Submitted:
06 March 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
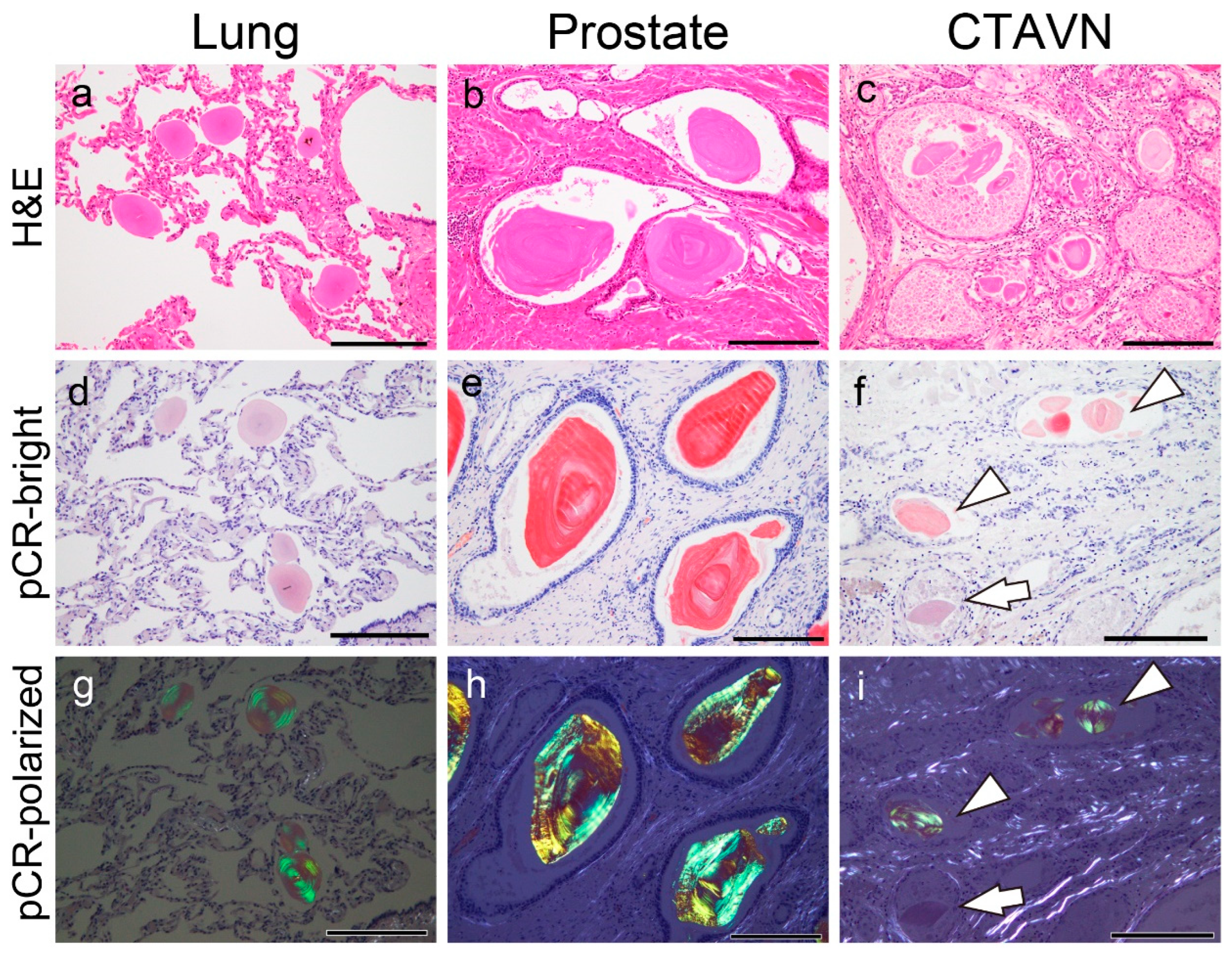
2.1. General Appearance of Pulmonary, Prostatic, and CTAVN-Associated CAs
2.2. General appearance of pulmonary, prostatic, and CTAVN-associated CAs
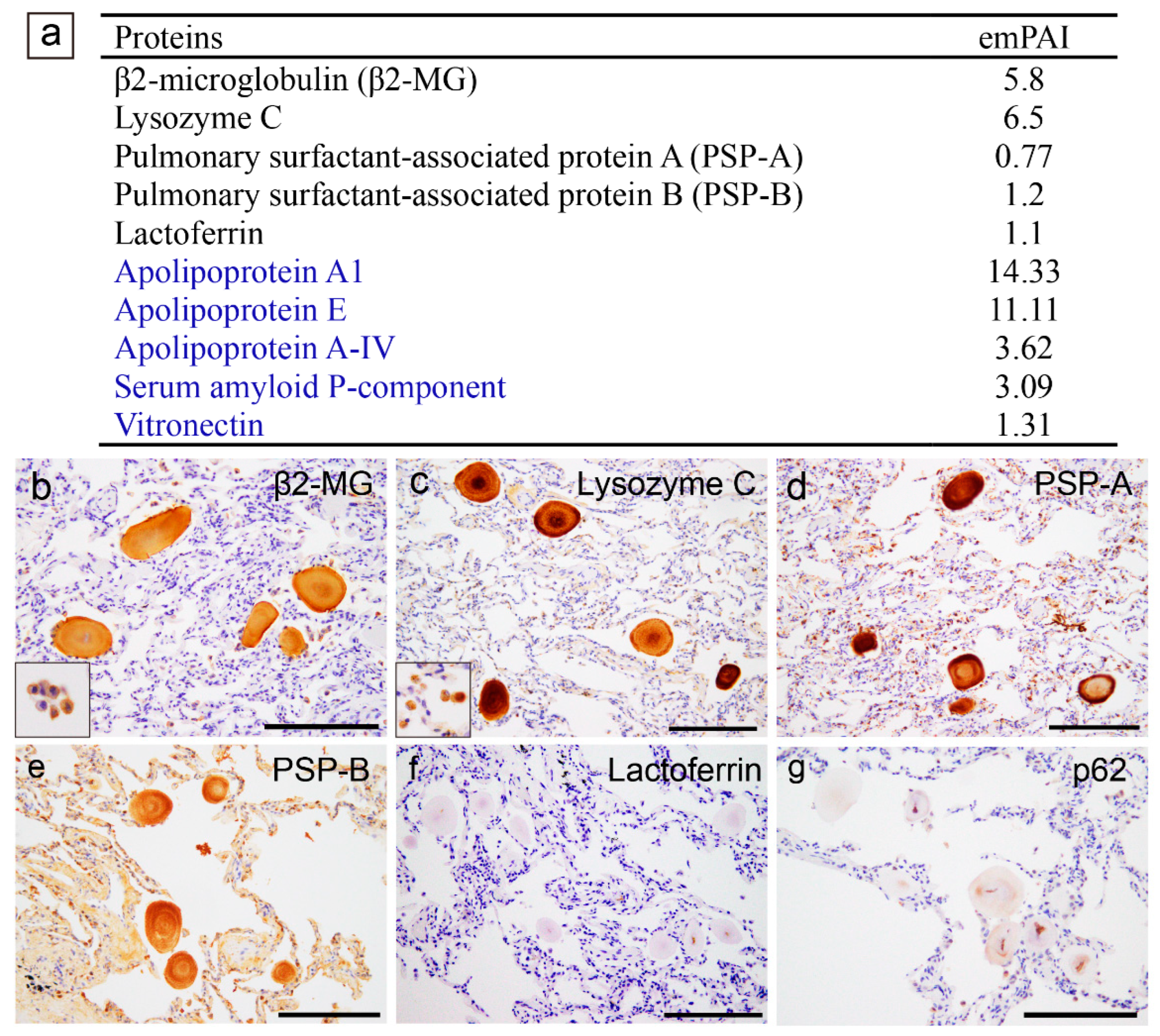
2.3. Proteomic and immunohistochemical features of the pulmonary CAs
2.4. Proteomic and immunohistochemical features of prostatic CAs
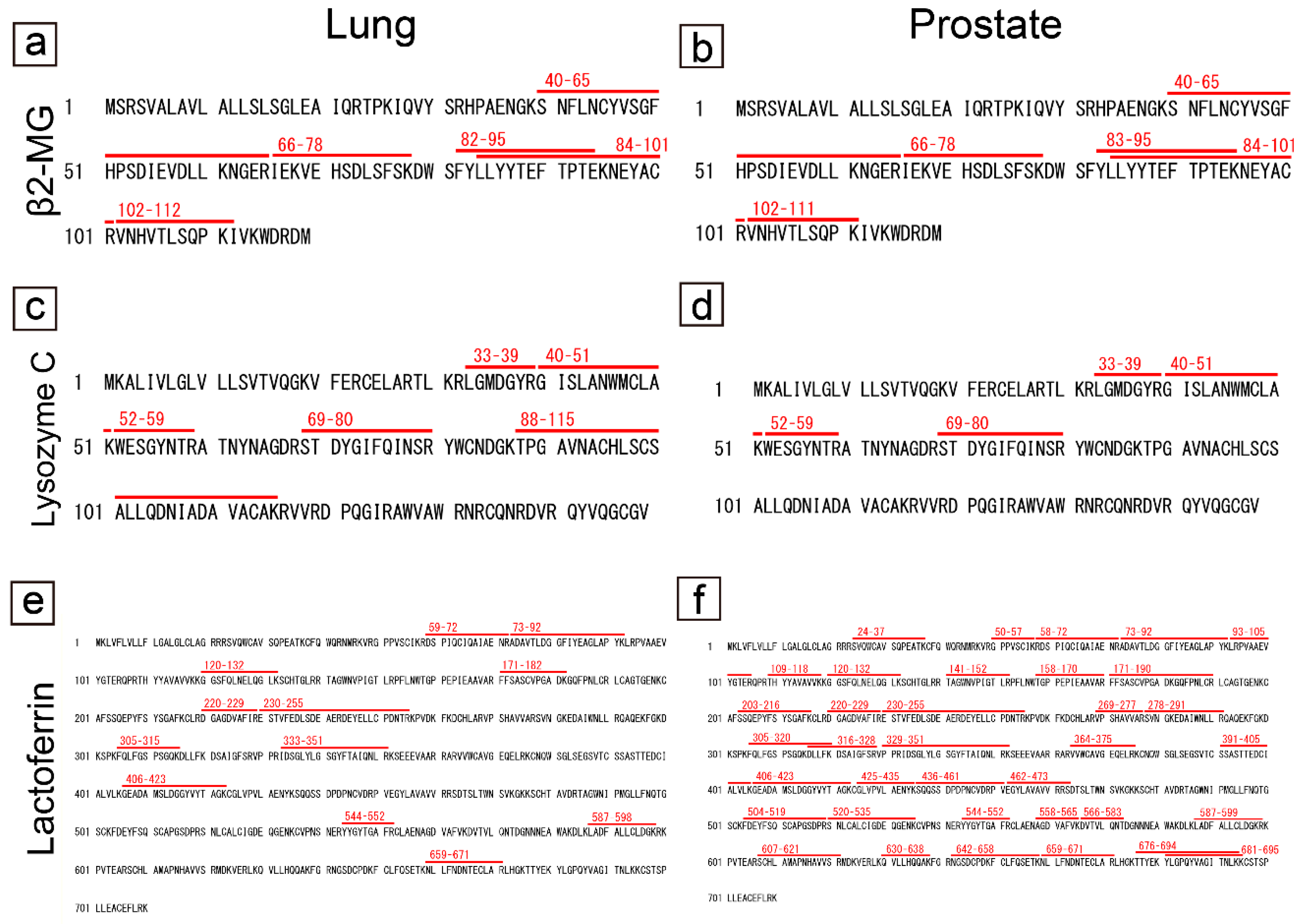
2.5. Comparison of the amino acid sequences of proteins commonly identified in pulmonary and prostatic CAs
2.6. Proteomic and immunohistochemical features of CTAVN-associated CAs
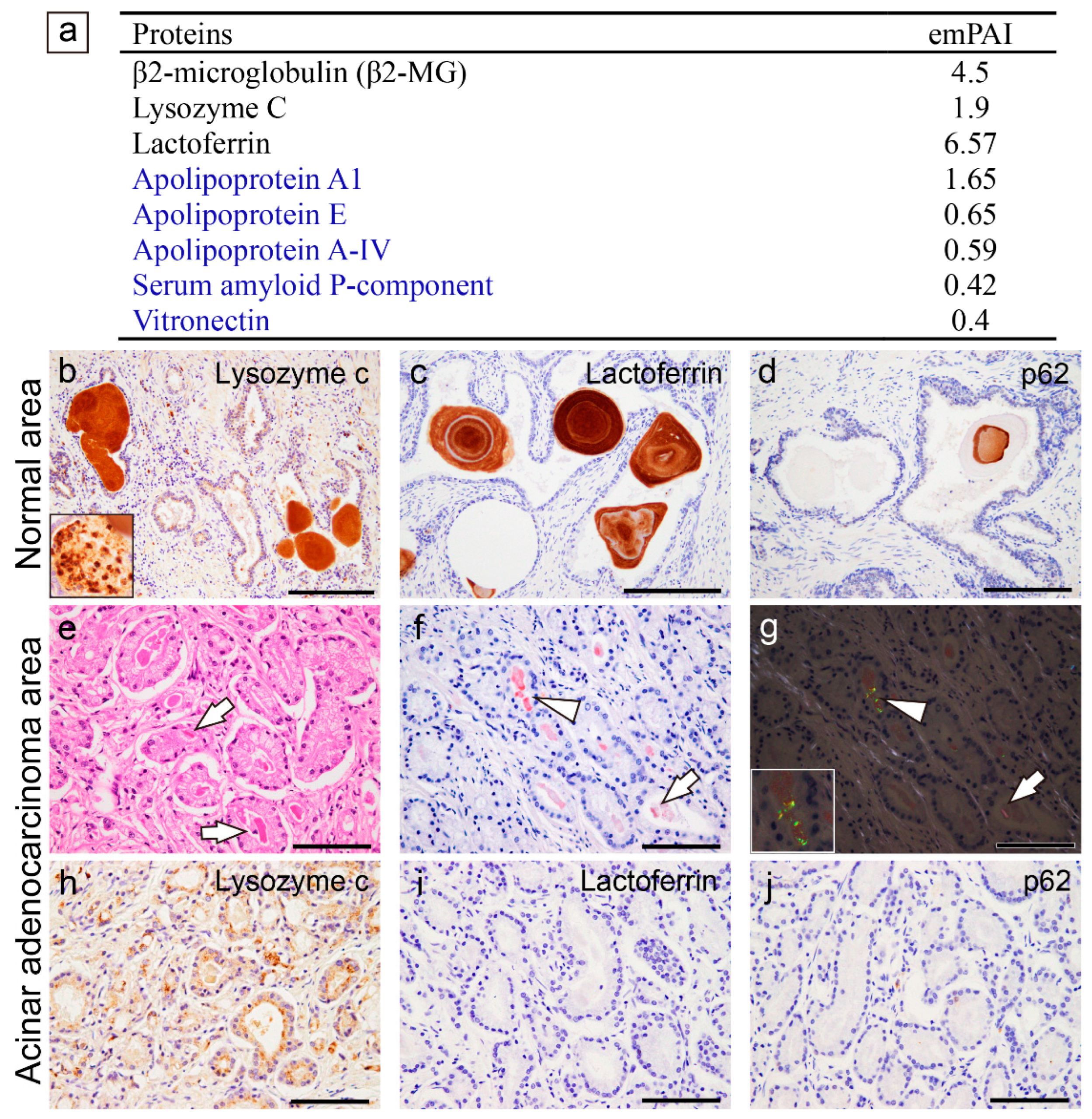
2.7. Proteomic and immunohistochemical features of STAD associated with systemic amyloid immunoglobulin light chain (AL) amyloidosis
2.8. Literature review of previous reports on STAD
3. Discussion
4. Materials and Methods
4.1. Tissue samples
4.2. Histopathological evaluation
4.3. Proteomics analysis using mass spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Riba, M.; Del Valle, J.; Auge, E.; Vilaplana, J.; Pelegri, C. From corpora amylacea to wasteosomes: History and perspectives. Ageing Res Rev 2021, 72, 101484. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Gordon, R.E.; McBride, R.B.; Harpaz, N. Corpora amylacea in gastrointestinal leiomyomas: a clinical, light microscopic, ultrastructural and immunohistochemical study with comparison to hyaline globules. J Clin Pathol 2013, 66, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Gordon, R.E.; Harpaz, N. Intramuscular corpora amylacea adjacent to ileal low-grade neuroendocrine tumours (typical carcinoids): a light microscopic, immunohistochemical and ultrastructural study. J Clin Pathol 2013, 66, 566–572. