1. Introduction
In the last 20 years, the rate of vineyards planted has increased dramatically all over the world. It was estimated to be 7.3 mha in 2021, with a world market of 34.3 bn EUR (Roca, 2022). The viticultural areas use a broad spectrum of soils, although experts suggest that the soil should be well drained and loamy, containing a mix of sand, silt, and clay. However, grapes also tolerate poor alkaline soils. Regarding climate, grapevines can grow in a wide range of climates, from mild tropical to arid or cold. However, grape quality is substantially affected by soil type and origin. Volcanic origin soils contain a wide range of minerals, have low water retention and organic content, are of great potential for viticultural use (Maltman, 2018; Szabo, 2016; Wilson et al., 2016). In Israel, the Golan Heights volcanic soils are basaltic clay soils found at an altitude of 400 to 1,200 meters above sea level. These soils are in demand by many vineries that consider the basaltic soils, temperature, and altitude as conditions in which grapes of high-quality grow.
The capacity of soil to function as a vital living ecosystem that fulfills all its nutrient supply functions is a fundamental requirement for the plants grown within it. These functions are dependent on the soil biota that maintains diverse soil organisms with high activity, retains and decomposes organic matter, which can meet the plant’s nutrient demands.
Soil quality is predominantly assessed by the physical structure and chemical nutrient levels, followed by its biological components (Jing et al., 2015; Paz-Ferreiro and Fu, 2013). The composition and function of the biotic components of the soil food web are determined by resources supplied by organic matter. In agricultural systems, organic and mineral amendments can be supplied from other sources that replace their enrichment role. The applied organic substrates are degraded by bacteria and fungi, that can potentially be used as indicators representing the nature of the organic matter. However, according to Ferris and Bongers (2006) available methods do not reliably indicate the soil microbial community composition, while the nematodes that regulate the microbial community and enhance plant nutrition, are a good indicator of substrate quality and nutrient release along the decomposition process (Christensen et al., 1992; Griffiths, 1994; Neher, 2001). The nematodes constitute a numerically important component of the soil biotic community, and their community composition follows the changes in food source availability depending on their trophic functions, which include bacteria, fungi, plant parasites, omnivores, and predators (Ferris and Bongers, 2006; Georgieva et al., 2005; Yeates, 2003; Yeates et al., 1993). Earlier studies had elucidated their importance due to their ability to reflect changes in soil physico-chemical properties and function based on nematode community structure (Neher et al., 1997). In agricultural management the use of diversity indices that combine between taxa richness and eveness may contribute to elucidate the frequency of less abundant trophic groups (Ludwig and Reynolds, 1988; Neher et al., 1997). According to Wardle and Yeates (1993), the most dominant interaction in the soil food web is determined by bacteria and fungi predation and competition, that may be limited by resource quality. In agro-ecosystems, where soil bacteria and fungi are closely linked with input of organic matter, the nematode community, and its trophic components are positively correlated to the amount and turnover of organic matter. Since nematodes occupy an important position in the detritus food web, they can be used as indicators for determining the effects of the different agro-managements on the soil detritus food web (Steinberger et al., 2001; Steinberger and Sarig, 1993). Application of organic materials from different sources to the soil will trigger changes in soil physical and chemical components, and in the bacterial and fungal communities, which will influence the trophic composition of the soil nematode community. Alteration between the different vineyard farming practices affect soil properties, some of them can have negative effects on soil quality (Coll et al., 2012). Recent studies conducted in vineyard soil have elucidate the importance in the use of nematodes in soil quality determination as playing a key role in soil organic matter decomposition. The use of soil free living nematodes that had presented to be a good bioindicators of vineyard soil quality is based on trophic groups diversity and characteristics demographic groups such as colonizers and persisters (Bongers, 1990; Yeates et al., 1993).
The present study aimed to determine the interaction between the soil free living nematodes community structure, from both a taxonomic and a functional aspect, and how it is affected by five long-term managements on basaltic soil in Northern Israel. The objective of the study was to compare the soil free-living nematode community in terms of density, diversity, and functionality between the five agro-managements in the vineyard rhizosphere of a basalt soil ecosystem. The rhizosphere is recognized as a hotspot for biota activity, functioning as a precursor to soil organic matter affecting community composition and diversity.
