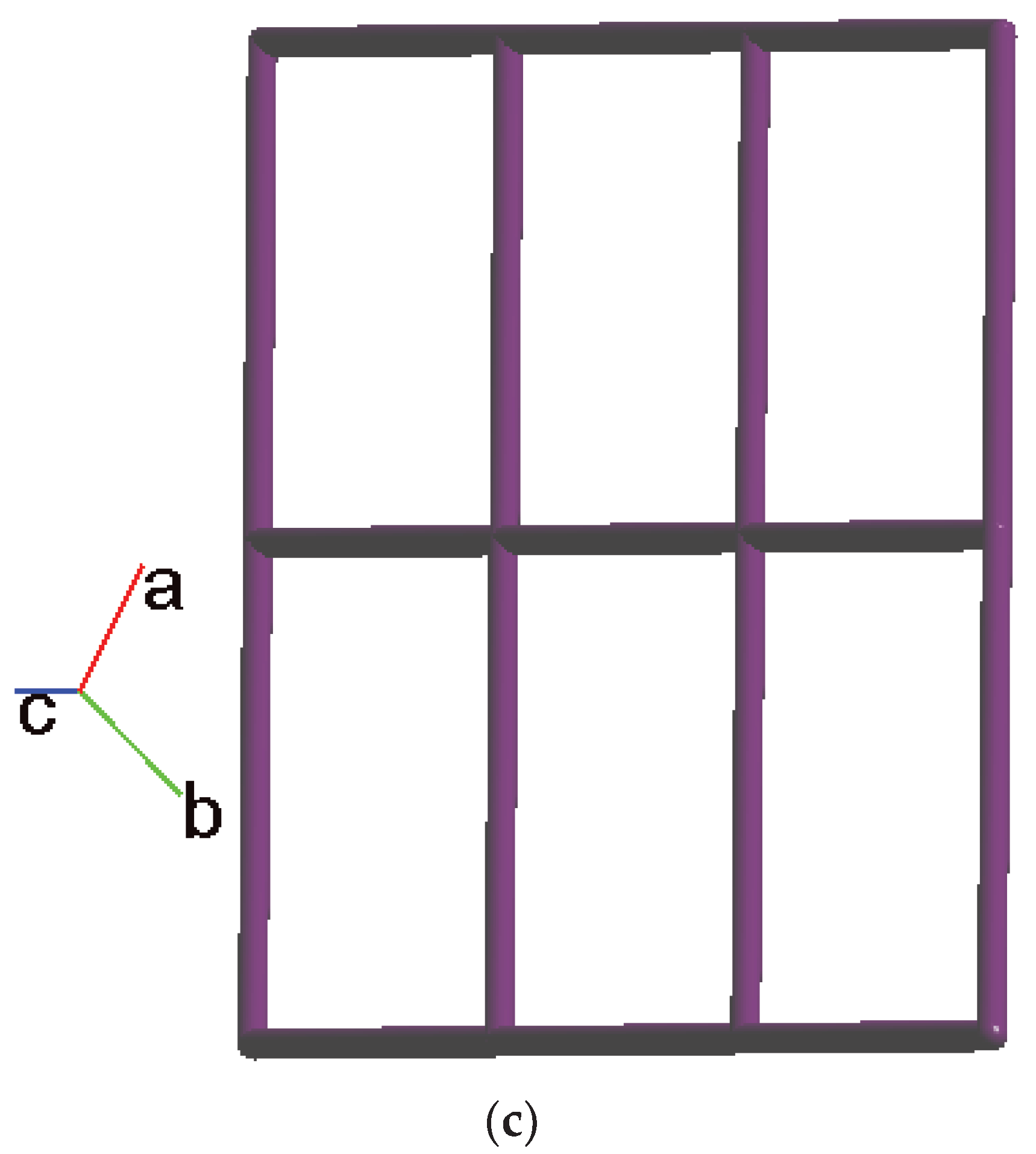
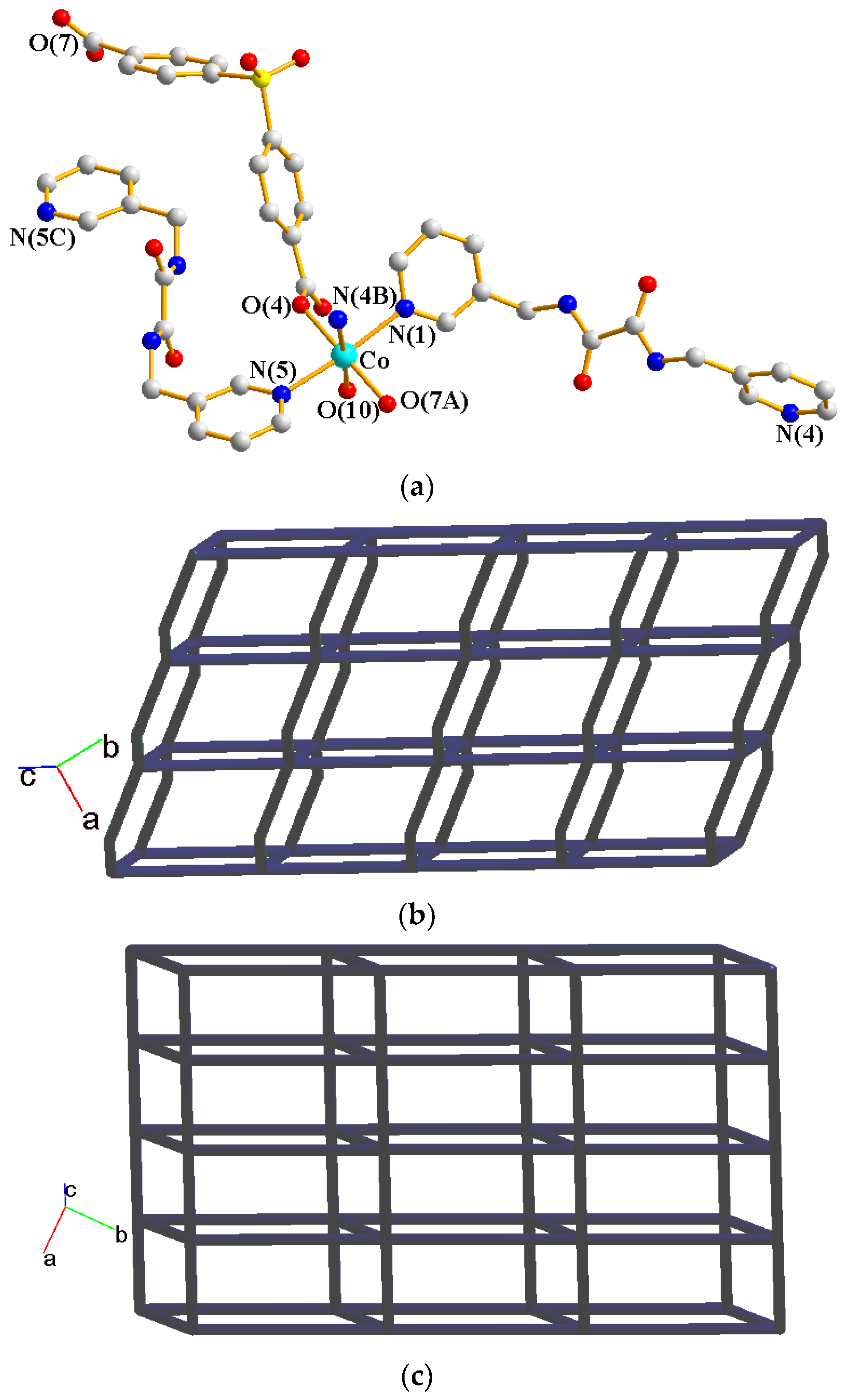
2.2. Structure of 1
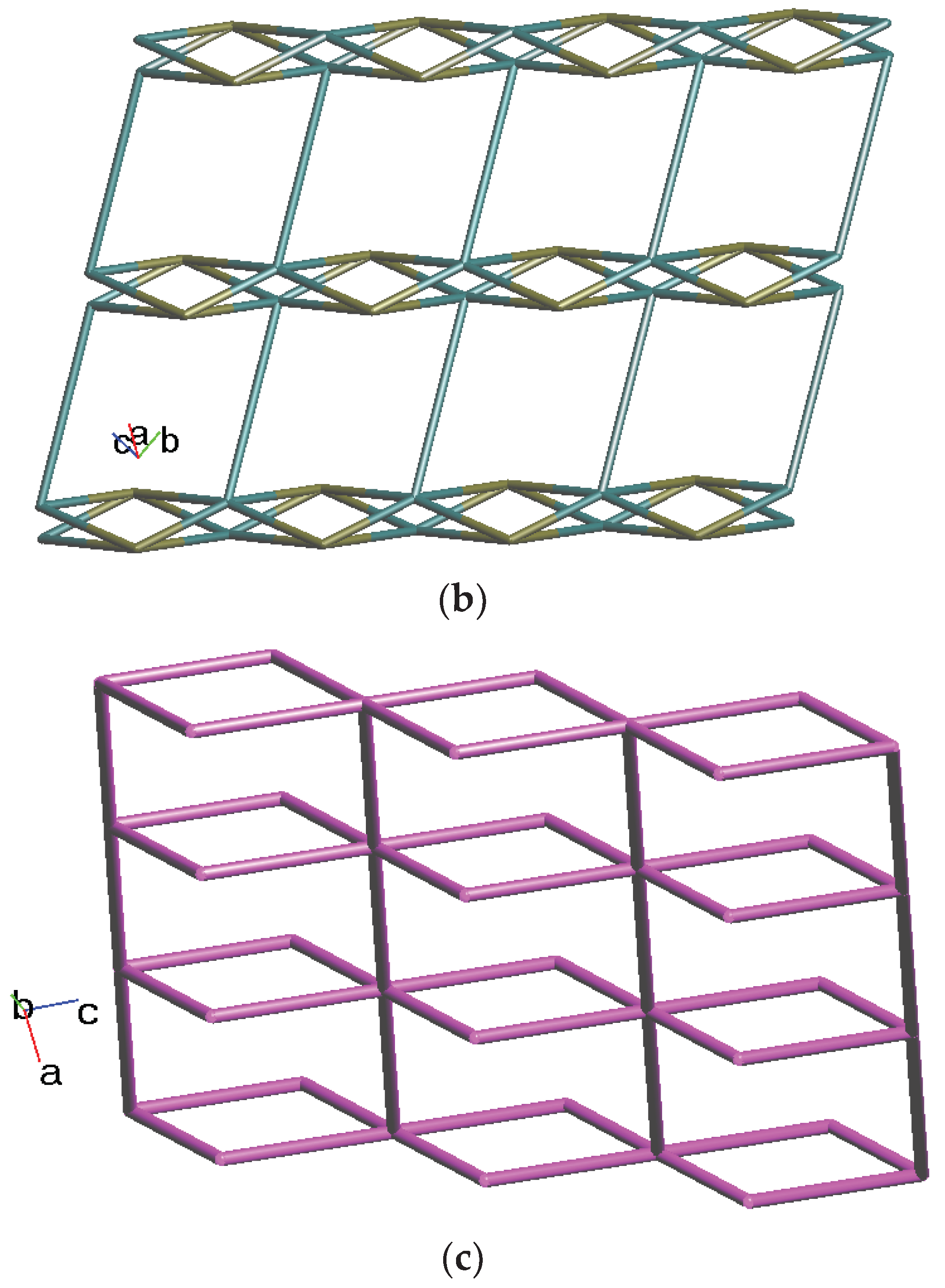
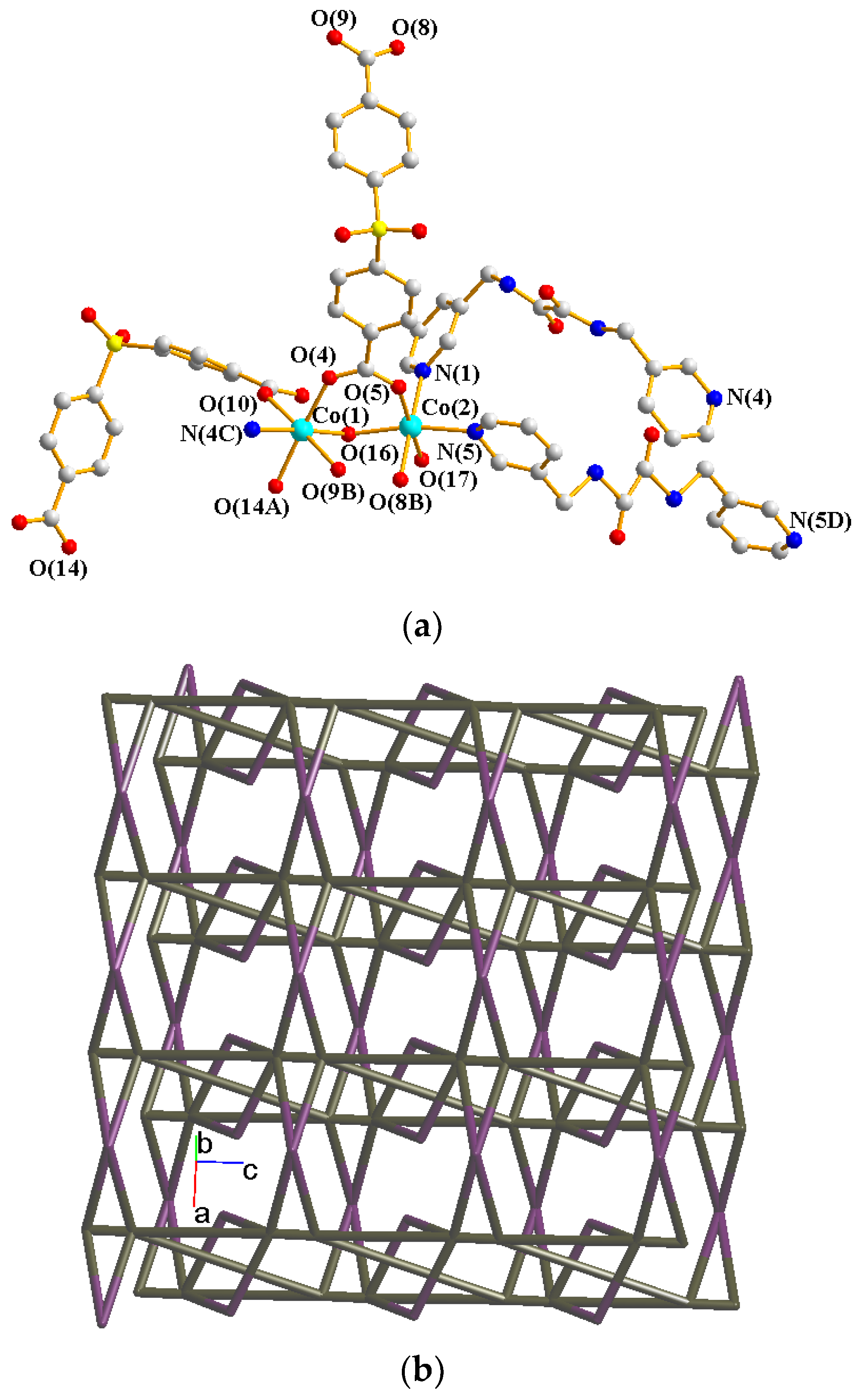
2.3. Structure of 2
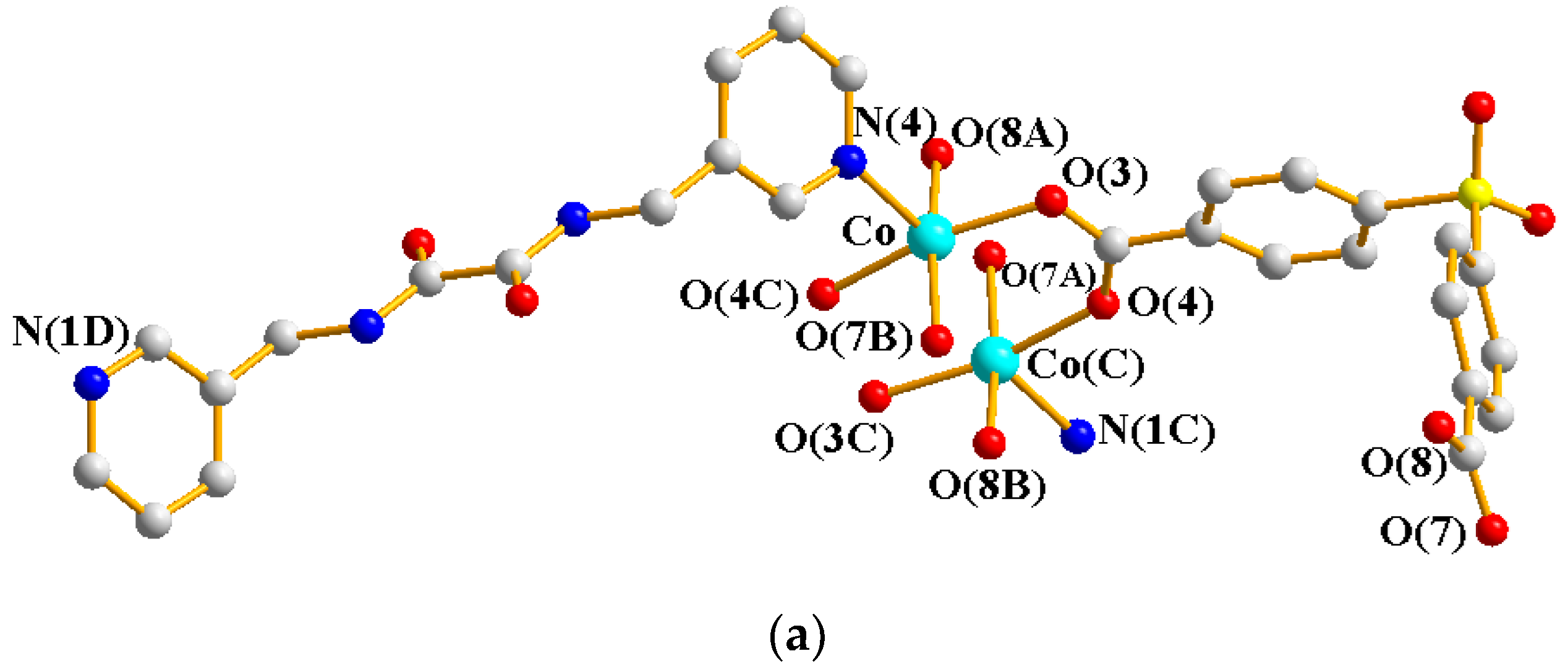
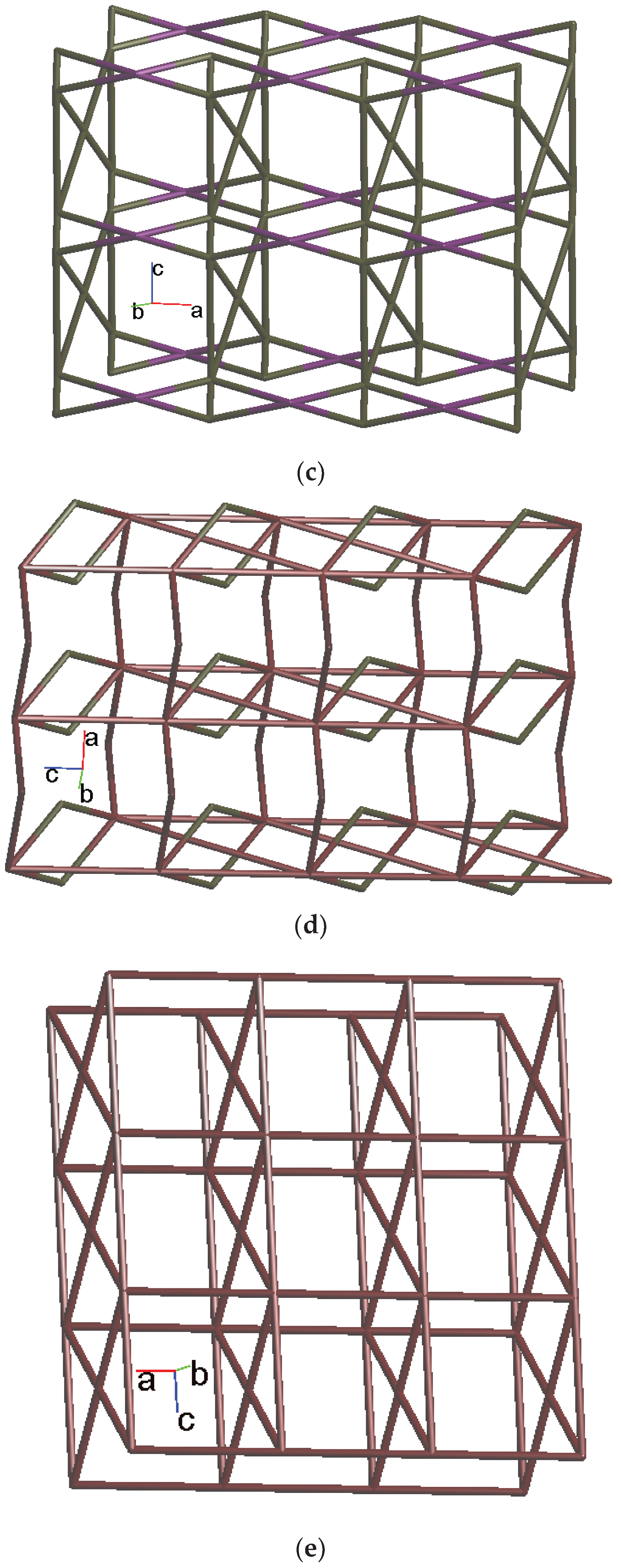
2.4. Structure of 3
2.5. Structure of 4
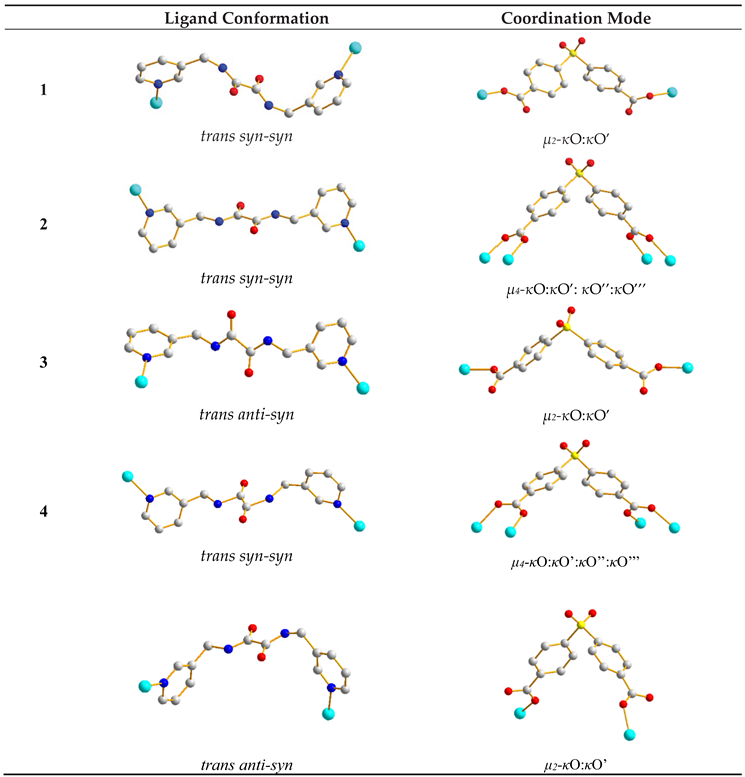
2.6. Ligand Conformations and Coordination Modes
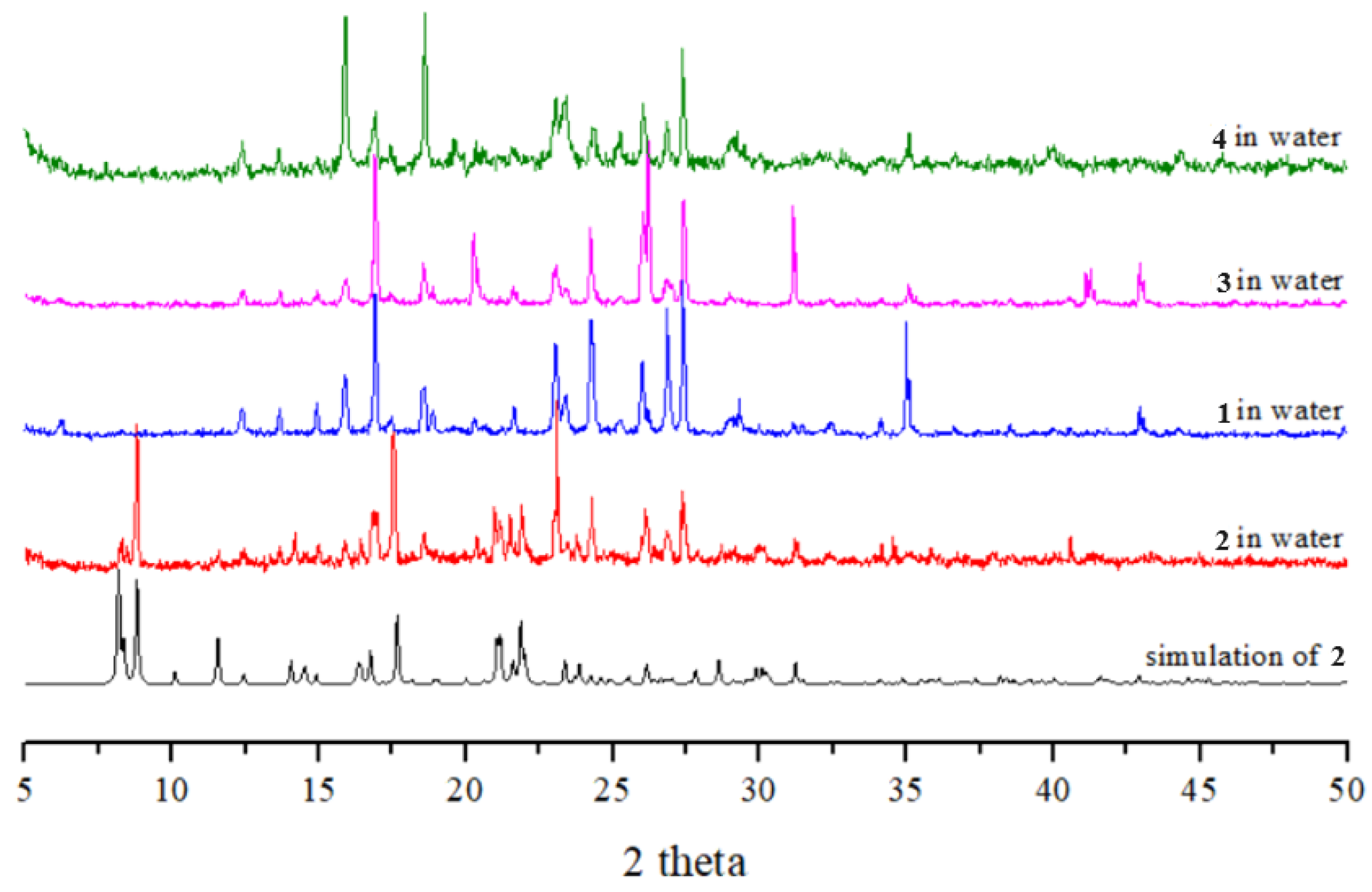
2.7. Powder X-ray Analysis
2.8. Thermal Properties
2.9. Chemical Stabilities
2.10. Attempts for Structural Transformation
3. Materials and Methods
3.1. General Procedures
Materials
3.2. Preparations
3.2.1. {[Co(L)(SDA)(H2O)2]·H2O·CH3OH}n, 1
3.2.2. {[Co(L)0.5(SDA)]·2H2O·0.5L}n, 2
