1. Introduction
Global warming is one of the major environmental problems facing mankind, and governments are taking a cumulative number of countermeasures to slow it down. China’s proposed targets of “carbon peaking” by 2030 and “carbon neutrality” by 2060 will promote the optimization, upgrading of the industrial structure and the adjustment of the energy structure, and lead the overall green transformation of the economy and society [
1]. The IPCC Fifth Assessment Report points out that greenhouse gas emissions from agricultural sources account for 24% of the total global greenhouse gas emissions [
2]. Agriculture is different from other industries in that, on the one hand, farmland ecosystems emit large quantities of greenhouse gases such as CO
2, N
2O, and CH
4, and at the same time, farmland which has the dual characteristics of a carbon source and a carbon sink fixes CO
2 through photosynthesis. Carrying out research on agricultural greenhouse gas emission reduction and carbon fixation and sink enhancement has become an important tool for promoting green development in agriculture.
Carbon footprint, as a quantitative greenhouse gas emissions research method, has received attention and recognition from many scholars and has been cited in the field of agriculture [
3]. The use of life cycle assessment (LCA) to assess the greenhouse gas emissions of the agricultural production process, and the production and use of agricultural materials is conducive to the identification of the carbon footprint of agricultural activities as well as the optimization of emission reduction measures [
4]. In recent years, many scholars have conducted research on carbon footprint in agriculture and achieved preliminary results. Zhu Q et al. studied the carbon footprint of alpine organic rice and compared the carbon emissions of the carbon footprint of a variety of rice products at various stages, concluding that the planting process is the production stage with the highest carbon emissions [
5]; Cao L et al. carried out a study on the rice production process, and concluded that nitrogen fertilizer application is considered to be the highest carbon-emitting stage in the production process [
6]. Chinese agricultural carbon footprint studies mainly focus on grain crops, such as Li C et al. [
7], Mao G [
8], and Long J [
9] studied the carbon footprints of grain crops such as wheat, maize, and millet, while the carbon footprint studies of cash crops are relatively weak. Due to the differences in natural conditions, crop species, and field management, the carbon footprint research results of grain crops cannot provide an accurate scientific basis for the green development of cash crops. At the same time, whether farmland ecosystems are net carbon sources or sinks remains controversial [
10]. Banana plantations are an important farmland ecosystem type in Hainan Province [
11], and conducting research on the carbon footprint of banana plantation ecosystems is of great significance in promoting the green development of tropical agriculture. In this study, Chengmai County was selected as the target area for the study. Through field research and one-year field location monitoring, the carbon footprint theory of agriculture and life cycle assessment were used to account for the carbon emissions of each link in the banana production process, to calculate the carbon sequestered by banana plants and farmland soils, to assess the carbon footprint of the tropical banana plantations, and the results of the study will provide theoretical basis and practical guidance for the optimization of the agricultural structure adjustment, the reduction of carbon emissions and the increase of carbon sinks in agriculture as well as the green development of agriculture.
2. Materials and Methods
2.1. Study Area
The study area is located in the northern part of Chengmai County, dominated by the tableland plains, with a tropical maritime monsoon climate, a multi-year average temperature of 23.2°C, an average annual precipitation of about 1700 mm, and obvious seasonal changes in precipitation, with the rainy season occurring from May to October, and the dry season occurring from November to April of the following year. The soil type is laterite with an average soil organic matter of 2.53% in the 0-30 cm layer. The soil texture is loamy-clay loam, deep and fertile, suitable for tropical crops such as bananas and coffee and other tropical crops.
Chengmai County is one of the main production areas of bananas in Hainan Province. The average annual banana harvesting area in the county in 2018-2020 is 9.17×103 hm 2, accounting for 20.95% of the banana planting area in Hainan Province, and the average annual production is 3.08×105 t, accounting for 24.70% of the province’s banana production. The main species of bananas planted are Musa paradisiaca AA and M. AAA Cavendish var. Brazil. The newly planted bananas are transplanted from the group seedling, the second and third planted bananas are from retained buds, and bananas are harvested three times in two years.
2.2. Research Methods
2.2.1. Carbon Emission Terms and Measurement Methods for Banana Plantations
Greenhouse gas emissions in banana plantation ecosystems mainly include CO
2 emissions from the manufacturing process of agricultural materials, N
2O emissions from the farmland due to fertilizer application, and CO
2 emissions due to soil respiration. Carbon emissions from the manufacturing process of agricultural materials mainly include carbon emissions from fertilizer and pesticide application, as well as energy consumption of agricultural machinery and agricultural irrigation. The formula is as follows [
12].
Where
is the CO
2 equivalent emission,the unit is t CO
2eq,
Ei is the carbon emission of each agricultural material in the agricultural production process,
Qi is the quantity of each agricultural material (including fertilizer, pesticide, agricultural machinery, agricultural irrigation and ploughing), and α
i is the carbon emission coefficient of each agricultural material, with reference to the relevant literature [
13,
14,
15,
16,
17,
18] (
Table 1).44/12 is the conversion factor of carbon equivalent (CE) to CO
2 equivalent.
N
2O emissions from banana plantations mainly come from nitrogen in fertilizers applied in field management, including: (1) direct emissions of N
2O from nitrogen in the process of nitrification and denitrification; (2) indirect emissions of N
2O due to the deposition of nitrogen-containing reactive substances after volatilization; (3) indirect emissions of N
2O due to nitrogen leaching and runoff. The formulae are as follows [
19]:
N
2O
direct, N
2O
deposition and N
2O
leaching represent N
2O direct emissions, N
2O indirect emissions due to deposition and N
2O indirect emissions due to leaching runoff, respectively; and N
total represents the total input of nitrogen. EF
direct, EF
deposition, and EF
leaching respectively represent N
2O direct emission coefficient, N
2O indirect emission coefficient due to deposition, and N
2O indirect emission coefficient due to leaching runoff. The coefficients are 1%, 1% and 1.1%, respectively; α
deposition is the rate of volatilization of nitrogen from agricultural land, with a value of 11%; and α
leaching is the rate of nitrogen leaching and runoff from banana plantations, with a value of 24% [
19].
is the CO
2 equivalent converted from total N
2O emissions, unit is t CO
2eq. 298 is a conversion coefficient based on the warming potential of N
2O converted to CO
2 equivalent [
14].