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Yajima, N.; Katayama, Y.; Nomoto, K.; Nishida, N. Sex-dependent expression of prostatic markers and hormone receptors in cystic tumor of the atrioventricular node: A histopathological study of three cases. Pathol Int 2021, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- DuPre, N.C.; Flavin, R.; Sfanos, K.S.; Unger, R.H.; To, S.; Gazeeva, E.; Fiorentino, M.; De Marzo, A.M.; Rider, J.R.; Mucci, L.A.; Transdisciplinary Prostate Cancer Partnership (ToPCaP). Corpora amylacea in prostatectomy tissue and associations with molecular, histological, and lifestyle factors. Prostate 2018, 78, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, M.; Yuda, F.; Narabayashi, M.; Imai, Y.; Isoda, N.; Obata, K.; Umetsu, A.; Ohgushi, M. Histopathological study of corpora amylacea pulmonum. Histol Histopathol 1989, 4, 153–165. [Google Scholar] [PubMed]
- Sfanos, K.S.; Wilson, B.A.; De Marzo, A.M.; Isaacs, W.B. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A 2009, 106, 3443–3448. [Google Scholar] [CrossRef]
- Kanenawa, K.; Ueda, M.; Isoguchi, A.; Nomura, T.; Tsuda, Y.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Histopathological and biochemical analyses of prostate corpora amylacea. Amyloid 2019, 26, 160–161. [Google Scholar] [CrossRef]
- David, R.; Hiss, Y. Corpora amylacea in mesothelioma of the atrioventricular node. J Pathol 1978, 124, 111–116. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Abe, R.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Sekijima, Y.; Ehara, T.; Nishida, N. An autopsy case of amyloid tubulopathy exhibiting characteristic spheroid-type deposition. Virchows Arch 2020, 477, 157–163. [Google Scholar] [CrossRef]
- Ichimata, S.; Aoyagi, D.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Uehara, T.; Shiozawa, S. A case of spheroid-type localized lactoferrin amyloidosis in the bronchus. Pathol Int 2019, 69, 235–240. [Google Scholar] [CrossRef]
