1. Introduction
Photosynthesis organ of plants is highly sensitive to the toxicity of organic pollutants. The toxic effects of pollutants on photosynthesis are usually probed by chlorophyll fluorescence and oxygen evolution rate [
1,
2,
3]. Measurement of photosynthetic oxygen evolution rate of plants under stress, which directly indicate the toxicity of pollutants to the oxygen evolution complex, is performed by a Clark oxygen electrode in a thermostatic closed chamber at a constant temperature. This conventional electrochemical method, however, cannot probe oxygen emission at a single stoma level and cannot give the spatial distribution of oxygen concentration in and around the stoma, which is sometimes important for understanding the responses of plants to environmental stresses.
The technique of scanning electrochemical microscopy (SECM) is often used to characterize chemical processes and spatial morphological features of the substrate when the tip is moved near the surface of a conductive or insulating bottom substrate in solution in which the current that flows through an ultramicroelectrode (UME)[
4].
The SECM tip can be moved at Å resolution so that the chemical concentration and morphology of the conductive or insulating bottom substrate surface can be imaged at atomic resolution when the tip is scanned in X-Y plane at constant height [
5]. SECM has a potential to examine response of photosynthetic oxygen evolution under environmental stresses because oxygen produced by the water splitting center in photosystem II is a mediator that can be reliably probed by UME [
6]. Since oxygen is released from the stoma during photosynthesis, SECM can simultaneously map oxygen concentration distribution and structure of stoma, which provide complementary data for the traditional liquid oxygen electrode method and chlorophyll fluorescence tests. Very limited studies showed the application potential of SECM for this purpose. Tsionsky et al. [
7] firstly monitored photoelectrochemistry and in-vivo topography of single stoma in stress-free
Tradescantia fluminensis Vell cv. Variegata by using SECM. [
8] firstly assessed by using a SECM probe to detect oxygen evolution and the changes to individual stoma structure in
Brassica juncea (L.) Czern. cv. AC Vulcan (Indian mustard) caused by Cd-stress. Besides its powerful function and extensive application in the field of biology [
9], SECM has not been systemically verified for probing toxic effects of pollutants from the perspective of ecotoxicology. The relevant protocols to get reproducible and reliable ecotoxicological data are still unavailable. Some problems must be solved before it is accepted by ecotoxicologist. For example, in the published literature, the leaf disc system for SECM detection is previously bubbled by air to ensure enough CO
2 in water for photosynthesis. However, CO
2 content in water can be significantly affected by temperature, air pressure and bubbling time and efficiency. The CO
2 in water after aeration may not be enough to maintain maximum photosynthesis performance for long-time SECM measurement. In addition, only one pollutant concentration was set in their experiments to probe the toxicity of pollutant, which cannot meet the requirement of the dose-response relationship in ecotoxicological experimental design and the toxicological indices such as the dose effect curve, the half maximal inhibitory concentration (IC
50) cannot be estimated.
2,4-dichlorophenol (2,4-DCP) has been listed by the U.S. Environment Protection Agency as a priority control pollutant because of its potential carcinogenicity and its high toxicity even at a low concentration [
10]. 2,4-DCP mainly arising from the extensively use of pesticides, herbicides and fungicides [
11].
M. pteropus is a common higher freshwater aquatic plant which has high demand in ornamentation and often use as a water pollution indicator [
12].
M. pteropus was chosen as the test material because previous research has shown that its multiple sites in PSII and PSI was highly sensitive to mercury exposure [
1].
The aim of the present study was to develop a reproducible experimental protocol for assessment of the ecotoxicity of 2,4-DCP exposure to M. pteropus based on nanoscale SECM visualization and quantification of photosynthetic oxygen evolution at single stoma level. The stoma structure upon exposure to 2,4-DCP treatment was simultaneously imaged and compared with those obtained by CLSM. The dose-response curve and derived IC50 were comparatively verified by the chlorophyll fluorescence method and the traditional Clark liquid oxygen electrode method.
2. Materials and Methods
2.1. Plant Materials
M. pteropus (wild type) [
13] seedlings were purchased from a market in Urumqi of China and cultivated in a glass aquarium (length 500 mm, width 300 mm, height 550 mm) filled with tap water at 25±2
oC under irradiance of 100 μmol photons m
-2s
-1 with a 12/12 h light/dark photoperiod [
1]. Then the seedlings were taken out from the glass aquarium after the adaptability of 4 d culture. The healthy seedlings without spores and dark spots, about 25±2 cm height, were selected for toxicological tests.