We hypothesized the following: (1) Organic and foliar managements, that increase organic matter, will stimulate bacteria and fungi feeder nematodes more than conventional, open field and natural pasture; (2) In undisturbed sites, there will be a decrease in predation pressure; (3) Guilds of nematodes (bacteria and fungi feeders - e.g., Cephalobidae, Aphelenchidae, Aphelenchoididae, respectively) will be responsive to changes in the abundance of their food.
2. Materials and Methods
2.1. Study Site and Sampling
The soil samples were collected from five different sites, northern Golan Heights (33°04′10″N 35°46′11″E) next to Mount Shifon at 820 m above sea level. This area has a humid Mediterranean climate, with cold winters (6-8°C range in January) and hot summers (24-26°C in August). The average multiannual rainfall is 760 mm y-1 (Israel Meteorological Service gov.il), and the topography of the area is of slopes with moderate gradient. On the surface, which is covered with a thick layer of basalt, tuff, and scoria, stands out a tall volcanic mound: Shifon Mountain, which creates a mountainous morphology (Dan et al., 1972). The soil is a Mediterranean reddish-brown basalt soil, on the slopes of the volcanic cone (Yaalon et al., 1974).
Five replicates of soil samples were collected by using a randomized sampling technique at a depth of the upper soil layer (0-10 cm) adjacent to the trunk of the vine plants rhizosphere, under five different plants in December 2021. Each soil sample were place in a individual plastic bag and placed in an insulation container transported to the laboratory and stored in 4° C.
After sampling, the soils were at the existing moisture to minimize changes in nematode populations. Subsamples were taken from each sample for estimation of nematode populations and different soil parameters. Soil moisture was determined gravimetrically by drying samples at 105°C for 48 h and expressed as a percentage of dry weight.
Soil was sampled from five sites: 1. Merom Golan Organic Vineyard (MO) (33°03’50.9”N 35°45’10.6”E). A certified organic vineyard planted in 2014. Fertilization is based on compost, applied once every 2 years; 2. Merom-Golan Intensive – Conventional Vineyard (MI) (33°03’29.5”N 35°44’57.4”E). An intensive vineyard that was planted in 2017. Fertilization is applied according to leaf analysis and compost is applied, on demand. Heliun (strong a long-lasting herbicide) was applied a year ago. 3. Natural pasture (NP) (33°03’44.4”N 35°45’06.9”E); 4. Foliar fertilization (FF) was planted in 2014 (33°04’45.8”N 35°46’21.8”E). Fertilization is based on foliar application of mainly N, K and micro-Mg, Zn and compost application, whiteout herbicide application. 5. Open field (OF), (33°04’45.6”N 35°46’22.5”E). Each sample included 3 replications.
2.2. Soil Free-Living Nematode Community Analysis:
Soil free-living nematodes were extracted using a modified Baermann funnel procedure(Cairns, 1960) with 200 grams of soil. The extracted nematodes were counted under a binocular to determine total nematode amount per 100 grams dry soil. The nematodes were transferred to a 1.5 ml tube and centrifuged for 10 min at 10,000 rpm to reduce the amount of remaining water. The obtained pellets were placed at –20°C until DNA extraction. Nematode DNA was extracted from the pellets using PureLink Genomic DNA Mini Kit (Invertogen, Thermo Fisher Scientific Inc., Waltham). The eluted DNA was stored at –20°C until PCR amplification. DNA wase amplified by PCR using SimpliAmptm thermal cycler (Thermofischer Scientific), by mixing 12.5 μl PCRBIO HS Taq Mix Red, 9.5 μl ultrapure water, 1 μl extracted DNA, 1 μl CS1-NF1 (ACACTGACGACATGGTTCTACAGGTGGTGCATGGCCGTTCTTAGTT) and 1 μl CS2-18sr2b (TACGGTAGCAGAGACTTGGTCTTACAAAGGGCAGGGACGTAAT). The thermal cycling program was set to: 98 C for 30 sec, 20 cycles of 98 C for 10 sec, 58 C for 30 sec, 72 C for 1.5 min, and after the cycles 72 C for 10 min. Sequencing (Miseq) was performed at the Hylabs Laboratory Ltd. (Rehovot, Israel), (
www.hylabs.co.il) sequencing facility using an Illumina sequencing platform (Illumina Inc., San Diego, CA, USA).