- Kim, M.J.; McCroskey, Z.; Piao, Y.; Belcheva, A.; Truong, L.; Kurtin, P.J.; Ro, J. Y. Spheroid-type of AL amyloid deposition associated with colonic adenocarcinoma: A case report with literature review. Pathol Int 2018, 68, 123–127. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Dispenzieri, A.; Eisenberg, D.S.; Fandrich, M.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Demirhan, B.; Bilezikci, B.; Kiyici, H.; Boyacioglu, S. Globular amyloid deposits in the wall of the gastrointestinal tract: report of six cases. Amyloid 2002, 9, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, P.R.; Topazian, M.D.; Gertz, M.A.; Abraham, S. C. Globular amyloid deposits isolated to the small bowel: a rare association with AL amyloidosis. Am J Surg Pathol 2007, 31, 141–145. [Google Scholar] [CrossRef]
- Acebo, E.; Mayorga, M.; Fernando Val-Bernal, J. Primary amyloid tumor (amyloidoma) of the jejunum with spheroid type of amyloid. Pathology 1999, 31, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Venugopal, S.; Ravindra, S. Unique spheroid deposits of amyloid in an ampullary neuroendocrine tumour. Indian J Pathol Microbiol 2022, 65, 226–228. [Google Scholar] [PubMed]
- Diaz Del Arco, C.; Fernandez Acenero, M.J. Globular amyloidosis of the colon. Arab J Gastroenterol 2018, 19, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Martín-Arranz, E.; Pascual-Turrión, J.M.; Martín-Arranz, M.D.; Burgos, E.; Froilán-Torres, C.; Adán-Merino, L.; Lorenzo, A.; Segura-Cabral, J.M. Focal globular amyloidosis of the colon. An exceptional diagnosis. Rev Esp Enferm Dig 2010, 102, 555–556. [Google Scholar] [CrossRef]
- Harris, J.C.; Zhang, Q.; Tondon, R.; Alipour, Z.; Stashek, K. Characterization of amyloidosis in the gastrointestinal tract with an emphasis on histologically distinct interstitial patterns of deposition and misinterpretations. Am J Surg Pathol 2024, 48, 302–308. [Google Scholar] [CrossRef]
- Makhlouf, H.R.; Goodman, Z.D. Globular hepatic amyloid: an early stage in the pathway of amyloid formation: a study of 20 new cases. Am J Surg Pathol 2007, 31, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Agaram, N.; Shia, J.; Klimstra, D.S.; Lau, N.; Lin, O.; Erlandson, R.A.; Filippa, D.A.; Godwin, T.A. Globular