2.2. Chemicals
All chemicals used in this study were at least of analytic grade. The 2, 4-DCP solutions were prepared by dissolving 2, 4-DCP solid ((99%, CAS#120-83-2, Sigma-Aldrich 105953-100G, HPLC: suitable) in tap water. The nominal concentration level gradients for 2,4-DCP treatment are set to 0.00, 0.01, 0.05, 0.1, 0.5, 1.0, 5.0, 10.0, 25.0, 50.0, 100.0, and 250.0 mg L
-1. The actual concentration of 2, 4-DCP in tap-water was measured by high performance liquid chromatography (HPLC, 890-0203 HITACHI, Hitachi, Japan)[
14] which were 0.01, 0.05, 0.10, 0.48, 1.00, 4.50, 9.53, 24.90, 49.81, 92.44 and 248.53 mg L
-1, respectively. 2, 4-DCP concentration in the tap-water was under the detection limit.
2.3. Ecotoxicity Exposure Experiments
Twelve groups of seedlings with three seedlings in one group were transferred to twelve 500 mL cylindrical glass cylinders (355 mm×50 mm) containing 600 mL tap-water with different measured concentrations of 2, 4-DCP (0-248.53 mg L-1). The seedlings grown in the tap water without addition of 2,4-DCP were used as the control. During the cultivation, some tap-water were irregularly added to the cylinders to compensate the loss of water due to evaporation. All the tap-water stood for at least 24 h before use in order to reduce or remove the disinfection substances. Control and 2,4-DCP -stress treatments were both set four replicates (per replicate containing three seedlings, ie, n=4×3=12). Leaves of the seedlings exposed to 2, 4-DCP for 24 h, 72 h, 96 h were used for SECM oxygen emission mapping, CLSM observation, chlorophyll fluorescence test and photosynthetic oxygen evolution rate measurement by the Clark oxygen electrode.
2.4. Stoma Oxygen Mapping by SECM
All stoma oxygen mapping and quantification were performed with a SECM workstation (920D, CH Instruments, Austin, TX, USA). The SECM instrument include three major constituent parts: the positioning system, the electrochemical system and the active data acquisition system. In general measurement, a potential was added to the SECM probe and a current was detected by the electrochemical system [
8]. The positioning system was used to displace the probe, and the data acquisition system were used to record the current and position data simultaneously. The electrochemical and positioning systems were built together in a Faraday cage to isolate external electrical noise. A well-polished 10 μm diameter platinum UME with RG 10 (HEKA Elektronik Dr. Schulze GmbH, Germany) was used as the SECM tip. Reliability of the UME was checked by capacitive current and steady-state current tests. A 0.5 mm platinum wire and Ag/AgCl were used as the corresponding the counter electrode and the reference electrode, respectively. In the voltammetry curve tests to characterize the electrodes, a 10 μm-diameter Pt UME as working electrode versus an Ag/AgCl reference electrode were simultaneously immersed in the solution as supporting electrolyte (containing 0.1 M KCl and 1.0 mM ferrocenemethanol).
All SECM experiments were performed at constant height mode at 25±2oC. The leaf disc at the bottom of the electrochemical cell was illuminated under 100 μmol photons m-2s-1 by a LED lamp. After 10 min dark adaptation of the leaf disc to be tested, the LED lamp was turned on and SECM measurement started. The phosphate buffered saline (PBS, pH 6.86) solution containing 0.1 M NaHCO3 was used as the supporting electrolyte which could provide excessive CO2 for photosynthesis during measurement.
Before starting test, the leaf disc (8 mm in diameter) was taken from M. pteropus seedlings with a punch. Then the leaf disc was placed centrally at the bottom of the Teflon cylinder electrochemical cell and fixed with a round piece of transparent glass (34mm in diameter, 4 mm in thickness) with a round hole (6 mm in diameter). The cylinder (diameter 34.8mm, depth 22mm) made of optically transparent polystyrene, used as an electrochemical cell with a flat bottom of uniform thickness. All the tested leaves were adapted in the dark for 10 min before transferred to the electrochemical cell.
In probe approaching experiments, the SECM tip was positioned over leaf disc and immobilized at a distance (generally 10-15 μm) above the substrate, and then was moved down to obtain the approach curves and the tip scanning height of the subsequent mapping experiments (about 1.5 μm). The current versus the moving distance toward a leaf was measured at the UME, which was biased at a potential (-0.6V) to obtain a steady-state current for the reduction of oxygen in the solution. The destination normalized current (the ratio of actual tip current, iT to iT, ∞) was set gradually to a low value (e.g., 0.6) to avoid crushing the tip.
In the scanning mapping experiments, the electrode was immediately scanned at a constant height (1.5 μm above the leaf disc) after probe approaching experiments. Firstly, an area of 200×200 μm (no leaf vein) of the leaf disc was scanned at a relatively faster speed (150 μm s-1) to find several stomas. Then a 100×100 μm area containing one stoma was scanned at high spatial resolution (50 μm s-1) to get a microscale image. The current of the stoma longitude perpendicular cross-sectional line current and the peak current of each stoma center was extracted. After each test, the UME was washed under ultrasonic irradiation twice and rinsed with deionized water several times, then checked with an optical microscope (IX71, Olympus Co., Japan) prior to use for further experiments.