2.2.1. Ecological Indices and Statistical Analysis
The characteristics of the nematode communities were described by means of indices. The classification of trophic groups was assigned to: (1) bacterivores; (2) fungivores; (3) plant-parasites; and (4) omnivores-predators (Liang et al., 2000; Steinberger and Sarig, 1993; Yeates et al., 1993). The nematode community was analyzed by the following approaches: (1) absolute abundance of individuals expressed per 100 g-1 dry soil; (2) trophic structure; (3) FB: (Fungi feeders/Bacteria feeders, or - FF/BF) reflects the structure of the microflora community. Bacteria-based food webs (lower values) exhibit higher decomposition rates than fungi-based webs (Twinn et al., 1974) ; (4) Wasilewska index (WI) = ratio of (fungivores+bacterivores) to plant parasites [(FF+BF)/PP], which repreesent substantial changes in the trophic structure of the community, indicates the dominant pathway of mineralization (Wasilewska, 1994, 1991); (5) Shannon index (H’), a species diversity measure which gives more weight to rare species, H’+Pi(ln Pi), where Pi is the proportion of each trophic group in the total population (Pielou, 1975); (6) trophic diversity, TD= 1/ P2 i, where Pi is the proportion of the individuals in the i-th trophic group (Higgins and Thiel, 1988; Neher, 2001) and describes the trophic group distribution; (7) genus dominance, λ = _P2i (Neher, 2001; Simpson, 1949); (8) modified maturity index (ΣMI), is the c-p value assigned by (Bongers, 1990) of the i-th taxon and ρi is the proportion of the i-th taxon in the nematode community. The c-p values describe the nematode life strategies and range from 1 (for colonizers and individuals tolerant to disturbance) to 5 (for persisters and individuals sensitive to disturbance), including plant-feeding nematodes (Yeates et al., 1994). ΣMI incorporates ecological characteristics of families based on a colonizer-to-persister scale of 1–5, where lower ΣMI values indicate more disturbed environments; (9) evenness J’ = H’/ln (S), where S is the number of taxa (Simpson, 1949) is highest when all species in a sample have the same abundance.
2.3. Statistical Analysis
The data were subjected to statistical analysis of variance using the SAS model (ANOVA and Duncan’s multiple range test, T-test, genera indicator analysis and Pearson correlation coefficients) to evaluate differences between the treatments, by using the statistical package Statistica 4.3. Differences obtained at levels of p<0.05 were considered significant.
The Indicator Species Analysis was run in R using the "multipatt" function from the "indicspecies" package. This function calculates the indicator value for each genus in relation to each type of land management. For those genera that were found to be significantly different (p <= 0.05), we provide bar plots created in R using gplot of their relative abundance across the different land management systems.
3. Results
3.1. Soil Nematode Community
3.1.1. Total Number of Nematodes
The mean total number of soil free living nematodes ranged between 108 individuals 100
-1 g dry soil in samples collected from the conventional management to 776 individuals 100
-1g dry soil (
Figure 1) in the foliar fertilization treatment. No significant differences were obtained in the total number of nematodes between the natural pasture (NP) and open field (OF) that represent control sites. The total number of nematodes in the organic (OM) management site (339.8 individuals 100
-1g dry soil) was almost two-fold lower compared to the FF management site (775.5 individuals 100
-1g dry soil).
The mean percentage of bacterial feeders (BF) from the five sampling sites were NP > OF > FF > MI > MO, where the two control NP and OF sites showed 72 and 60% bacterial feeders out of the total population, followed by about 50% in the soil obtained from the foliage management. Lower values for bacteria feeders and fungi feeders were obtained in the MO (organic farming). However, the highest percentage of omnivore–predator and plant parasite nematodes were present in the MO compared to the other managements.