hepatic amyloid: a diagnostic peculiarity that bears clinical significance. Hum Pathol 2005, 36, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Pilgaard, J.; Fenger, C.; Schaffalitzky de Muckadell, O.B. Globular amyloid deposits in the liver. Histopathology 1993, 23, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Osick, L.A.; Lee, T.P.; Pedemonte, M.B.; Jacob, L.; Chauhan, P.; Navarro, C.; Comer, G.M. Hepatic amyloidosis in intravenous drug abusers and AIDS patients. J Hepatol 1993, 19, 79–84. [Google Scholar] [CrossRef] [PubMed]
- French, S.W.; Schloss, G.T.; Stillman, A.E. Unusual amyloid bodies in human liver. Am J Clin Pathol 1981, 75, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Chandan, V.S.; Shah, S.S.; Lam-Himlin, D.M.; Petris, G.D.; Mereuta, O.M.; Dogan, A.; Torbenson, M.S.; Wu, T.T. Globular hepatic amyloid is highly sensitive and specific for LECT2 amyloidosis. Am J Surg Pathol 2015, 39, 558–564. [Google Scholar] [CrossRef]
- Kumar, B.; Pant, B.; Kumar, V.; Negi, M. Sinonasal globular amyloidosis simulating malignancy: A rare presentation. Head Neck Pathol 2016, 10, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Drut, R.; Giménez, P.O. Acinic cell carcinoma of salivary gland with massive deposits of globular amyloid. Int J Surg Pathol 2008, 16, 202–207. [Google Scholar] [CrossRef]
- Michaels, L.; Hyams, V.J. Amyloid in localised deposits and plasmacytomas of the respiratory tract. J Pathol 1979, 128, 29–38. [Google Scholar] [CrossRef]
- Pambuccian, S.E.; Horyd, I.D.; Cawte, T.; Huvos, A.G. Amyloidoma of bone, a plasma cell/plasmacytoid neoplasm. Report of three cases and review of the literature. Am J Surg Pathol 1997, 21, 179–186. [Google Scholar] [CrossRef]
- Bauer, W.H.; Kuzma, J.F. Solitary tumors of atypical amyloid (paramyloid). Am J Clin Pathol 1949, 19, 1097–1112. [Google Scholar] [CrossRef]