3.2.3. {[Co(L)1.5(SDA)(H2O)]·H2O}n, 3
3.2.4. {[Co2(L)1.5(SDA)2(H2O)2]·4H2O}, 4
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiekink, E.R.; Vittal, J.J. Frontiers in Crystal Engineering; John Wiley & Sons: West Sussex, England, 2006. [Google Scholar]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers Design, Analysis and Application; The Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Zhang, W.H.; Abrahams, B.F.; Braunstein, P.; Lang, J.P. Fabrication of Photoactuators: Macroscopic Photomechanical Responses of Metal-Organic Frameworks to Irradiation by UV Light. Angew. Chem. Int. Ed. 2019, 58, 9453–9458. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Chen, H.H.; Zhang, W.H.; Day, G.S.; Lang, J.P.; Zhou, H.C. Photoinduced nonlinear contraction behavior in metalorganic frameworks. Chem. Eur. J. 2019, 25, 8543–8549. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Pamela, B.S.; Chen, H.-T.; Lin, C.-H. De novo synthesis and particle size control of iron metal organic framework for diclofenac drug delivery. Microporous Mesoporous Mater. 2020, 309, 110495. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- Vittal, J.J. Supramolecular structural transformations involving coordination polymers in the solid state. Coord, Chem. Rev. 2007, 251, 1781–1795. [Google Scholar] [CrossRef]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Hu, J.-H.; Lee, W.-T.; Yang, X.-K.; Chen, J.