3.1.2. Ecological Parameters
A total of nine ecological indices (
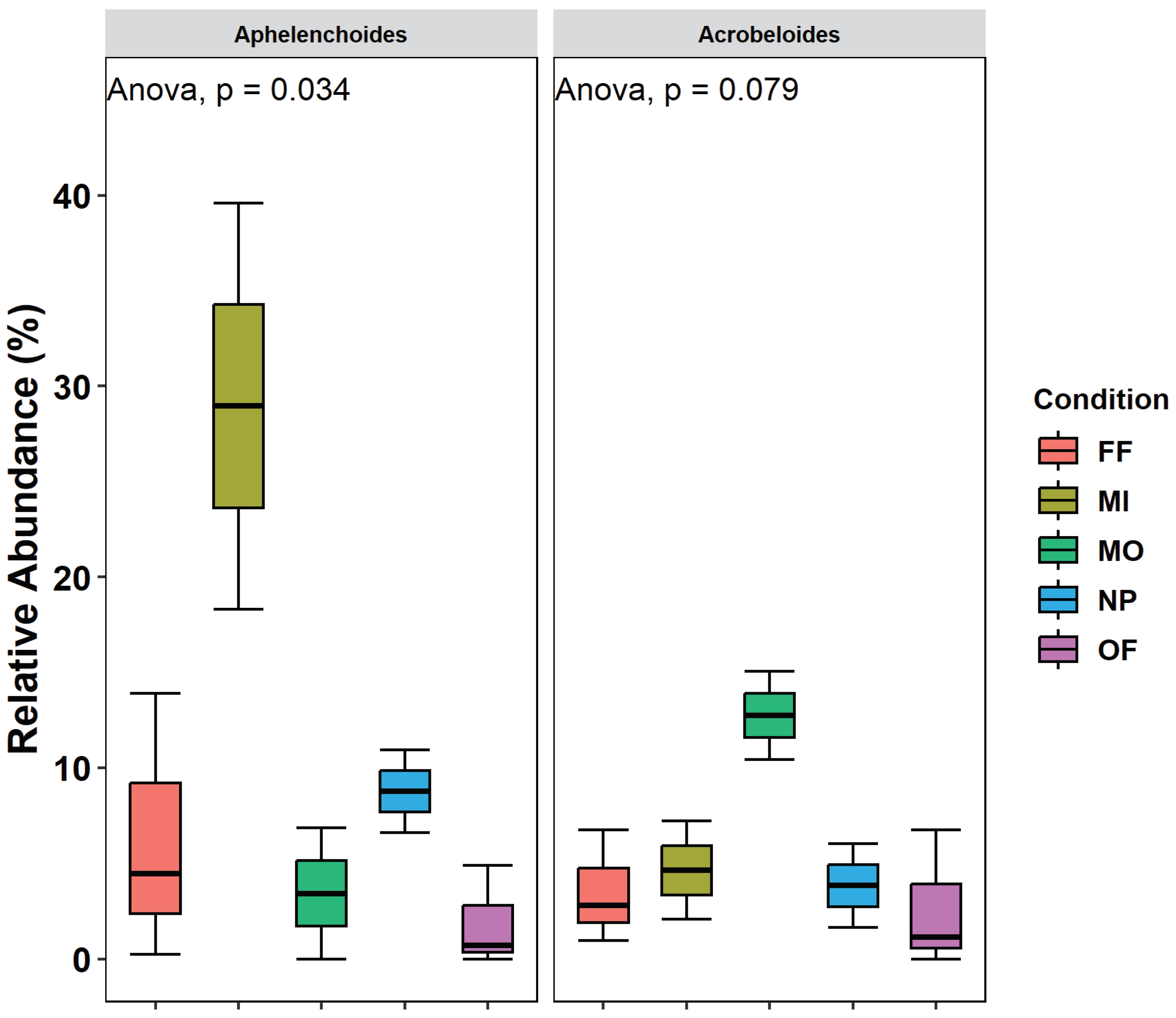
Table 1) were used to assess management differences between the sites. The relative high variability in data obtained we had observed significant difference in the bacterivores between MI and NP (p< 0.0053) were no significant differences in FF, OP, and PP were obtained between the treatments. Moreover, as result of it no significant difference was found in H’ (Shanon indices). From all the indices only the dominance (λ) that measure the information energy elucidating that only one of a few representatives’ individuals in the genera that are found in high numbers will have more dominance. In this case the λ had been found to show significant difference between MO and MI (p< 0.007). Only two genera showed a specific signal (
Figure 2) the Aphelenchoides in representing the MI treatment with a p=0.034, and in the MO treatment the Acrobeloides were the p= 0.079 showing difference in comparison to the other four treatments.