CO
2 absorbed from the air by plants through photosynthesis, part of which is returned to the atmosphere through root respiration and leaf respiration, and the other part is stored in the plant as organic matter, i.e., net primary production (NPP) [
10]. To avoid double counting of CO
2 released by root respiration, banana plant roots should be physically isolated when monitoring soil respiration fluxes in agricultural fields. The formula is as follows.
CSR represents CO2 emissions due to soil respiration in t CO2eq, T represents time in a, A represents the area of banana plantation in hm 2, and Rsoil is the soil respiration flux from agricultural land in μmol·m-2·s-1.
2.2.2. Carbon Fixation Term and Measurement Methods for Banana Plantations
The carbon pool of banana plantations mainly consists of two parts: banana plants and banana plantation soils. The carbon fixation of banana plants is calculated as follows.
Where C plant denotes the carbon fixation of a single banana plant in g C; i denotes an organ of the banana; Ci denotes the carbon fixation of the organ in g C; Gi denotes the weight of the organ in g; ti denotes the carbon content of the organ and wi denotes the water content of the organ of the banana.
Soil carbon fixation is calculated as follows [
20].
where C
soil denotes soil carbon content in g C; γ denotes soil bulk density in g·cm
-3; d denotes soil depth in cm; C
SOC denotes soil organic carbon content in %; S denotes area in cm
2; ΔC
soil denotes soil carbon increase in g C; C
soil t denotes soil carbon content in period t, and C
soil 0 denotes soil carbon content in the base period, and t-o denotes the length of time in years.
2.2.3. Experiments and Data Collection
(1) Data on carbon emissions from agricultural materials used in agricultural production were derived from the 2018-2020 household survey, in which 30 households each of Musa paradisiaca AA growers and M. AAA Cavendish var. Brazil were selected for the study, with continuous tracking and investigation of the production information of the growers over the two-year production process. The research farmers all had more than 2 years of experience in banana planting and had more than 3 hm2 of cultivated area, and the total area of the research banana plantation was about 450 hm2. The questionnaire mainly consisted of banana varieties, planting area and density, yield, amount of fertilizer, pesticide and bagging, electricity consumption for irrigation, and diesel consumption of farming machinery, and other production information.
(2) Soil respiration carbon emissions were continuously monitored from October 2019 through December 2020 for the second and third banana growing cycles. Two banana plantations close to the average fertilizer application were selected as sample plots for soil CO2 flux measurements, and soil rings were installed at three measurement points in each sample plot, and a 60 cm deep trench was dug around the soil rings, with a double-thick plastic film placed inside the trench to isolate the surrounding plant roots and to monitor soil respiration without root respiration of the banana plants. Using the Li-8100A soil respiration monitoring system, the measurement points were monitored once a month for a continuous period of 48h, with 1h intervals between measurements, and each measurement was repeated three times.
(3) Measuring banana biomass. During the banana ripening and harvesting period, 50 representative plants were selected from each of the eight near-average fertilizer-applied plantations of M. AAA Cavendish var. Brazil and Musa paradisiaca AA. Biological parameters such as plant height, diameter at breast height and leaf length were measured and the mean values were calculated. Each of the eight households selected one banana plant close to the average value of each parameter as a standard plant, the whole plant was cut down and above-ground organs such as pseudo stems, leaves, and fruits were weighed separately, and the underground parts were excavated, washed and weighed, and samples were taken back to the laboratory for the determination of its water content and carbon content, respectively. Water content was determined by drying method, and the samples were dried to constant weight at a constant temperature of 60°C to calculate water content of each organ. Banana plant samples were dried and ground into powder, passed through a 0.25 mm sieve, and the carbon content of the samples was determined by potassium dichromate-sulfuric acid oxidation method to derive the amount of carbon sequestered in the dry matter of a single banana plant, and the amount of carbon sequestered by banana plants in the banana plantations was calculated based on the density of banana plantations.
(4) Measuring soil carbon fixation. Soil organic carbon pools were accounted for on a two-year cycle, with September 2018 before banana planting as the base period and October 2020 when plantains were harvested as the end period.
Five sampling points were selected between plants and rows of each banana plantation sample plot, and soil samples were taken at 0-10 cm, 10-20 cm, and 20-30 cm depths for each sampling point to determine the soil organic carbon content by using the potassium dichromate oxidation-spectrophotometry method, and the soil bulk weight was determined by using the ring knife method at each sampling point. Accordingly, the soil carbon contents of the base period and the final period were calculated, and the difference between the two soil carbon contents was divided by 2, which was the average annual soil carbon fixation.