2.5. Three Conventional Ecotoxicological Assessment Methods
Autofluorescence imaging of leaf disc: The autofluorescence (excited by a 488 nm laser) of the leaf discs treated with various concentrations of 2,4-DCP were observed and imaged using a CLSM (FV1000, Olympus Co., Japan).
Measurement of photosynthetic oxygen evolution rate: The photosysnthetic oxygen evolution rate of the leaf discs treated with various concentrations of 2,4-DCP were measured using a Clark oxygen electrode (Oxygraph, Hansatech Instruments, United Kingdom). The reaction chamber was filled with 2.0 mL PBS (pH 6.86) solution containing 0.1 M NaHCO3. The measurements were performed at 25oC under 100 μmol photons m-2s-1 irradiance. The measured photosynthetic oxygen evolution rate was converted to the rate of oxygen release per minute of leaf (nmol cm-2 min-1).
Chlorophyll fluorescence test: The maximal PS II quantum yield (Fv/Fm) of the leaf discs treated with various concentrations of 2,4-DCP were measured by the double modulation chlorophyll fluorometer (Dual-PAM-100 system, Heinz Walz GmbH, Effeltrich, Germany). The chlorophyll fluorescence test was performed after the leaf discs were adapted in the dark for 5 min [
1,
15].
2.6. Calculation and Statistics
Each 2,4-DCP -stress treatment was quadruplication (three seedlings each repeat). Means, standard deviations (SD.) were calculated using Microsoft Excel 2016. Plottings were performed with the OriginPro 9.0. The Best-fit values and the 95% Confidence Intervals of IC50 value was estimated by ‘normalized Response-Variable slope’ (GraphPad Prism 5) through the Mean, SD and N (calculated with Microsoft Excel 2016 in advance). The statistical significance between 2,4-DCP -treatments and control treatment were performed by one-way ANOVA (SPSS V21.0) through the least significant difference (LSD) test. The statistical significance of IC50 value among SECM, Clark oxygen electrode and chlorophyll fluorescence data were performed by one-way ANOVA (SPSS V21.0) through Duncan’s Multiple Range Test (DMRT) difference test.
3. Results
3.1. SECM Mappings of Stoma Oxygen Emission
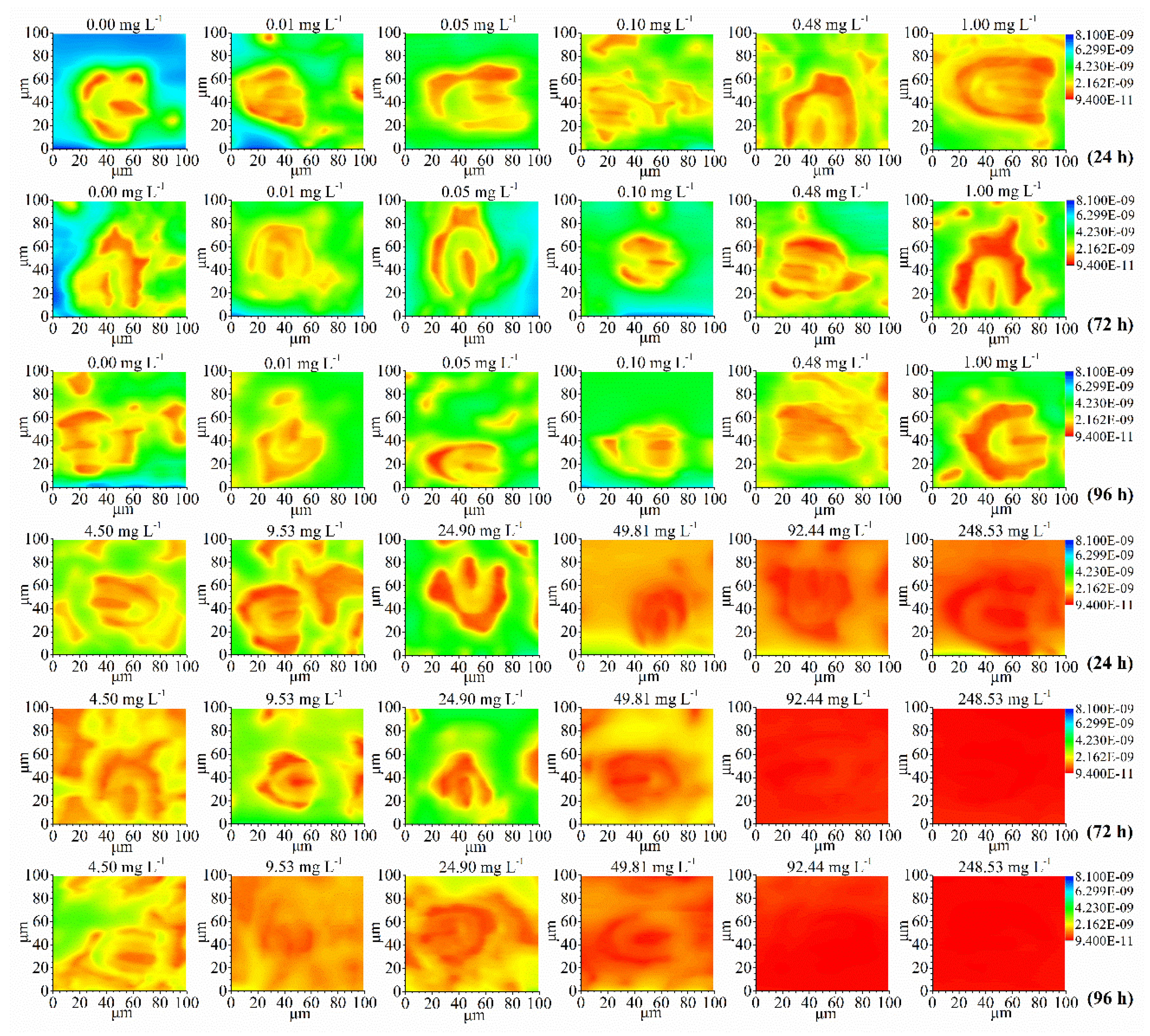
The Typical 100×100 μm spatial distribution images of oxygen concentration current above an individual stoma of the leaf discs (8 mm in diameter) under 2,4-DCP treated and untreated
M. pteropus recorded by SECM were shown in
Figure 1.