Running a multilevel pattern analysis at a significant level (alpha) at level p=0.05 – on the total number of 42 genera, the number of selected genera were found to be tow (2), were number of associate genara were two in the first group, in comparison to the other groups were 0 (zero). The group number one (MI treatment) in the present case were represented by Aphelenchoides p = 0.0278, and for MO treatment p=0.0329.
In both above cases the two genera were found to determine the differences between the treatments.
The Wasilevska index (WI) was significantly (p< 0.05) higher in the FF management compared to the other managements, except for the conventional (MI) samples. This shows the significant low number of plant parasite individuals in FF compared to MO managements. Moreover, the H’ (Shannon index) showed a significant difference between the MO (organic management) and OF (open field – natural system), without any significant difference relative to the other managements. No significant differences between the managements were observed in trophic diversity (TD). The ΣMI values in the MO management reached a maximum value which was significantly higher compared to the other managements, elucidating a higher disturbance in the MO management compared to the other management sites. Evenness values indicated a higher abundance and greater biodiversity in the MO management relative to the other four managements, with the lowest value in the OF site.
3.1.3. Nematode Taxa
A total of six orders, 22 families, 42 genera and 89 species were detected in total from all soil samples collected from the five different managements. Eleven families were found to be dominant: Plectidae, Aporcelaimidae, Panagrolaimidae, Cephalobidae, Tylenchina, Qudsianematidae, Monhysteridae, Aphelenchoididae, Mononchidae, Anguinidae, and Aphelenchidae (
Table 2).
A total of 25, 23, 27, 29 and 31 genera were present in the MO, MI, NP, FF and OF soil samples, respectively, from the total of 42 genera (
Table 2). Eleven of the genera belonged to seven families of bacteria feeders, six genera belonged to 3 families of fungi feeders, six families represented, by 8 genera belonged to omnivores-predators and six families are represented by 17 genera belonging to plant parasites. The bacteria feeders genera that were present in all samples included
Acrobloides,
Panagrolaimuns and
Plectus. Out of the 6 fungi feeders genera found in our study, four were present in all managements:
Ditylechus,
Aphelenchus,
Paraphelenchus, and
Aphelenchoides. The omnivores-predators genera present in all soil samples included
Aporceliamellus,
Allodorylaimuns and
Thonus. Five out of a total of 17 genera of plant parasites were present in all of the soil samples:
Anguina,
Mothotylenchidae,
Aglenchus,
Basiria, and
Tylenchus.
4. Discussion
In the recent years agricultural practices had overgone enormous changes trying to give answers to increasing demand for higher primary production, soil health maintenance, use of organic amendment more natural and favorable practices, that should go hand in hand with global requests. In order to do so the agroecosystem consequently try to develop novel approaches in developing and improving organic amendments. The effect of organic amendments from different sources had been reported to improve biological, microbiological, and biochemical soil characteristics such as water and nutrient holding capacity.
In the present study, the response of soil free living nematodes community, its density, functional composition, and additional attributes that are known to be good environmental indicators, were used to reveal the differences between long-term forms of managements (organic, conventional, natural pasture, foliage, and open field). Studies conducted by Werner and Dindal (Werner and Dindal, 1990) on conversion of organic agriculture on soil biota had shown that soil nematodes were the most abundant in organic plots. Our results had been found to demonstrate that organic farming contribute in improving the density of microbial feeding that tend to improve nutrient resource availability. As being one of the primary grazers of saprophytic community the nematodes increase nutrient availability to primary producers (Matlack, 2001). The changes in nematodes community features reflect the changes in the below-ground soil milieu, including the below-ground plant rhizosphere biosphere (Bongers and Ferris, 1999; Fitoussi et al., 2016; Neher et al., 2005). Nematode feeding groups indices elucidate the impact of the different managements on accessibility and immediate utilization of food sources (bacteria and fungi) (Yeates et al., 1993). Although the determined nematode indices cannot help in understanding or differentiating between management performances in all cases, we should prolong the use the nematode community food web structure as proposed by Martin et al. (Martin et al., 2022). This will increase our understanding of scale row-crop agriculture.