-D. Structural Transformations of Cobalt(II) Coordination Polymers Constructed from N,N’-Di-3-pyridyladipoamide and Tetracarboxylic Acids: Disentanglement upon Water Coordination. Cryst. Growth Des. 2020, 20, 7211–7218. [Google Scholar] [CrossRef]
- Yang, X.-K.; Chen, J.-D. Crystal-to-crystal transformation and linker exchange in Cd(II) coordination polymers based on flexible bis-pyridyl-bis-amide and 1,4-naphthalenedicarboxylate. CrystEngComm 2019, 21, 7437–7446. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Huang, W.-C.; Yang, X.-K.; Yang, C.-T.; Chhetri, P.M.; Chen, J.-D. Entanglement and Irreversible Structural Transformation in Co(II) Coordination Polymers Based on Isomeric Bis-pyridyl-bis-amide Ligands. Cryst. Growth Des. 2019, 19, 1728–1737. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Lee, W.T.; Liao, T.T.; Chen, J.-D. Nickel (II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. Int. J. Mol. Sci. 2022, 23, 3603. [Google Scholar] [CrossRef] [PubMed]
- Bruker, A.X.S. APEX2, V2008.6, SADABS V2008/1, SAINT V7.60A, SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Hou, Y.-F.; Liu, B.; Yue, K.-F.; Zhou, C.-S.; Wang, Y.-M.; Yan, N.; Wang, Y.-Y. Five solvent-induced cadmium coordination polymers (CPs) based on the same mixed ligands. CrystEngComm 2014, 16, 9560–9567. [Google Scholar] [CrossRef]
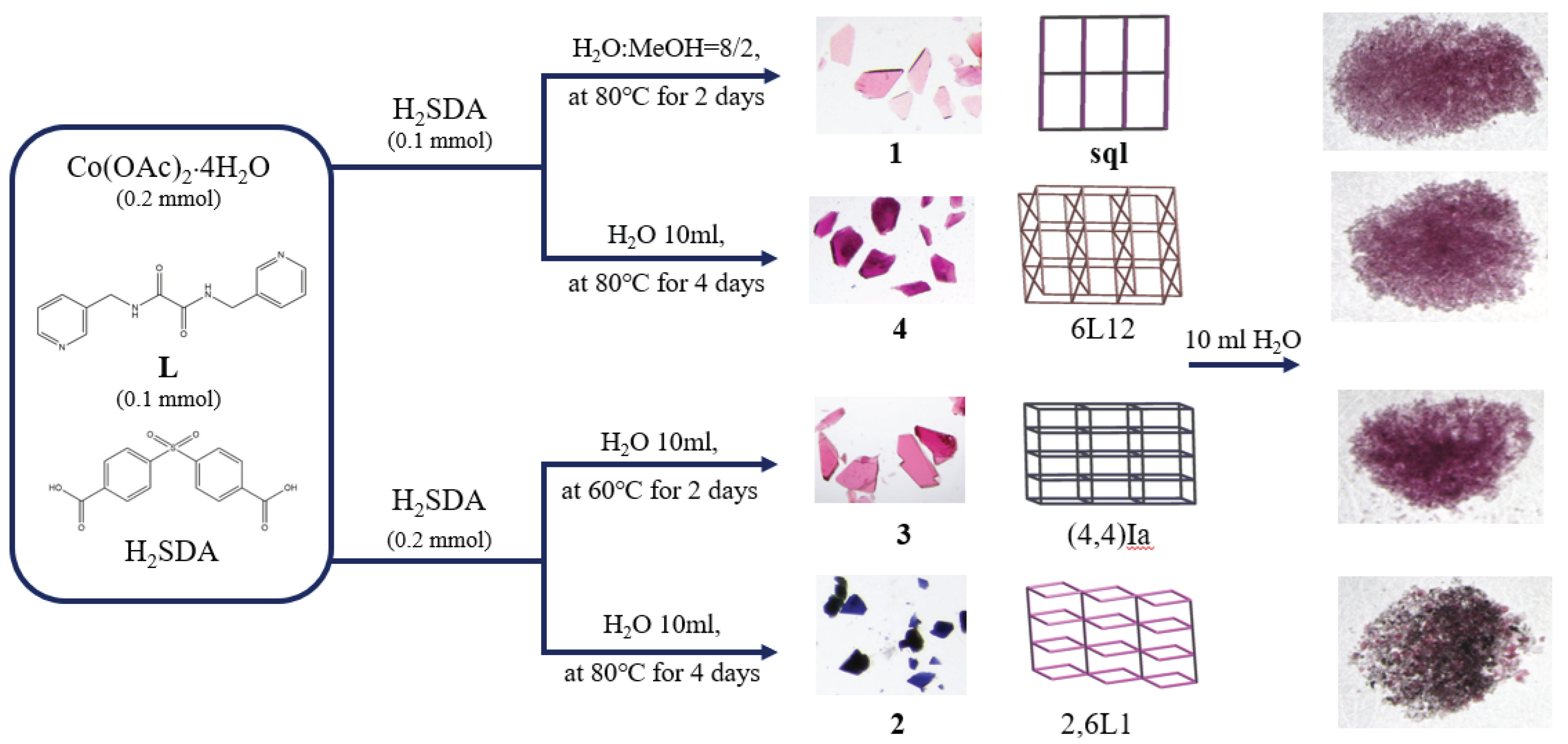
| 0.20 mmol Co(OAc)2⋅4H2O + 0.10 mmol H2SDA + x L |
Solvent |
Temperature (°C)/ Time (days) |
Complex | Yield (%) |
|---|---|---|---|---|
|