Each image was obtained at 50 μm s-1 (n=12). The probe-to-substrate distance, d, was initially set at 1.5μm. The UME was biased at -0.60 V. The electrolyte solution was the PBS solution (pH 6.86) containing 0.1 M NaHCO3. In each image, relatively high concentrations of oxygen were in deep blue to green; raised topographical features were in yellow to red.
The color around the stoma was dominated by green and blue for the control and the lower concentrations treated samples, then gradually turned to green, yellow and red as 2, 4-DCP concentration increased up to nearly 100-250 mg L
-1 indicating the oxygen concentration in stoma decreased with increasing 2,4-DCP concentrations. There were clear three-tined-fork-shaped stoma for the control and the samples treated with up to about 50 mg L
-1. However, When the concentration of 2, 4-DCP continued to increase to 250 or 500 mg L
-1, the outline of the three-tined-fork-shaped stoma became increasingly blurred. These results were in good agreement with the CLSM fluorescence images of the stroma (
Figure 2). The stomatal oxygen release concentration gradually decreased with the exposure time extending, which is consistent with the variation rule (
Figure 2).
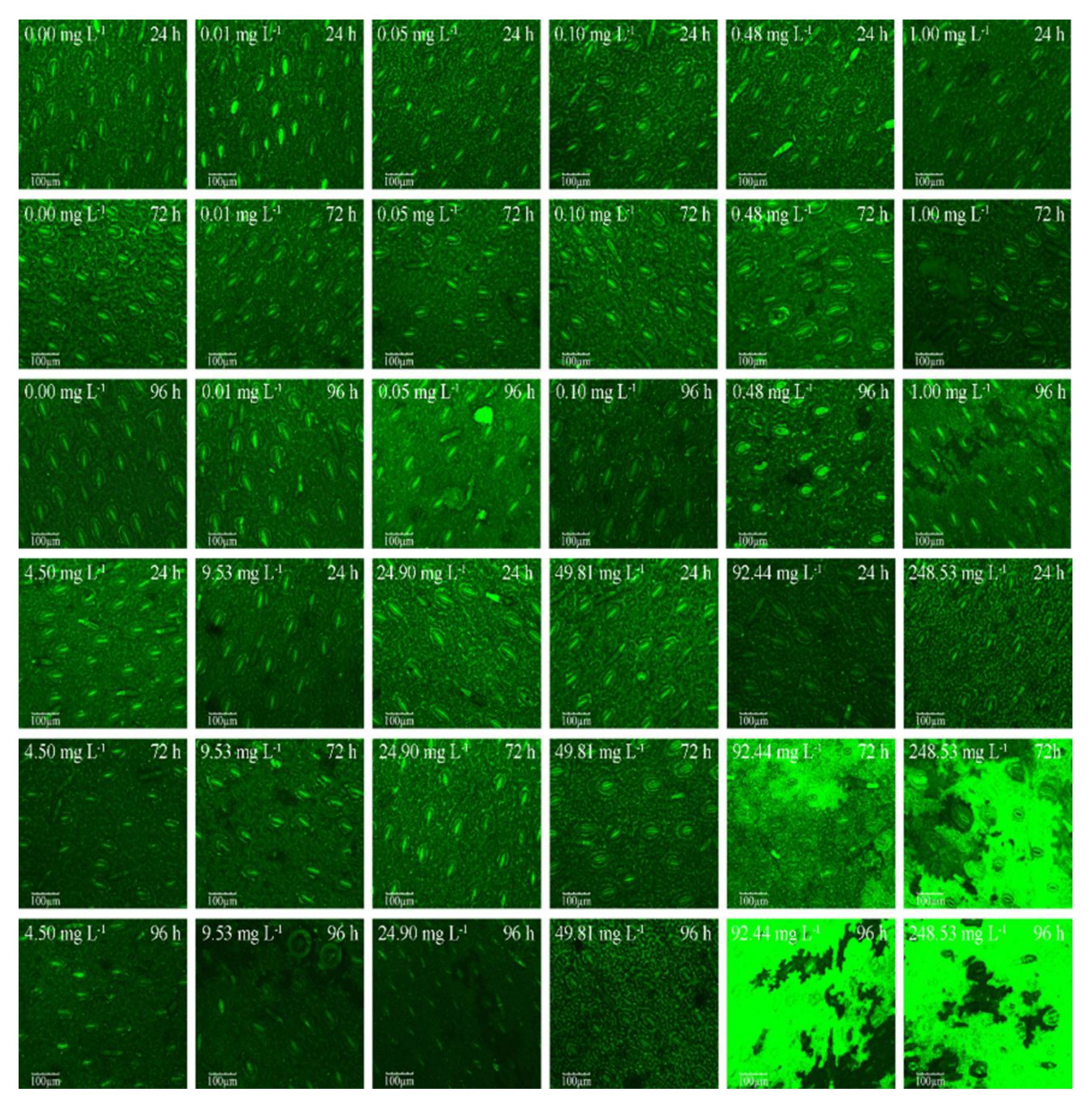
3.2. CLSM Images of M. pteropus Stoma
CLSM images (at 488 nm) of
M. pteropus stoma under various concentration of 2,4-DCP are shown in
Figure 2. The concentrations of 2,4-DCP are given in the legend. The scale bar indicates 100 μm.
Figure 2 shows that the stoma changed from clear and intact to blurry and damaged with increasing 2, 4-DCP concentration (n=12). The boundaries between the stomata and the surrounding tissues were clear in the stoma and mesophyll tissue of the control and the lower concentrations treated samples, and the stomata density was relatively evenly distributed, and the brightness was more obvious.
However, when the concentration of 2, 4-DCP continues to increase to 100-250 mg L
-1, the degree of damage in the leaves was deepened, and black spots and black holes appeared in the leaf tissue; Meanwhile, the outline of the stoma itself and the boundary between stomata and tissue became increasingly blurred (
Figure 2). These results are also consistent with the image results of the SECM corresponding concentration in
Figure 1.