Manipulating managements in vineyards to reduce disease and improve yields is a popular and accepted practice. Various studies had tried to link cover crops and the soil nematode community – both beneficial and pathogenic – in order to enhance the control over parasitic nematodes. Our results indicated differences in soil food web function between managements and shifting between the three main feeding groups (bacterivores, fungivores, and omnivores-predators) were found to be according to resource availability. Soil organic matter and organic residues are supporting fungi development which in turn is followed by fungivore nematodes abundance that were showed high presence of omnivores and predators community development. These findings are similar to those reported by Ferris (Ferris, 2010; Ferris and Bongers, 2006) and Ugarte et al,. (2013). Nematode indices which are developed based on the various functional guilds (Ferris and Bongers, 2006) were inconsistent comparing the managements, except the Wasilevska index which showed a sharp increase in foliage management, thus elucidating the management effect on plant parasites.
Plant endoparasitic or ectoparasitic nematodes present in grapevine are having a strong effect on plant growth and virus transmission which are widespread all over the globe without any exception of geographical region or soil textures (Aballay et al., 2009). Plant parasitic nematode families followed by their genera were found to vary between the five managements. In the Pratylechidae family, the genus Pratylenchus was present in organic-related management (MO, FF) and in the open field (OF) that can be attributed to development of primary production along time (Coll et al., 2012). It is known as a root lesion and pin nematode, pathogen of grapevine as reported by (Zasada et al., 2012) and (Howland et al., 2014). The Nothotylenchidae family, represented by genus Nothatylenchidae, was present in relatively high numbers in all managements. The Nothotylenchidae (Thorne, 1941) is a redescription of Tylenchorhynchus (Elmiligy, 1955) and is known to be a pathogen present in the vicinity of clover roots (Trifolium alexandrinum L.), guava (Pisidium guave L.), squash (Cucurbit moscbata Duch.), turnip (Brassica rapa L.), and near grapevine based on our results from the present study. They can also predispose roots to invasion by other plant pathogens. The main damage of plant pathogen nematodes is in a disturbance of nutrient and water uptake, thus decreasing and yield.
Soil free living plant-parasitic nematodes are very commonly found in vineyards (Schlüter et al., 2022; Zasada et al., 2012). Permanent grass cover, different types of managements, soil aggregate and pore size distribution affect the community structure, diversity, and abundance (Ferris et al., 2001; Neher, 2001; van den Hoogen et al., 2019). Long-term organic, intercrops (grass cover) negatively influence diversity, while positively affecting omnivore-predators that are sensitive to disturbance determined by habitat constrains related to resource availability. Studies of plant parasitic nematodes had showed that competition with arbuscular mycorrhizal and endophytic fungi and predator nematodes can control their community size.
The present study has important implications for understanding the effect of different management types on the soil free living nematode community composition (Siddiqui and Mahmood, 1999). This study emphasized the importance of the bacterial and fungal community that can be used as a front line against pathogens such as nematodes. As the plant rhizosphere determine habitat structure the different management contribute to the physical soil pore structure, moisture availability and resource distribution. The new and the different habitat’s structure promoted by the different agro-management stimulate new conglomerates of soil biota composition. This supports our findings that organic managements suppress most parasitic nematode similar to the finding reported by McSorley (2011). These results provide a new perspective on a time axis, where a deeper study to link grapevine plant phenology, soil biota dynamics with emphasis on soil nematode community and its functionality, and physico-chemical components are needed to evaluate and avoid the impact of these pests on vineyard productivity.
Author Contributions
Conceptualization, Y.S. and N.R.; methodology, Y.S.; software, T.D.; validation, C.S., and N.R.; formal analysis, Y.S. and I.A.; investigation, C.S. and I.A.; resources, Y.S.; data curation, T.D.; writing—original draft preparation, Y.S.; writing—review and editing, I.A.; visualization, Y.S.; supervision, Y.S.; funding acquisition, Y.S. and N.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the Grapevine Council in Israel and by Faculty of Live Science Bar Ilan University by providing funds for a M.Sc. student activity.