3. Results and Analysis
3.1. Carbon Emission of Banana Plantation Ecosystems
3.1.1. Carbon Emission of Agricultural Material
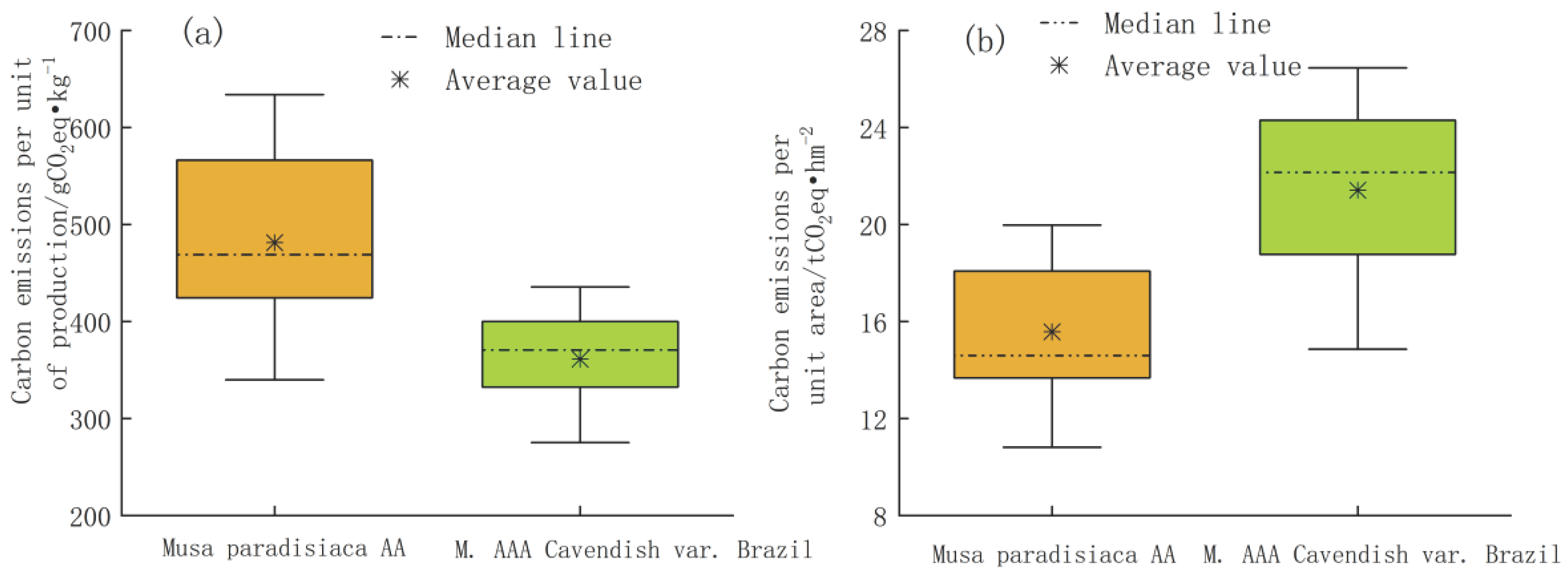
As shown in
Figure 1, there were large differences in carbon emissions from agricultural materials per unit of production and per unit of area for different varieties of bananas. The carbon emission per unit of production of Musa paradisiaca AA was 464.05 g CO
2 eq·kg
-1, and the carbon emission per unit of planted area was 15.03 t CO
2 eq·hm
-2. The carbon emission of M. AAA Cavendish var. Brazil was 395.88 g CO
2 eq·kg
-1 and 23.46 t CO
2 e /hm
-2, respectively.
As shown in
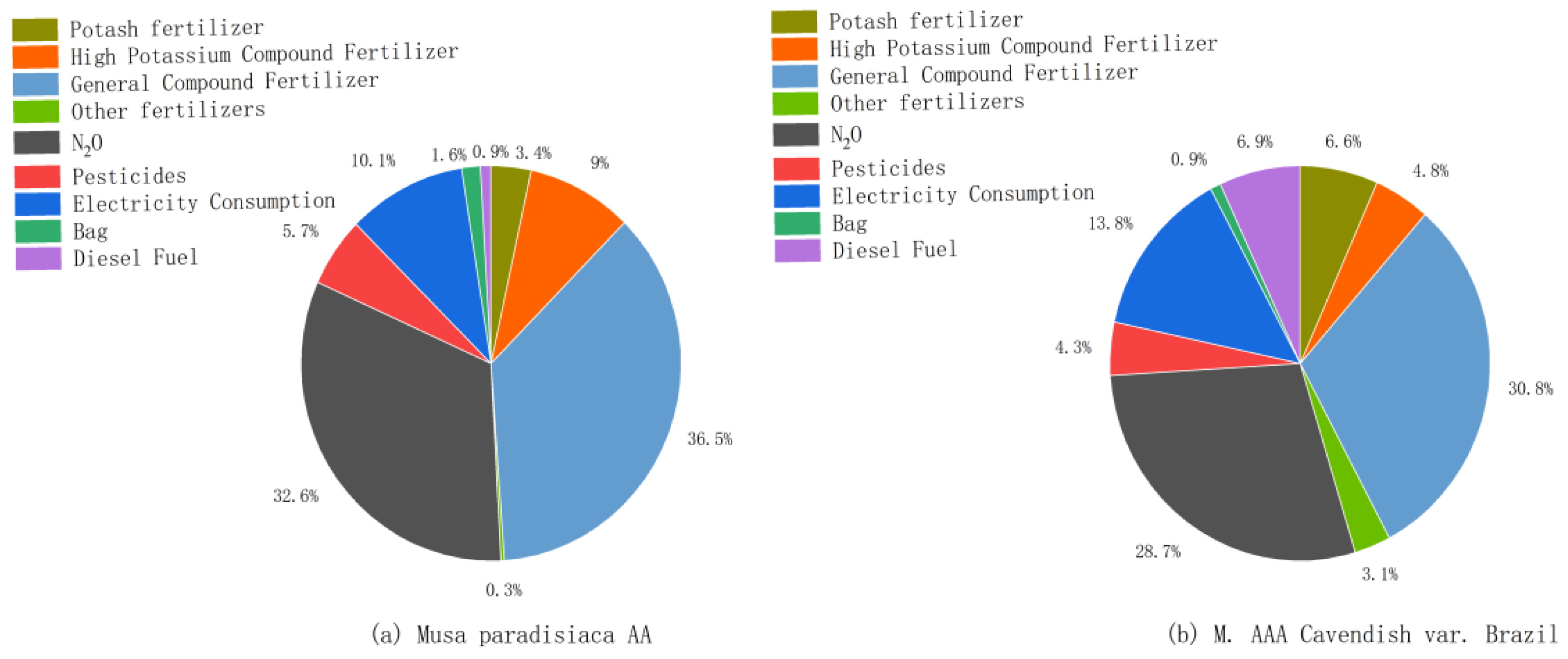
Figure 2, differences in the ranking of carbon emission sources from agricultural materials and the contribution of carbon emission components were found between the Musa paradisiaca AA and M. AAA Cavendish var. Brazil. The largest source of carbon emissions for both Musa paradisiaca AA and M. AAA Cavendish var. Brazil was the production of compound fertilizers, which accounted for 36.50% and 30.80% of the carbon emissions from total agricultural materials, respectively. The second largest source of emissions was both N
2O emissions due to the application of nitrogenous fertilizers and nitrogenous composite fertilizers, with Musa paradisiaca AA and M. AAA Cavendish var. Brazilian contributing 32.60% and 28.70% of the carbon emissions, respectively. The contribution of carbon emissions from agricultural materials of potash fertilizers (including potassium chloride, potassium sulphate and high-potassium compound fertilizers) for Musa paradisiaca AA was 12.40%, which was slightly higher than that of M. AAA Cavendish var. Brazilian at 11.40%. Carbon emissions from agricultural energy consumption as a percentage of carbon emissions from total agricultural materials varied greatly between Musa paradisiaca AA, which accounted for 11.00%, and M. AAA Cavendish var. Brazilian, which accounted for 20.70%, respectively. The difference was due to the fact that all the plantations in the study area were irrigated with