In addition, the CLSM results also confirmed that the toxicity and damage of 2, 4-DCP to the stomata of M. Pteropus also increased as the concentrations increased and the culture time prolonged.
3.3. Estimation of IC50 from SECM and Other Methods
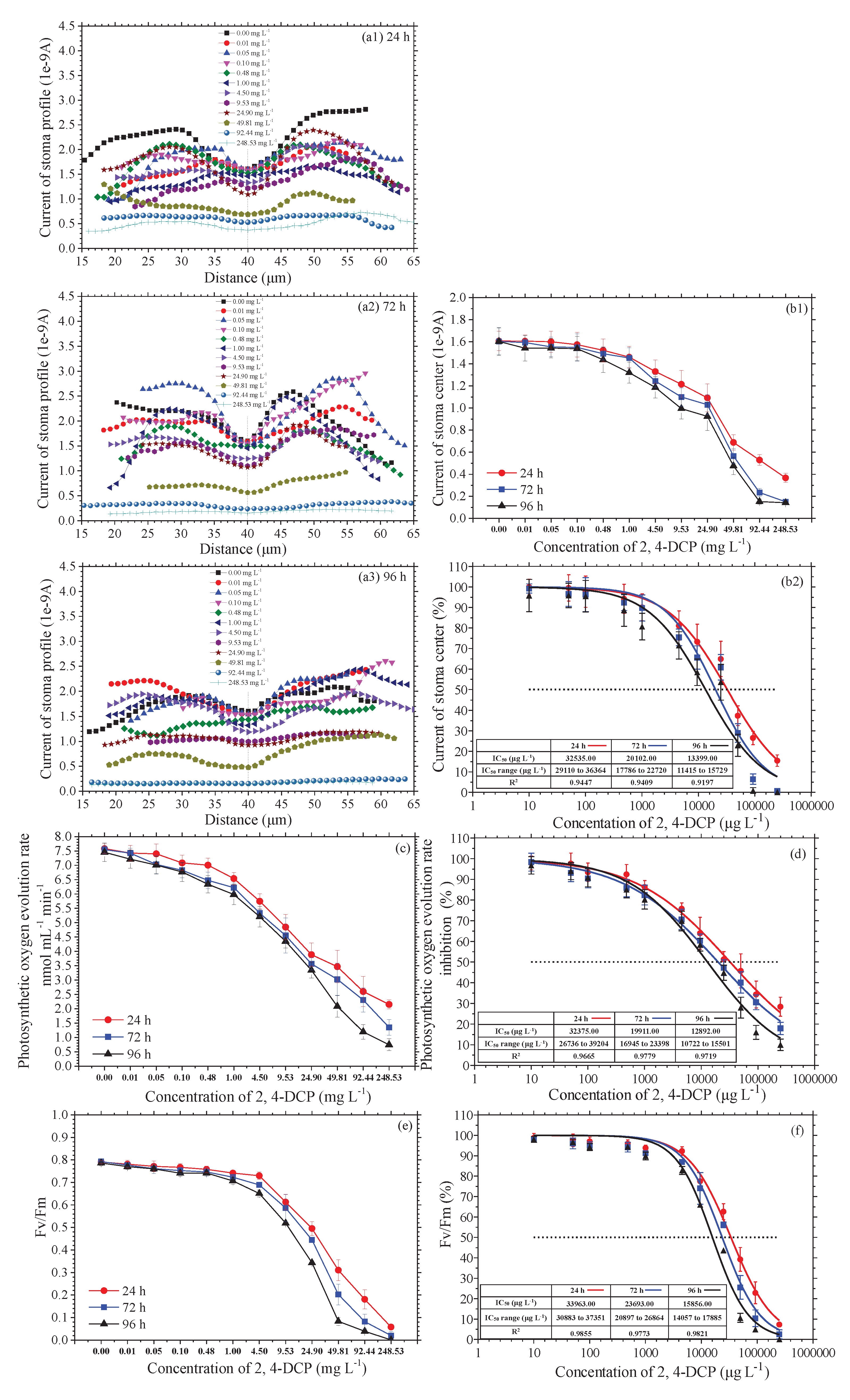
Figure 3 shows the current profile curves (a1, a2, a3), the current curves of stoma center (b1), the photosynthetic oxygen evolution rate curves(c), the Fv/Fm curves (e), and their corresponding dose-effect curves (with attachments for IC
50 value) (b2, d, f) of
M. pteropus stoma under various concentrations of 2, 4-DCP for 24 h, 72 h and 96 h. The error bars represented standard deviation; Each treatment was replicated four times (n=12). The IC
50 value was calculated by the GraphPad Prism 5 software.
Figure 3(a1,a2,a3) show the typical current curve along a line section of one stoma perpendicular to longitude. Oxygen concentration current at the center of the stoma was used to estimate IC
50 because oxygen is directly emitted from the stoma core with least influence of other factors. In order to estimate the validity of IC
50 derived from the Oxygen concentration current from SECM data, IC
50 was also calculated from photosynthetic oxygen evolution data collected by the Clark liquid-phase electrode and the Fv/Fm.
It can be seen from
Figure 3(b1,c,e) that Oxygen concentration current at the center of the stoma, the photosynthetic oxygen evolution rate and the Fv/Fm gradually decreased as the concentrations of 2,4-DCP increased. we found (
Figure 3(b2,d,f)) that the Best-fit values of IC
50 curve fit the reverse S-shape, and the IC
50 value of by SECM is close to the IC
50 value obtained by the photosynthetic oxygen evolution rate and the Fv/Fm.
The very close statistical significance of IC
50 value between SECM method and two traditional measurement methods (Clark oxygen electrode and PAM) through DMRT test (P=0.949>0.05) illustrated that there was no significant difference between the results obtained by SECM method and that obtained by two traditional methods (
Table 1). In other words, the determination of SECM method is reliable and can introduced it into the ecotoxicology field.
4. Discussion
Stomata are the main routes for leaf transpiration,gas exchange and controlling CO
2 uptake. Stomatal damage or closure reduces CO