- Unal, F.; Hepgül, K.; Bayindir, C.; Bilge, T.; Imer, M.; Turantan, I. Skull base amyloidoma: Case report. J Neurosurg 1992, 76, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Bommannan, B.K.K.; Sonai, M.; Sachdeva, M.U. Bone marrow amyloid spherulites in a case of AL amyloidosis. Blood Cells Mol Dis 2016, 58, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Mantoo, S.; Hwang, J.S.; Chiang, G.S.; Tan, P.H. A rare case of localised AA-type amyloidosis of the ureter with spheroids of amyloid. Singapore Med J 2012, 53, e77–e79. [Google Scholar]
- Gibbons, D.; Lindberg, G.M.; Ashfaq, R.; Saboorian, M.H. Localized amyloidosis of the uterine cervix. Int J Gynecol Pathol 1998, 17, 368–371. [Google Scholar] [CrossRef]
- Gondo, T.; Ishihara, T.; Kawano, H.; Uchino, F.; Takahashi, M.; Iwata, T.; Matsumoto, N.; Yokota, T. Localized amyloidosis in squamous cell carcinoma of uterine cervix: electron microscopic features of nodular and star–like amyloid deposits. Virchows Arch A Pathol Anat Histopathol 1993, 422, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Aho, H.J.; Talve, L.; Mäenpää, J. Acantholytic squamous cell carcinoma of the uterine cervix with amyloid deposition. Int J Gynecol Pathol 1992, 11, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Nakayama, T.; Ohata, K.; Wakasa, K.; Miki, Y. Computed tomography and magnetic resonance imaging appearance of prolactinoma with spheroid-type amyloid deposition. J Comput Assist Tomogr 2011, 35, 313–315. [Google Scholar] [CrossRef]
- Gul, S.; Bahadir, B.; Dusak, A.; Kalayci, M.; Edebali, N.; Acikgoz, B. Spherical amyloid deposition in a prolactin-producing pituitary adenoma. Neuropathology 2009, 29, 81–84. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Lee, S.K.; Kim, T.S. Squash smear findings of spherical amyloid in pituitary prolactinoma. A case report. Acta Cytol 2004, 48, 447–450. [Google Scholar] [CrossRef]
- Hassan, T.; Ikeda, H.; Yoshimoto, T. Salmon roe-like amyloid deposition in a prolactinoma: a case report. Brain Tumor Pathol 2003, 20, 89–92. [Google Scholar] [CrossRef]