water and fertilizer, and deep wells were mostly pumped for irrigation during the dry season; the former required relatively less water, whereas the latter had a high frequency of fertilizer application and consumed a large amount of water. Secondly, the latter had a high yield per plant, and during the fruiting period it is necessary to use a diesel engine to punch holes to fix brackets to support the stalks, which increases energy consumption. Third, pesticide consumption account for a relatively small percentage of carbon emissions for both Musa paradisiaca AA and M. AAA Cavendish var. Brazilian, 5.70% for the former and 4.33% for the latter, because of the dense foliage of the latter, growers mostly use diesel engines to spray pesticides, while the former growers usually spray pesticides manually, which increases the energy consumption of M. AAA Cavendish var. Brazilian. The combination of the three factors resulted in the contribution of carbon emissions from agricultural energy consumption of M. AAA Cavendish var. Brazil being nearly one times higher than that of Musa paradisiaca AA.
3.1.2. Carbon Emission of Soil Respiration
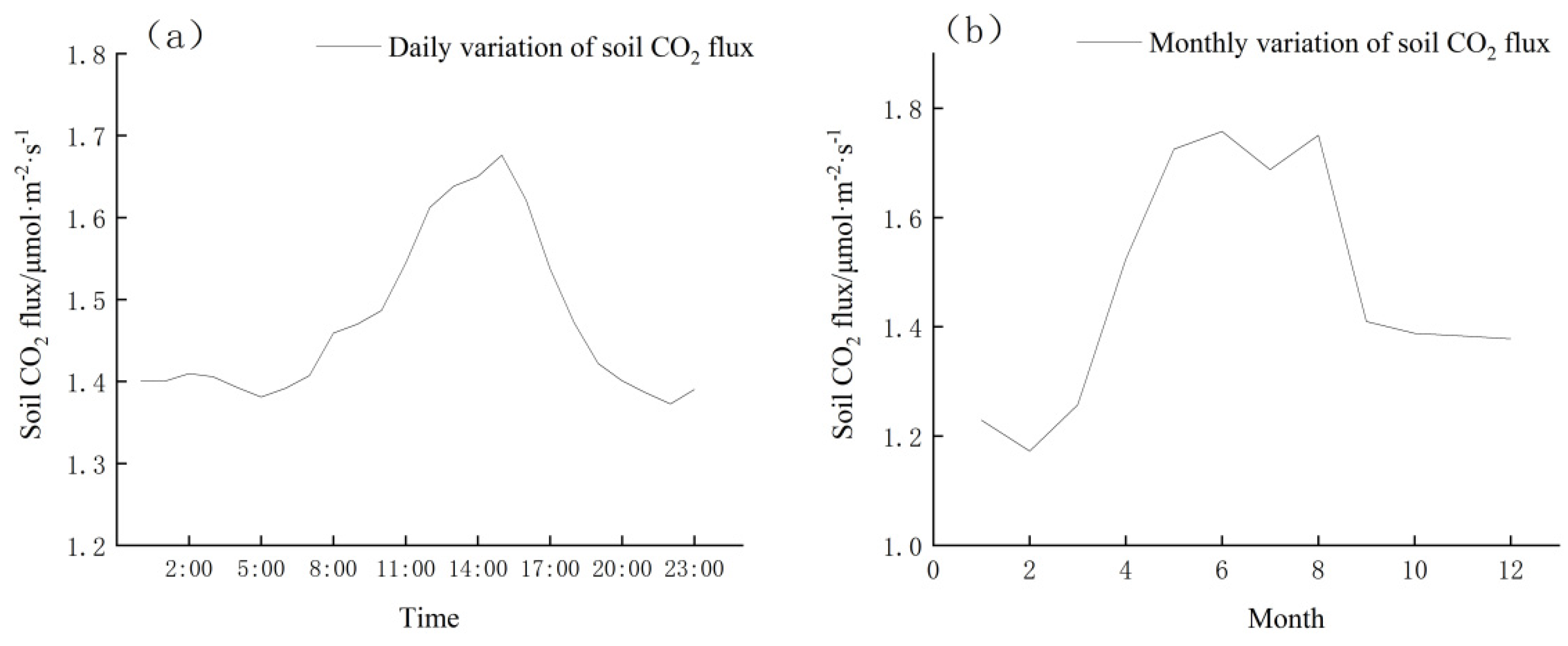
As shown in
Figure 3, daily and monthly changes in soil respiration in sample banana plantations showed a single-peaked curve. Soil respiration in the banana plantation continued to rise after 6:00 a.m. and reaching a peak of about 1.68 μmol·m
-2·s
-1 at 15:00 a.m. Soil respiration continued to decline after the peak, and fell to a lower level of about 1.37 μmol·m
-2·s
-1 around 22:00 at night and continued until around sunrise. Soil respiration was higher from April to August, with the maximum value occurring in June at about 1.76 μmol·m
-2·s
-1 and soil respiration was lower from September to March of the following year, with the minimum value occurring in February at about 1.17 μmol·m
-2·s
-1. The average value of soil respiration in banana plantations for the whole year was 1.47 μmol·m
-2·s
-1, and the CO
2 emission from soil respiration in banana plantations in the study area was about 20.37 t·hm
-2·a
-1.