2 uptake, thereby weakens photosynthesis and photosynthetic oxygen evolution rate. Literatures show that photosynthetic oxygen evolution rate (Reflecting photosynthetic rate) determined by the conventional oxygraph respirometers is a reliable indicator to quantify the inhibition of photosynthesis by pollutants [
16]; Fv/Fm (Reflecting efficiency and physiological status of photosynthetic organs), another well validated indicator based on photosynthetic electron transport rate, can be detected rapidly and non-invasively by chlorophyll fluorometer [
1]; Gas exchange(Characterization of photosynthetic efficiency), the third common technique of photosynthesis research can be mensurated by measuring CO
2 fixation efficiency); In addition, there are some in vitro physiological and biochemical methods by extraction and isolation of photosynthetic organs. However, the above methods cannot provide spatial distribution of the bioindicators. CLSM can acquire images, but the spatial resolution is at best submicrometer resolution and in most cases cannot provide accurate concentration information of the bioindicators.
This study shows SECM provides a solution to these limitations by mapping concentration data at high spatial resolution using UME and precise positioning and scanning system. The greatest superiority of SECM is its ability to probe chemical/electrochemical information of electron and ion transfer processes at the interfaces [
8]. When the probe (usually a Pt disc electrode with diameter < 25 μm) is rastered above a plane approaching to the substrate, SECM is possible to map out the surface topography and/or chemical redox reactions by monitoring the current perturbation. The accurate tip position and the exact current measured determine the high quality of SECM images. The principle of SECM to probe toxic effects on photosynthetic oxygen evolution is based on the role of the oxygen produced by water splitting as a mediator. The changes in the mediator concentration induced by environmental stress can be very sensitively detected as change in the current by the UME of SECM in the ‘substrate-generation and tip-collection mode’ [
17]. Therefore, monitoring oxygen concentration profiles affords a possible pathway for direct and real-time detection of oxygen evolution response to the stress. SECM can in-vivo and in-situ image the cell respiration rate and plant photosynthetic oxygen evolution, which supply supplementary information for traditional liquid oxygen electrode method.