Data Availability Statement
The genetic data generated for this study can be found in NCBI under accession number PRJNA908721.
Acknowledgments
We would like to thank Ido Bar from the Merom Golan vineyard, and Steve Applebaum from the Ortal vineyard, for kindly allowing us to use their vineyard for our study and providing us with relevant information. The authors thanks Ms. May Levi for her great help with the lab work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aballay, E.; Persson, P.; Martensson, A. Plant-parasitic nematodes in chilean vineyards. Nematropica 2009, 39, 85–97. [Google Scholar]
- Bongers, T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Cairns, E.J. Methods in nematology, in: Sasser, J.N., Jenkins, W.R. (Eds.), Nematology, Fundamentals and Recent Advances with Emphasis on Plant Parasitic and Soil Forms. University of North Carolina Press Cha- pel Hill, 1960; pp. 33–84.
- Christensen, H.; Griffiths, B.; Christensen, S. Bacterial incorporation of tritiated thymidine and populations of bacteriophagous fauna in the rhizosphere of wheat. Soil. Biol. Biochem. 1992, 24, 703–709. [Google Scholar] [CrossRef]
- Coll, P.; Le Cadre, E.; Villenave, C. How are nematode communities affected during a conversion from conventional to organic farming in southern French vineyards? Nematology 2012, 16, 665–676. [Google Scholar] [CrossRef]
- Dan, J.; Yaalon, D.H.; Koyumdjisky, H.; Raz, Z. The Soil Association Map of Israel. Isr. J. Earth-Science.
- Elmiligy, I.A. Redescription of Tylenchorhynchus clarus Allen. Nematologica 1955, 15, 288–290. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil. Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode Indicators of Organic Enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil. Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Fitoussi, N.; Pen-Mouratov, S.; Steinberger, Y. Soil free-living nematodes as bio-indicators for assaying the invasive effect of the alien plant Heterotheca subaxillaris in a coastal dune ecosystem. Appl. Soil. Ecol. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Georgieva, S.; Christensen, S.; Petersen, H.; Gjelstrup, P.; Thorup-Kristensen, K. Early decomposer assemblages of soil organisms in litterbags with vetch and rye roots. Soil. Biol. Biochem. 2005, 37, 1145–1155. [Google Scholar] [CrossRef]
- Griffiths, B.S. Microbial-feeding nematodes and protozoa in soil: Their effectson microbial activity and nitrogen mineralization in decomposition hotspots and the rhizosphere. Plant Soil. 1994, 164, 25–33. [Google Scholar] [CrossRef]
- Higgins, R.P.; Thiel, H. 1988. Introduction to the Study of Meiofauna. Smithsonian Institution Press. [CrossRef]
- Howland, A.D.; Schreiner, R.P.; Zasada, I.A. Spatial Distribution of Plant-Parasitic Nematodes in Semi-Arid Vitis vinifera Vineyards in Washington. J. Nematol. 2014, 46, 321–330. [Google Scholar]
- Jing, X.; Sanders, N.J.; Shi Yu Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi Yue Jiang, Y.; He, J.S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Liang, W.; Pinhasi-Adiv, Y.; Shtultz, H.; Steinberger, Y. Nematode population dynamics under the canopy of desert halophytes. Arid. Soil. Res. Rehabil. 2000, 14, 183–192. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Reynolds, J.F. , 1988. Statistical ecology: a primer on methods and computing. John Wiley & Sons, Ltd, New York. [CrossRef]
- Maltman, A. 2018. Vineyards, rocks, and soils: The wine lover’s guide to geology. Oxford University Press.