x = 0.10 mmol |
8 ml H2O and 2ml MeOH | 80 / 2 | 1 | 33.70 |
| 10 ml H2O |
80 / 2 |
2 3 4 |
3.29 0.0010 13.55 |
|
| 80 / 4 |
2 4 |
2.09 17.34 |
||
|
x = 0.20 mmol |
60 / 2 | 3 | 27.53 | |
| 80 / 2 |
2 3 4 |
4.18 9.28 6.45 |
||
| 80 / 4 |
2 4 |
26.21 6.86 |
 |
| Complex | Weight Loss of Solvent °C (Calc/Found), % |
Weight Loss of Ligand °C (Calc/Found), % |
|---|---|---|
| 1 | 3H2O + MeOH ~ 170 (11.95/9.23) |
L + (SDA2-) 290 - 800 (80.06/82.72) |
| 2 | 2 H2O ~ 130 (5.38/4.65) |
L + (SDA2-) 270 - 800 (86.02/88.15) |
| 3 | 2 H2O ~ 170 (4.48/4.56) |
1.5 L + (SDA2-) 200 - 800 (88.36/85.91) |
| 4 | 6 H2O ~ 130 (8.71/8.23) |
1.5 L + 2 (SDA2-) 300 - 800 (82.02/78.76) |