3.2. Carbon Fixation of Banana Plantation Ecosystem
3.2.1. Plant Carbon Fixation of Banana Plantations
Table 2 shown mean water content and carbon content varied considerably between the various organs of Musa paradisiaca AA and M. AAA Cavendish var. Brazil, while the differences between Musa paradisiaca AA and M. AAA Cavendish var. Brazil were small for the same organ. The water content of each organ of banana of the two varieties in descending order was pseudo stem, root system, leaves, and fruit. The carbon content in descending order was leaves, fruit, pseudo stem, and root system. The two varieties of bananas did not differ much in water content and carbon content of each organ, and Musa paradisiaca AA had slightly lower water content and slightly higher carbon content than M. AAA Cavendish var. Brazil.
Carbon fixation of Musa paradisiaca AA per plant and per unit area was smaller than that of M. AAA Cavendish var. Brazil. The former had a smaller individual biomass than the latter, and carbon fixation per plant was 36.77% smaller than the latter. The former fixed an average of 2.31 kg CE per plant, which was 8.46 kg CO
2 eq, while the latter fixed an average of 3.65 kg CE per plant, which was 13.38 kg CO
2 eq. The carbon fixation per hectare of the former was about 24.72 t CO
2 eq and that of latter was about 34.66 t CO
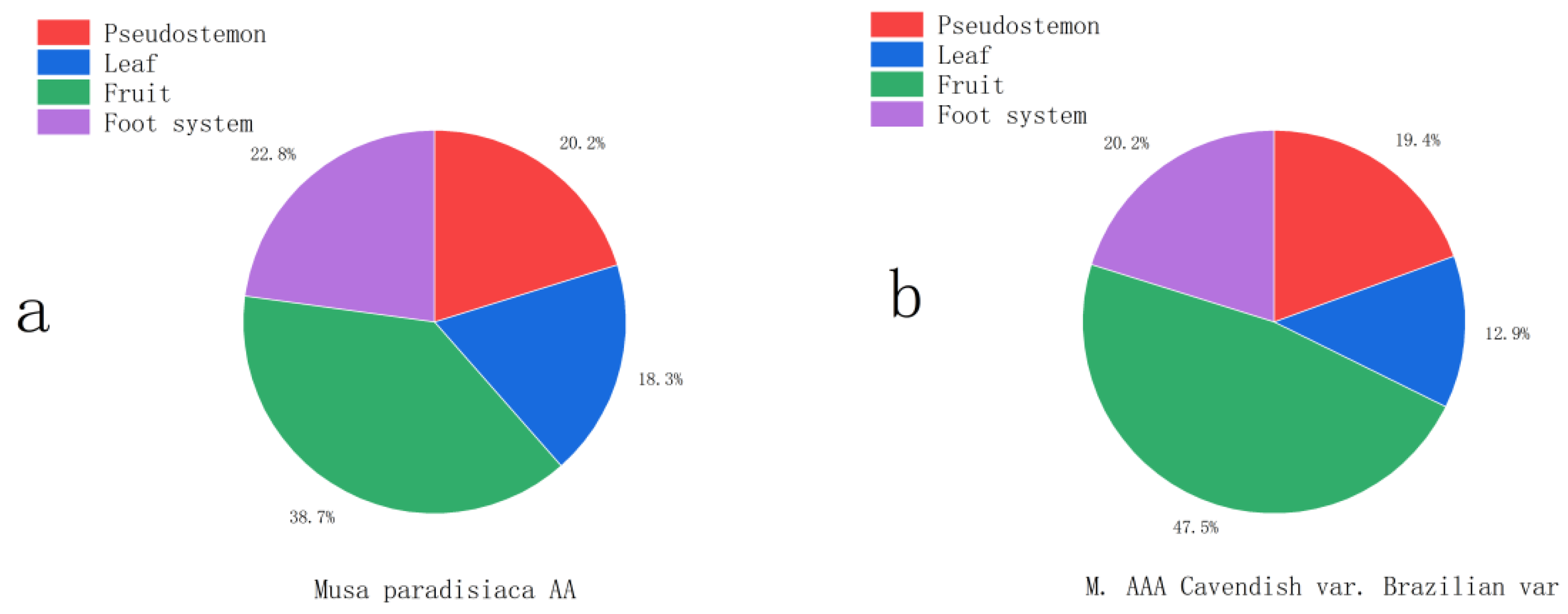
2 eq, that is, carbon fixation per unit area of the former was 28.68% smaller than that of the latter. As shown in
Figure 4, the ordering of the contribution rates of carbon sink in the organs of Musa paradisiaca AA and M. AAA Cavendish var. Brazil was consistent, and was fruit, root system, pseudo stem, and leaf in descending order. Fruits were the most important component of carbon sinks for Musa paradisiaca AA and M. AAA Cavendish var. Brazil, accounting for 38.65% and 47.53%, respectively, and leaves accounted for the smallest portion of carbon sinks, 18.32% and 12.88%, respectively. Musa paradisiaca AA and M. AAA Cavendish var. Brazil had intermediate percentages of pseudo stem and root carbon sinks and small differences between species.