- Martin, T.; Wade, J.; Singh, P.; Sprunger, C.D. The integration of nematode communities into the soil biological health framework by factor analysis. Ecol. Indic. 2022, 136, 108676. [Google Scholar] [CrossRef]
- Matlack, G.R. Factors determining the distribution of soil nematodes in a commercial forest landscape. For. Ecol. Manage. 2001, 146, 129–143. [Google Scholar] [CrossRef]
- Mcsorley, R. Overview of organic amendments for management of plant-parasitic nematodes, with case studies from Florida. J. Nematol. 2011, 43, 69–81. [Google Scholar] [PubMed]
- Neher, D.A.; Easterling, K.N.; Fiscus, D.; Campbell, C.L. Comparison of Nematode Communities in Agricultural Soils of North Carolina and Nebraska. Ecol. Soc. Am. 1997, 8, 213–223. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar] [PubMed]
- Neher, D.A.; Wu, J.; Barbercheck, M.E.; Anas, O. Ecosystem type affects interpretation of soil nematode community measures. Appl. Soil. Ecol. 2005, 30, 47–64. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological Indices for Soil Quality Evaluation Perspectives and Limitations. pdf. L. Degrad. Dev. 2013, 27, 14–25. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological diversity. Limnol. Oceanogr. 1975, 22, 174. [Google Scholar] [CrossRef]
- Roca, P. State of the world vine and wine Sector 2021. Int. Organ. Vine Wine Intergov. Organ. 2022, 1–19. [Google Scholar]
- Schlüter, S.; Gil, E.; Doniger, T.; Applebaum, I.; Steinberger, Y. Abundance and community composition of free-living nematodes as a function of soil structure under different vineyard managements. Appl. Soil. Ecol. 2022, 170, 104291. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Role of bacteria in the management of plant parasitic nematodes: A review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- Simpson, E. Measurment of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Steinberger, Y.; Liang, W.; Savkina, E.; Meshi, T.; Barness, G. Nematode community composition and diversity associated with a topoclimatic transect in a rain shadow desert. Eur. J. Soil. Biol. 2001, 37, 315–320. [Google Scholar] [CrossRef]
- Steinberger, Y.; Sarig, S. Response by soil nematode populations and the soil microbial biomass to a rain episode in the hot, dry Negev Desert. Biol. Fertil. Soils 1993, 16, 188–192. [Google Scholar] [CrossRef]
- Szabo, J. 2016. Volcanic Wines: Salt, Grit and Power. Jacqui Small.
- Thorne, G. Some nematodes of the family Tylenchidae which do not possess a valvular median esophageal bulb. Gt. Basin Nat. 1941, 2, 37–85. [Google Scholar]
- Twinn, D.C.; Pugh, G.J.F.; Dickinson, C.H. 1974. Biology of plant litter decomposition. Academic Press. [CrossRef]
- Ugarte, C.M.; Zaborski, E.R.; Wander, M.M. Nematode indicators as integrative measures of soil condition in organic cropping systems. Soil Biol. Biochem. 2013, 64, 103–113. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Yeates, G.W. The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 1993, 93, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, L. The effect of age of meadows on succession and diversity in soil nematode communities. Pedobiologia 1994, 38, 1–11. [Google Scholar] [CrossRef]
- Wasilewska, L. Long-term changes in communities of soil nematodes on fen peat meadows due to the time since their drainage. Ekol. Pol. 1991, 39, 59–104. [Google Scholar]
- Werner, M.R.; Dindal, D.L. Effects of conversion to organic agricultural practices on soil biota. Am. J. Altern. Agric. 1990, 5, 24–32. [Google Scholar] [CrossRef]
- Wilson, S.G.; Lambert, J.-J.; Dahlgren, R.A. Seasonal Phosphorus Dynamics in a Volcanic Soil of Northern California. Soil Sci. Soc. Am. J. 2016, 80, 1222–1230. [Google Scholar] [CrossRef]
- Yaalon, D.H.; Brenner, I.; Koyumdjisky, H. Weathering and mobility sequence of minor elements on a basaltic pedomorphic surface, galilee, israel The distribution and reorganization of minor and trace elements during weathering and soil formation depend on their mode of occurrence in the host roc. Geoderma 1974, 12, 233–244. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera-An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Yeates, G.W.; Orchard, V.A.; Speir, T.W.; Hunt, J.L.; Hermans, M.C.C. Impact of pasture contamination by copper, chromium, arsenic timber preservative on soil biological activity. Biol. Fertil. Soils 1994, 18, 200–208. [Google Scholar] [CrossRef]
- Zasada, I.A.; Riga, E.; Pinkerton, J.N.; Wilson, J.H.; Paul Schreiner, R. Plant-Parasitic Nematodes Associated with Grapevines, Vitis vinifera, in Washington and Idaho. Am. J. Enol. Vitic. 2012, 63, 522–528. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).