| Complex | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Formula | C29H32CoN4O12S | C28H26CoN4O10S | C35H33CoN6O11S | C49H49Co2N6O21S2 | |
| Formula weight | 719.57 | 669.52 | 804.66 | 1239.92 | |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | |
| Space group | Pī | Pī | Pī | Pī | |
| a, Å | 12.0281(8) | 10.537(3) | 11.6913(8) | 13.9967(9) | |
| b, Å | 12.3628(9) | 12.378(2) | 12.5564(8) | 14.4949(8) | |
| c, Å | 12.5821(9) | 12.849(2) | 13.2862(8) | 14.9094(8) | |
| α, ° | 110.490(3) | 61.476(8) | 105.7159(18) | 104.0893(14) | |
| β, ° | 99.692(3) | 72.697(10) | 105.6037(18) | 93.1620(15) | |
| γ,° | 108.754(3) | 85.374(14) | 91.3885(19) | 114.1365(14) | |
| V, Å3 | 1574.1(2) | 1402.0(5) | 1798.4(2) | 2636.1(3) | |
| Z | 2 | 2 | 2 | 2 | |
| Dcalc, Mg/m3 | 1.518 | 1.586 | 1.486 | 1.562 | |
| F(000) | 746 | 690 | 832 | 1278 | |
| µ(Mo Kα), mm−1 | 0.682 | 0.754 | 0.605 | 0.795 | |
| Independent reflection | 7756 [R(int) = 0.0276] |
6938 [R(int) = 0.0202] |
7082 [R(int) = 0.1016] |
10361 [R(int) = 0.0473] |
|
| Data/restraint/parameter | 7756 / 0 / 452 | 6938 / 0 / 397 | 7082 / 0 / 497 | 10361 / 2 / 776 | |
| quality-of-fit indicator c | 1.048 | 1.063 | 1.004 | 1.019 | |
| Final R indices [I > 2σ(I)] a,b |
R1 = 0.0413, wR2 = 0.1070 |
R1 = 0.0296, wR2 = 0.0825 |
R1 = 0.0535, wR2 = 0.0871 |
R1 = 0.0495, wR2 = 0.1133 |
|
| R indices (all data) | R1 = 0.0440, wR2 = 0.1089 |
R1 = 0.0323, wR2 = 0.0841 |
R1 = 0.1193, wR2 = 0.1061 |
R1 = 0.0811, wR2 = 0.1364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