3.2.2. Soil Carbon Fixation of Banana Plantations
Table 3 shown that Soil organic carbon content in the 0-30 cm soil layer of banana plantations increased from 2018 to 2020, and the increase between rows was significantly higher than that between plants, and the annual average soil carbon change was the soil carbon fixation in banana plantations. There were temporal and spatial differences in soil carbon content between rows and plants in banana plantations. The soil organic carbon content between plants and rows in the 0-30 cm soil layer decreased with increasing depth, and the organic carbon content of all soil layers between rows was higher than that between plants. The soil organic carbon content of the soil layer between plants in the depth of 0-30 cm increased by an average of 13.47% in the period from 2018 to 2020, whereas the average increase between rows amounted to 24.48%. The soil bulk density between rows was slightly higher than that between plants in banana plantations, and the change in soil bulk density was small during the study period. The soil carbon content of banana plantations in the sample site was 45.62 t C·hm
-2 in the base period of 2018 and 52.99 t C·hm
-2 in the end period of 2020, with an average annual increase in soil organic carbon of 3.68 t C·hm
-2, or 13.51 t CO
2 eq·hm
-2.
3.3. Carbon Footprint of Banana Plantation Ecosystems
The carbon sources of banana plantation ecosystems mainly included agricultural material use and soil respiration. The carbon emissions of Musa paradisiaca AA were 35.40 t CO2 eq·hm-2, and the carbon emissions caused by agricultural material and soil respiration accounted for 42.46% and 57.54%, respectively. The carbon emissions from M. AAA Cavendish var. Brazil were 43.84 t CO2 eq·hm-2, accordingly, agricultural material and soil respiration accounted for 53.53% and 46.47%, respectively. The carbon sinks of banana plantation ecosystems mainly included plant and soil carbon fixation. Musa paradisiaca AA orchard plantation fixed 38.23 t CO2 eq·hm-2, with 64.66% plant carbon fixation and 35.34% soil carbon fixation. M. AAA Cavendish var. Brazil plantation fixed 48.17 t CO2 eq·hm-2, with 71.95% plant carbon fixation and 28.05% soil carbon fixation.
4. Discussion
4.1. Comparison of Carbon Emissions from Agricultural materials of Banana and Other Crops
The carbon emissions from agricultural materials per unit of production of Musa paradisiaca AA and M. AAA Cavendish. var. Brazil were 464.05 g CO
2 eq·kg
-1 and 395.88 g CO
2 eq·kg
-1 and the annual carbon emissions per unit of area were 15.03 t CO
2 eq·hm
-2·a
-1 and 23.46 t CO
2 eq·hm
-2·a
-1.
Table 4 shown that Musa paradisiaca AA and M. AAA Cavendish var. Brazil had higher carbon emissions per unit area than wheat, maize, cotton and other crops, while carbon emissions per unit of production were lower. Carbon emissions per unit area in banana plantations were lower than that of pomelo plantations, while carbon emissions per unit of production were slightly higher [
21].
The composition of carbon emissions from the banana production process was similar to that of other crops, with fertilizer (especially nitrogen fertilizer) application being the main source of CO
2 and N
2O emissions from farmland, contributing 81.73% of the carbon emissions per unit of production of Musa paradisiaca AA, and 74.00% of M. AAA Cavendish var. Brazil. It was higher than that of winter wheat-summer maize rotation farmland, where CO
2 and N
2O emissions from fertilizer application accounted for 68.56% of the total carbon emissions from all agricultural materials [
23]. High temperatures generally produce higher direct emissions of CO
2 and N
2O from fertilizer application in tropical regions than those in subtropical and temperate regions [
19,
20,
24], which is the reason for the high carbon emission contribution of banana plantations in this study.
4.2. Factors Affecting Carbon Fixation of Banana Plantation Ecosystems
Banana fruits and plants were the main contributors to carbon fixation in banana plantation ecosystems, and carbon fixation in banana plantations was influenced by factors such as biomass, water content, carbon content and planting density. Despite the fact that the water content of banana fruits reached 80%, the water content of plants was as high as 90%, and the carbon content of dry matter was less than 40%, the carbon fixation per unit area of banana was higher because of the large biomass of banana single fruits and plants, and because bananas were planted with 2,700 to 3,000 plants per hectare at a higher planting density.
Conservation tillage could reduce the loss of soil organic carbon and increases soil carbon fixation [
25,
26]. Field management of banana plantations in the study area was mostly 3 crops in 2 years, with the 2nd and 3rd crops being left to bud, and no-tillage and less-tillage to reduce soil disturbance and decrease the rate of mineralization of soil organic carbon, which promoted the increase of soil organic carbon, and the soil organic matter showed an increasing trend in the banana plantations in the study area. Previous long-term positioning experiments on winter wheat land, as well as other types of farmlands, also found that the rate of carbon sequestration was higher in no-tillage than in tilled land, and that the soil organic carbon content of no-tillage was significantly higher than that of conventional tillage patterns [
27,
28].