Zhu, R.K. [
8] firstly assessed using a SECM probe to detect oxygen evolution and the changes in individual stoma structure in
Brassica juncea (L.) Czern. cv. AC Vulcan caused by Cd stress. These limited pioneering studies demonstrated the great potential of application of SECM in the field of ecotoxicology. In the present study, the results show that the photosynthetic oxygen evolution rate and Fv/Fm of
M. pteropus all significantly decreased with increasing 2, 4-DCP concentration (
Figure 3(c,e)). This indicates the photosynthesis of
M. pteropus are stressed upon 96 h exposure to 2, 4-DCP. The SECM data and the Clark oxygen evolution data indicate the toxicity of 2,4-DCP to water-splitting center, while the Fv/Fm data implies that the electron transport was inhibited by 2, 4-DCP on the donor side or/and on the acceptor side (
Figure 3). The close values of IC
50 between stoma center current by SECM and two conventional methods (O
2 evolution and Fv/Fm) verifies that SECM method is a reliable method to probe the toxicity of 2, 4-DCP to photosynthesis on the primary electron donor side.
In addition, the clear shape of the stoma and regular change of its structure from the SECM images show that SECM has the advantage of simultaneously mapping topography of stoma and photosynthesis activity. Before its wide application to ecotoxicology, the protocols to ensure reliability of SECM needs to be checked with more higher plants and photosynthesis microbes exposed to more pollutants (heavy metals, saline-alkali, and compound contaminants).
The resolution of SECM mainly depends on the size and shape of the probe and the distance between the probe and the substrate, so the quality of the probe is very important to SECM imaging [
18]. We suggest to use high quality UME with good C-V curve and proper RG value. In addition, the resolution of the SECM is determined by the parameters of the instrument (including shock resistance and thermal stability), scanning speed, appropriate electrolyte solution, as parallel as possible between the end face of the probe and the substrate surface, as well as the gas escape from the electrode reaction. With the continuous improvement and development of SECM instrument itself (including the improvement of resolution) [
19,
20] and its combination with other measurement methods, it will also become a major development trend of ecotoxicology.
5. Conclusions
We present a novel ecotoxicological method describing the detection process based on nanoscale electrochemical mapping of photosynthetic oxygen evolution of aquatic plants by SECM. In this technology, a well-polished 10 μm diameter platinum UME was used as the SECM tip, a 0.5 mm platinum wire was served as the counter electrode, Ag/AgCl as served as the reference electrode. the phosphate buffered saline (PBS, pH 6.86) solution containing 0.1 M NaHCO3 was used as the supporting electrolyte which could provide excessive CO2 for photosynthesis during measurement.
As shown previously, such approach can be used for simultaneous topographical and electrochemical scanning experiments of stoma oxygen emission of M. pteropus under 2, 4-DCP stress, which indicates that SECM can be a powerful and noninvasive tool used for ecotoxicological study. Furthermore, more ecotoxicological experimental protocols on more higher plants and more pollutants (heavy metals, other organics, saline-alkali, and compound contaminants, etc) can be done at a later stage.
Author Contributions
Conceptualization, N.Z. and D.Z.; methodology, N.Z. and D.Z.; software, N.Z.; validation, N.Z. and D.Z.; formal analysis, N.Z.; investigation, N.Z. and D.Z.; resources, D.Z.; data curation, N.Z.; writing—original draft preparation, N.Z. and D.Z.; writing—review and editing, D.Z.; visualization, N.Z.; supervision, D.Z.; project administration, D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: National Natural Science Foundation of China (No. U1703243, U1503281) and Ministry of Education of the People’s Republic of China (No. 230818010207279).
Data Availability Statement
Data are available from the authors by request.