- Wiesli, P.; Brändle, M.; Brandner, S.; Kollias, S.S.; Bernays, R.L. Extensive spherical amyloid deposition presenting as a pituitary tumor. J Endocrinol Invest 2003, 26, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.R.; Polk, R.K.; Linse, K.D.; Weiss, M.H.; Kovacs, K.; Garner, J.A. Characterization of spherical amyloid protein from a prolactin-producing pituitary adenoma. Acta Neuropathol 1997, 93, 43–49. [Google Scholar] [CrossRef]
- Kuratsu, J.; Matsukado, Y.; Miura, M. Prolactinoma of pituitary with associated amyloid-like substances. Case report. J Neurosurg 1983, 59, 1067–1070. [Google Scholar] [CrossRef]
- Taniyama, H.; Kitamura, A.; Kagawa, Y.; Hirayama, K.; Yoshino, T.; Kamiya, S. Localized amyloidosis in canine mammary tumors. Vet Pathol 2000, 37, 104–107. [Google Scholar] [CrossRef]
- Yanamandra, K.; Alexeyev, O.; Zamotin, V.; Srivastava, V.; Shchukarev, A.; Brorsson, A.C.; Tartaglia, G. G.; Vogl, T.; Kayed, R.; Wingsle, G.; Olsson, J.; Dobson, C.M.; Bergh, A.; Elgh, F.; Morozova-Roche, L.A. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLOS ONE 2009, 4, e5562. [Google Scholar] [CrossRef]
- Tekin, B.; Dasari, S.; Theis, J.D.; Vrana, J.A.; Murray, D.L.; Oglesbee, D.; Thompson, R.H.; Leibovich, B.C.; Boorjian, S.A.; Whaley, R.D.; Hernandez, L.H.; Jimenez, R.E.; Cheville, J.C.; Karnes, R.J.; Sukov, W.R.; Gupta, S. Mass spectrometry-based assessment of prostate cancer-associated crystalloids reveals enrichment for growth and differentiation factor 15. Hum Pathol 2023, 135, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cross, P.A.; Bartley, C.J.; McClure, J. Amyloid in prostatic corpora amylacea. J Clin Pathol 1992, 45, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Palangmonthip, W.; Wu, R.; Tarima, S.; Bobholz, S.A.; LaViolette, P.S.; Gallan, A.J.; Iczkowski, K.A. Corpora amylacea in benign prostatic acini are associated with concurrent, predominantly low-grade cancer. Prostate 2020, 80, 687–697. [Google Scholar] [CrossRef]