4.3. Scientific management and development of agricultural land
The problem of over-fertilization is common in banana plantations, and studies have shown that scientific fertilization can reduce the amount of chemical fertilizer by 1/3 without decreasing banana yields [
29,
30]. Fertilizer application was the main source of carbon emissions from farmland, and fertilizer application accounted for 80-90% of the carbon emissions from agricultural materials in banana plantations, which showed that there was a greater potential for controlling the amount of fertilizer application in the green development of agriculture. The degree of modernization of field management also affected carbon emissions from agricultural materials per unit of production. Field management of Musa paradisiaca AA plantations was managed traditionally and manually, while field management of M. AAA Cavendish var. Brazil plantain plantations was more modernized, with the former emitting an average of 464.05 g CO
2 eq·kg
-1 of carbon per unit of production, and the latter emitting an average of 395.88 g CO
2 eq·kg
-1 of carbon per unit of production. Similar findings have been reported for vegetable plots, orchards, and other crops [
31,
32]. Farmland fertilizer reduction, agricultural product yield enhancement and farmland soil carbon increase have become research hotspots for green development of agriculture [
33]. Moderate input of chemical fertilizers into farmland has contributed to increased net primary productivity and reduced net CO
2 emissions [
11]. The net farmland carbon sink per unit area of M. AAA Cavendish var. Brazil is higher than that of Musa paradisiaca AA, and summer maize is higher than that of winter wheat [
34], suggesting that optimization and adjustment of agricultural planting structure can increase farmland carbon sinks [
35]. In addition, the comprehensive application of measures such as energy saving, pesticide reduction, and water-saving irrigation can also reduce greenhouse gas emissions and promote green development of agriculture [
36].
5. Conclusion
Throughout the life cycle of banana cultivation, N 2O emissions from the application of nitrogenous fertilizers and carbon emissions from the production and transportation of fertilizers are the most important sources of greenhouse gas emissions, and banana fruits and plants are the main contributors to carbon fixation in the banana production process. Both Musa paradisiaca AA and M. AAA Cavendish var. Brazil farmland systems have annual carbon emissions lower than annual carbon fixation, and both have negative carbon footprints per unit area, and the banana ecosystem has the function of carbon sequestration. Scientific management in the field can achieve carbon reduction and sink increase, reduce farmland carbon footprint, and promote green development of tropical agriculture.
Acknowledgments
This research was funded by the Hainan Natural Science Foundation, grant number 420MS043 and the National Nature Science Foundation of China, grant number 41361006.
References
- Zhou, H.C. Researcher Zhou Hongchun from the Development Research Center of thCarbon Peaking and Carbon Neutrality Goals Leading the Comprehensive Green Transformation in China’s Economy and Society: An Interview withe State Council. J. Poyang Lake 2022, 5–14+125. [Google Scholar]
- Chen, Z.D.; Li, F.B.; Feng, J.F.; Zhou, X.Y.; Xu, C.C.; Ji, L.; Fang, F.P. Study On Carbon Footprint For Rice-Wheat Rotation System In The Lower Reaches of Yangtze River—Based On The Life Cycle Assessment. Chin. J. Agric. Resour. Reg. Plan. 2019, 40, 81–90. [Google Scholar]
- WIEDMANN, T.; MINX J, A. Definition of “Carbon Footprint”. J. Royal Soc. Med. 2007, 92, 193–195. [Google Scholar]
- Zhou, Z.H. Estimates of Carbon Footprint of Crop Production by Life Cycle Assessment (LCA) Method. MA thesis, Chinese Academy of Agricultural Sciences Dissertation, Beijing, 2018. [Google Scholar]
- Zhu, Q.; Duan, J.H.; Qian, Y.H.; Lou, Y.L. Carbon Footprint of Organic Rice Based On Life Cycle Theory—Case Of Mountain Organic Rice In Jinzhai County. J. Arid Land Resour. Environ. 2019, 33, 41–46. [Google Scholar] [CrossRef]
- Cao, L.M.; Li, M.B.; Wang, X.Q.; Zhao, Z.P.; Pan, X.H. Life cycle assessment of carbon footprint for rice production in Shanghai. Acta Ecol. Sin 2014, 34, 491–499. [Google Scholar] [CrossRef]
- Li, C.X.; Luo, T.T.; Yan, G.X.; Xu, S.; Zong, J.J.; Shao, Y. Carbon footprint analysis of wheat-maize double cropping system in different ecological regions of Henan province. Ecol. & Environ 2020, 29, 918–925. [Google Scholar] [CrossRef]
- Mao, G.H. Carbon Footprint Evaluation of Agricultural Products Based On Lca And Research Of Carbon Tag Evaluation Method. MA thesis, Taiyuan University Of Technology, Taiyuan, 2017. [Google Scholar]
- Long, J. Integration of Environmental Footprints And Its Impact Analysis Of Beijing Crops Based On Life Cycle Assessment. MA thesis, Beijing Forestry University, Beijing, 2020. [Google Scholar]
- Liu, X.H.; Xu, W.X.; Li, Z.J.; Chu, Q.Q.; Yang, X.L.; Chen, F. The Missteps,Improvement And Application Of Carbon Footprint Methodology In Farmland Ecosystems With The Case Study Of Analyzing The Carbon Efficiency of China’s Intensive Farming. Chin. J. Agric. Resour. Reg. Plan 2013, 34, 1–11. [Google Scholar]
- Xie, J.H. Fruit scientific research in New China in the past 70 years: Banana. J. Fruit Sci 2019, 36, 1429–1440. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Qin, M.Z. Temporospatial Variation of Partial Carbon Source/Sink of Farmland Ecosystem in Coastal China. J. Ecol. Rural Environ 2007, 23, 1–6+11. [Google Scholar]
- Chen, S.; Lu, F.; Wang, X.K. Estimation of greenhouse gases emission factors for China’s nitrogen, phosphate, and potash fertilizers. Acta Ecol. Sin 2015, 35, 6371–6383. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, F.; Huang, Z. ; Gang.; Chen, S.; Wang, X.K. Estimations of application dosage and greenhouse gas emission of chemical pesticides in staple crops in China. Chin. J. Appl. Ecol 2016, 27, 2875–2883. [Google Scholar] [CrossRef]
- Ministry Policy Paper. China Low Carbon Yearbook—2013 China Regional Grid Baseline Emission Factors, 1rd ed.; Metallurgical Industry Press: Beijing, China, 2014; pp. 189–191. [Google Scholar]
- IPCC. Climate change 2007: Synthesis report. Contribution of working groups i, ii and iii to the fourth assessment report of the intergovernmental panel on climate change. In (International Conference on Climate Change, Geneva, Switzerland, May 29-31, 2007. 29 May.