Acknowledgments
We are grateful to the reviewers for their valuable comments that significantly improved our manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deng, C.N.; Zhang, D.Y.; Pan, X.L.; Chang, F.Q.; Wang, S.Z. Toxic Effects of Mercury on Psi and Psii Activities, Membrane Potential and Transthylakoid Proton Gradient in Microsorium Pteropus. J Photochem. Photobiol. B 2013, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Pan, X.L.; Zhang, D.Y. Psi Showed Higher Tolerance to Sb(V) Than Psii Due to Stimulation of Cyclic Electron Flow around Psi. Curr. Microbiol. 2015, 70, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.A.; Ahmed, H.M.I.; Misra, M.; Sharma, P.; Misra, A.N. Nitric Oxide Alleviates Photochemical Damage Induced by Cadmium Stress in Pea Seedlings. Phyton-International Journal of Experimental Botany 2022, 91, 959–975. [Google Scholar] [CrossRef]
- Kong, D.; Li, X.; Tang, Y.; Sui, M.; Li, J.; Ma, Y.; Wang, G.; Gu, W.; Guo, X.; Yang, M. A Highly Parallel Dtt/Mb-DNA/Au Electrochemical Biosensor for Trace Hg Monitoring by Using Configuration Occupation Approach and SECM. Ecotoxicol. Environ. Saf. 2022, 234, 113391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Ning, X.M.; Ma, Q.L.; Qin, D.D.; Lu, X.Q. Recent Advances in Electrochemistry by Scanning Electrochemical Microscopy. Trends Analyt. Chem. 2016, 80, 242–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Takahashi, Y.; Fukuma, T.; Matsue, T. Scanning Electrochemical Microscopy for Biosurface Imaging. Current Opinion in Electrochemistry 2021, 29, 100739–100736. [Google Scholar] [CrossRef]
- Tsionsky, M.; Cardon, Z.G.; Bard, A.J.; Jackson, R.B. Photosynthetic Electron Transport in Single Guard Cells as Measured by Scanning Electrochemical Microscopy. Plant Physiol. 1997, 113, 895–901. [Google Scholar] [CrossRef]
- Zhu, R.K.; Macfie, S.M.; Ding, Z.F. Cadmium-Induced Plant Stress Investigated by Scanning Electrochemical Microscopy. J. Exp. Bot. 2005, 56, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, I.; Kuss, S.; Mauzeroll, J.; Geissler, M. Biological Scanning Electrochemical Microscopy and Its Application to Live Cell Studies. Anal. Chem. 2011, 83, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Li, Y. Enhanced 2, 4-Dichlorophenol Degradation at pH 3-11 by Peroxymonosulfate Via Controlling the Reactive Oxygen Species over Ce Substituted 3d Mn2O3. Chem. Eng. J. 2019, 355, 448–456. [Google Scholar] [CrossRef]
- Li, R.; Jin, X.; Megharaj, M.; Naidu, R.; Chen, Z. Heterogeneous Fenton Oxidation of 2,4-Dichlorophenol Using Iron-Based Nanoparticles and Persulfate System - Sciencedirect. Chem. Eng. J. 2015, 264, 587–594. [Google Scholar] [CrossRef]
- Makevita, M.; Athauda S.Pahalawattarachchi V. Development of in Vitro Sterilization Procedure for Java Fern (Microsorum Pteropus)[C]. FAuRS-2018. 2018.
- Miyoshi, S.; Kimura, S.; Ootsuki, R.; Higaki, T.; Nakamasu, A. Developmental Analyses of Divarications in Leaves of an Aquatic Fern Microsorum Pteropus and Its Varieties. PLoS ONE 2019, 14, e0210141. [Google Scholar] [CrossRef]
- QIN, Y.; GENG, S.; JIAO, W.; LIU, Y. Deep Oxidation Degradation of Aniline Wastewater by O3 /Fe( Ⅱ) Process Enhanced Using High-Gravity Technology. Chinese Journal of Energetic Materials 2018, 26, 448–454. [Google Scholar] [CrossRef]
- Wang, S.Z.; Chen, F.L.; Mu, S.Y.; Zhang, D.Y.; Pan, X.L.; Lee, D.J. Simultaneous Analysis of Photosystem Responses of Microcystis aeruginoga under Chromium Stress. Ecotoxicol. Environ. Saf. 2013, 88, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Jiang, X.H.; Li, K.; Wu, M.; Zhang, R.F.; Zhang, L.; Chen, G.X. Photosynthetic Responses of Oryza sativa L. Seedlings to Cadmium Stress: Physiological, Biochemical and Ultrastructural Analyses. BioMetals 2014, 27, 389–401. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Pemaiah, B.; Natesan, R.; Padmavathy, S.R.; Pachiappan, J. Real-Time Mapping of Salt Glands on the Leaf Surface of Cynodon dactylon L. Using Scanning Electrochemical Microscopy. Bioelectrochem. 2015, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Zoski, C.G. Review-Advances in Scanning Electrochemical Microscopy (SECM). J. Electrochem. Soc. 2016, 163, 3088–3100. [Google Scholar] [CrossRef]
- Sciutto, G.; Zangheri, M.; Prati, S.; Guardigli, M.; Mirasoli, M.; Mazzeo, R.; Roda, A. Immunochemical Micro Imaging Analyses for the Detection of Proteins in Artworks. Top. Curr. Chem. 2016, 374, 32–59. [Google Scholar] [CrossRef] [PubMed]
- Junjie, Z.; Tong, Z.; Jinxin, L.; Wenxuan, F.; Fei, L. Recent Advances of Scanning Electrochemical Microscopy and Scanning Ion Conductance Microscopy for Single-Cell Analysis. Current Opinion in Electrochemistry 2020, 22. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).