- Riba, M.; Campo-Sabariz, J.; Tena, I.; Molina-Porcel, L.; Ximelis, T.; Calvo, M.; Ferrer, R.; Martin-Venegas, R.; Del Valle, J.; Vilaplana, J.; Pelegri, C. Wasteosomes (corpora amylacea) of human brain can be phagocytosed and digested by macrophages. Cell Biosci 2022, 12, 177. [Google Scholar] [CrossRef]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.; Simon, R.; Schubert, D.; Eisenberg, D.; Rivier, J.; Sawchenko, P.; Vale, W.; Riek, R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Pioselli, B.; Salomone, F.; Mazzola, G.; Amidani, D.; Sgarbi, E.; Amadei, F.; Murgia, X.; Catinella, S.; Villetti, G.; De Luca, D.; Carnielli, V.; Civelli, M. Pulmonary surfactant: A unique biomaterial with life-saving therapeutic applications. Curr Med Chem 2022, 29, 526–590. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Thyberg, J.; Naslund, J.; Eliasson, E.; Johansson, J. Amyloid fibril formation by pulmonary surfactant protein C. FEBS Lett 1999, 464, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, H.; Yoshinouchi, T.; Watanabe, R.; Fujita, J.; Takahara, J.; Ohtsuki, Y. Immunohistochemical study of a patient with diffuse pulmonary corpora amylacea detected by open lung biopsy. Intern Med 1999, 38, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.; Riek, R. Functional amyloids. Cold Spring Harb Perspect Biol 2019, 11, a033860. [Google Scholar] [CrossRef]
- Ishii, W.; Matsuda, M.; Nakamura, N.; Katsumata, S.; Toriumi, H.; Suzuki, A.; Ikeda, S. Phenol Congo red staining enhances the diagnostic value of abdominal fat aspiration biopsy in reactive AA amyloidosis secondary to rheumatoid arthritis. Intern Med 2003, 42, 400–405. [Google Scholar] [CrossRef]
- Kametani, F.; Haga, S. Accumulation of carboxy-terminal fragments of APP increases phosphodiesterase 8B. Neurobiol Aging 2015, 36, 634–637. [Google Scholar] [CrossRef]
- Abe, R.; Katoh, N.; Takahashi, Y.; Takasone, K.; Yoshinaga, T.; Yazaki, M.; Kametani, F.; Sekijima, Y. Distribution of amyloidosis subtypes based on tissue biopsy site—Consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol Int 2021, 71, 70–79. [Google Scholar] [CrossRef]