- Zhou, T.; Gao, M.; Xie, D.T.; Wei, C.F. Research on Carbon Source/Sink and Carbon Footprint of Cropland Ecosystem in Chongqing. J. Southwest Agric. Univ 2014, 36, 96–102. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, R.Z. Estimation of Energy Consumption And Carbon Emissions Of China’s Pulp & Paper Industry. Chin. Pulp Paper 2014, 32, 50–55. [Google Scholar] [CrossRef]
- Department of Climate Change. National Development and Reform Commission. Guidelines for the preparation of provincial greenhouse gas inventories (for trial implementation). In State Council Executive Meeting, Beijing, China, May 1, 2011. 1 May.
- Han, B.; Wang, X.K.; Lu, F.; Duan, X.N.; OuYang, Z.Y. Soil carbon sequestration and its potential by cropland ecosystems in China. Acta Ecol. Sin 2008, 612–619. [Google Scholar]
- Xu, X.Z. Comprehensive evaluation of carbon emissionand optimum fertilization in Guanxi Pomelo Production. MA thesis, Fujian Agriculture And Forestry University, Fuzhou, 2019. [Google Scholar]
- Wang, Z.B.; Wang, M.; Chen, F. Carbon Footprint Analysis of Crop Production in North China Plain. Sci. Agric. Sin 2015, 48, 83–92. [Google Scholar] [CrossRef]
- Zhu, Y.C.; L, Y.e.; Jiang, D.F.; Zou, X.X. Life Cycle Assessment on Carbon Footprint of Winter Wheat -Summer Maize Cropping System Based on Survey Data of Gaomi in Shandong Province, China. J. Agric. Resour. Environ 2017, 34, 473–473. [Google Scholar] [CrossRef]
- LAURA, R.; AZIZ, E.; ALMUDENA, H. Carbon footprint along the Ecuadorian banana supply chain: methodological improvements and calculation tool. J. Clean. Prod 2016, 112, 2441–2451. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Reicosky, D.C.; Allmaras, R.R.; Sauer, T.J; Venterea, R. T.; Dell, C.J. Greenhouse gas contributions and mitigation potential of agriculture in the central USA. Soil Tillage Res 2005, 83, 73–94. [Google Scholar] [CrossRef]
- Yang, Y. X.; Gao, G. W.; Zhang, Z. M.; Chen, C. Y.; Sui Peng, S. P. Reducing agricultural carbon footprint through diversified crop rotation systems in the North China Plain. J. Clean. Prod. 2014, 76, 131–139. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yan, C.R.; Zhang, Y.Q.; Wang, J.B.; He, W.Q.; Chen, B.Q.; Liu, E.K. Effect of no tillage on carbon sequestration and carbon balance in farming ecosystem in dryland area of northern China. Trans. Chin. Soc. Agric. Eng 2015, 31, 240–247. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Li, S.S.; Liu, B.Y.; Zhang, Y.; Latif Virk, A.; Wang, X.; Zhao, X.; Zhang, H.L. Effects of no till and residue retention on carbonsequestration and yield in China. J. China Agric. Univ 2020, 25, 1–12. [Google Scholar]
- Zang, X.P.; Jng, T.; Zhou, D.B.; Wang, W.; Ding, Z.L.; Wang, B.Z.; X, J.H.; Ma, W.H. Effects of Reducing Nitrogen, Phosphorus and Potassium Fertilizer on Yield, Nutrient Absorption and Economic Benefit of Banana under Drip Irrigation. J. Irrig. Drain 2019, 38, 22–27. [Google Scholar] [CrossRef]
- Song, S.S.; Duan, J.L.; Zou, X.J.; Yang, Z.; Ou, Z.W.; Wang, B. Parameter optimization and test of variable fertilizer apparatus based on root distribution pattern of bananas. Trans. Chin. Soc. Agric. Eng 2020, 36, 11–18. [Google Scholar] [CrossRef]
- Song, b. Carbon Footprint, Low-carbon and Ecological Compensation Mechanism of Vegetable Production. PhD dissertation, China Agricultural University, Beijing, 2016. [Google Scholar]
- LU, H. Comparative Study Based On The Bifferent System Simulation Model Kiwifruit Orchard Ecosystem Carbon Sink Capacity----Bo Xiang Village, Qinglong Township Yingjing as an example. MA thesis, Sichuan Agricultural University, Chengdu, 2012. [Google Scholar]
- Xiao, G.M.; Ru, S.H.; Hou, L.M.; Wang, C.; Zhao, O.Y.; Sun, S.Y.; Wang, L.; Liu, L.; Zhang, G.Y. Carbon Footprint Analysis of Wheat-maize Production System in Hebei Province. J. Ecol. Rural. Environ 2022, 38, 1388–1395. [Google Scholar] [CrossRef]
- Chen, S.; Yang, S.; Zhang, B.; Wang, L.; Hu, T. Carbon balance in summer maize /winter wheat farmland ecosystem under different water and fertilizer conditions. T. Chin. Soc. Agr. Mach. 2021, 52, 229–238. [Google Scholar] [CrossRef]
- Ye, W.W.; Wang, C.C.; Zhao, C.J.; Zheng, X. Spatial and temporal patterns of the carbon footprint of tropical farmland ecosystems on Hainan Island in the last 20 years. Chin. J. Agric. Resour. Reg. Plan. 2021, 42, 114–126. [Google Scholar]
- Huang, B.B.; Zhang, S.Y.; Zhang, J.Q. Study on the carbon reduction effect and application drivers of low-carbon planting technologies. J. Ecol. Rural. Environ 2018, 34, 1082–1090. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).