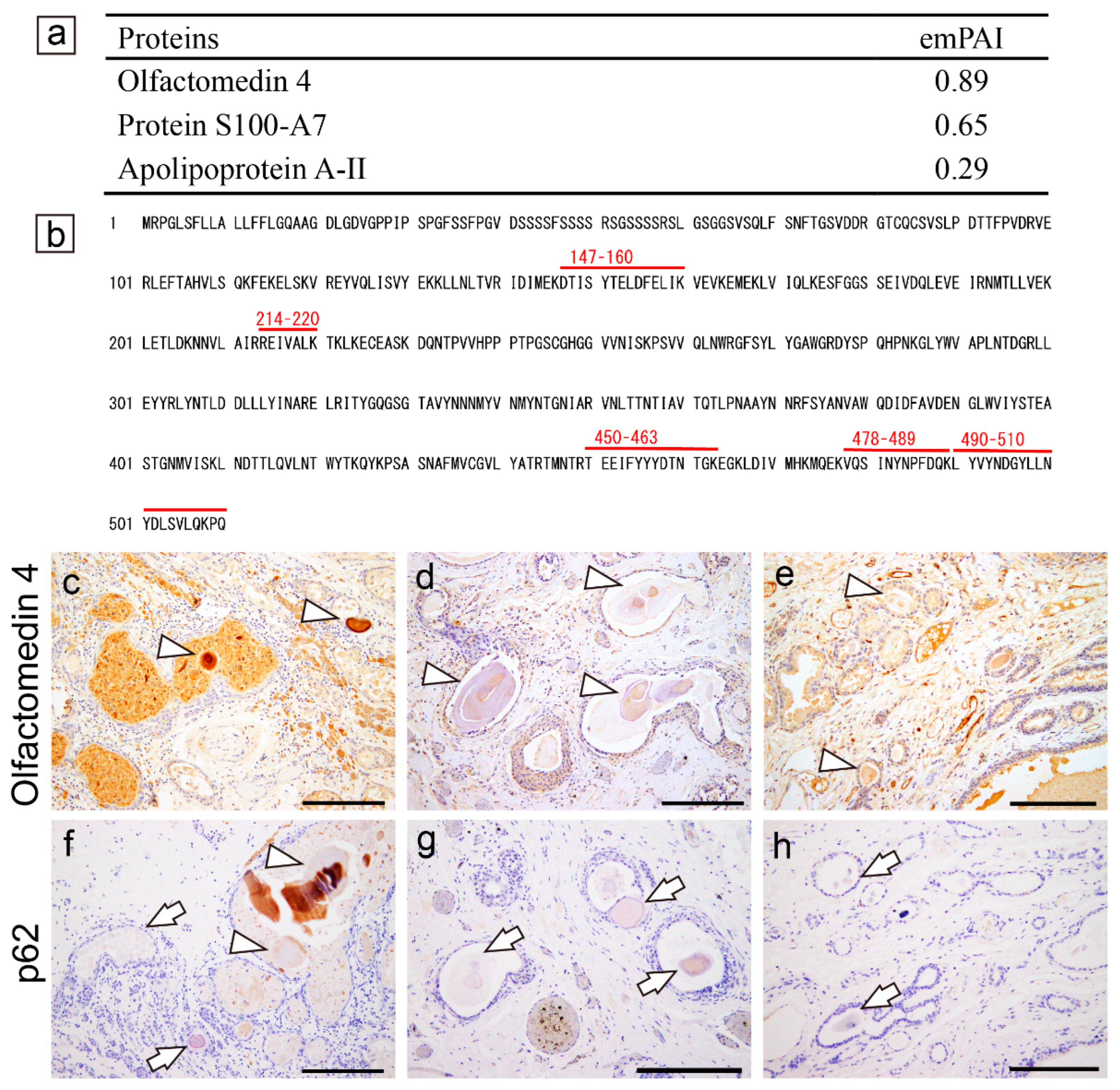
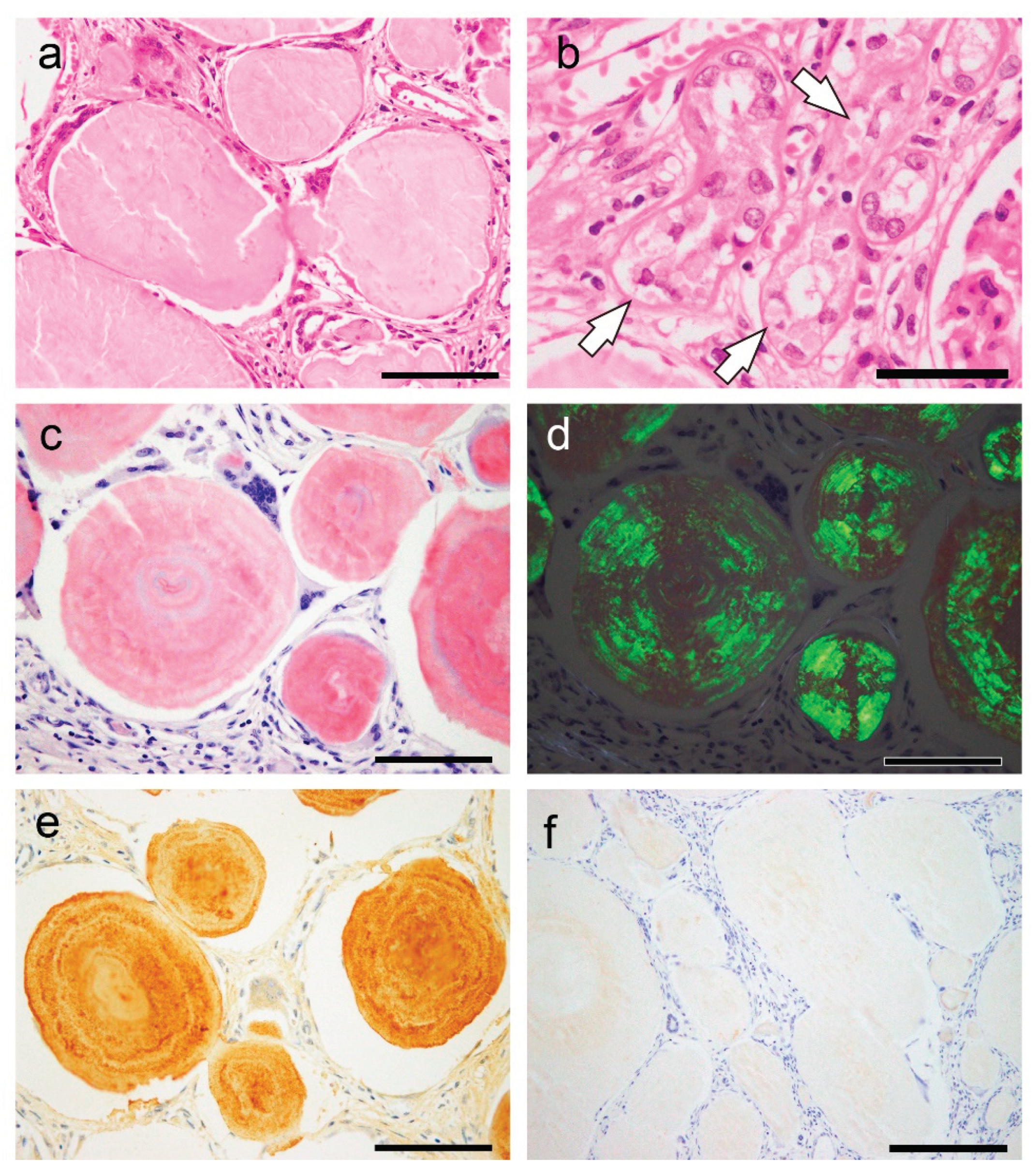
| Pulmonary CA cases | Prostatic CA cases | CTAVN cases | |||||||
| Case # | Case 1* | Case 2 | Case 3 | Case 1* | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 |
| Age | 75 | 90 | 85 | 75 | 88 | 45 | 36 | 45 | 76 |
| Sex | M | F | F | M | M | M | F | M | M |
| Cause of death | HyT | MN | Drowning | HyT | ACD | KA | SCD | SCD | SCD |
| Dialysis | None | None | None | None | None | None | None | None | None |
| Location | S/L | Concomitant findings | Immunohistochemical analysis | Proteomic analysis |
| Stomach and small intestine [14] | S | Bronchiectasis, FMF, and renal failure | Pos: AA; Neg: β2MG, TTR, Igκ, Igλ, CD68 | NE |
| Small intestine [15,16] | L | Polypoid lesions | Pos: Igλ; Neg: AA, β2MG, TTR, Igκ [15] Pos: AP, AA, Igκ, Igλ (uneven) [16] |
NE |
| Vater ampulla [17] | L | NET | NE | NE |
| Colon, TI [12] | L | Adenocarcinoma | NE | ALλ |
| Colon [18,19,20] | L | Ulcerative lesions [18]and rounded lesions [19] | Pos: Igλ [18] | NE |
| Liver [21,22,23,24,25,26] | S/L | Various diseases (See [21,22]) | Pos: AP, AA; Neg: Igκ, Igλ [21] Pos: none; Neg: AP, AA, UB, TTR, Igκ, Ig [23] Pos: AA; Neg: β2MG, TTR, Igκ, Igλ [24] Pos: LECT2 or Ig; Neg: AA, β2MG, TTR [26] |
ALECT2 or AL [26] |
| Sino-nasal tract [27] | L | Nasal mass | Pos: Igκ and Igλ (κ>λ) | NE |
| Parotid gland [28] | L | AACC | NE | NE |
| URT [29] | S/L | Plasmacytoma | NE | NE |
| Bronchus [11] | L | Erythematous mass | Pos: Lac; Neg: AA, β2MG, TTR, Igκ, Igλ | ALac |
| Bone [30,31,32] | L | Myeloma, malignant lymphoma (see [30]) | Pos: Igκ or Igλ; Neg: AA [30] | NE |
| Bone marrow [33] | S | PCP | Pos: Igλ | NE |
| Ureter [34] | L | Hydronephrosis | NE (likely AA) | NE |
| Kidney [10] | S | PCP | Pos: Igλ; Neg: AA, β2MG, TTR, Igκ | ALλ |
| Uterine cervix [35,36,37] | L | Smooth mass [36], SCC [37],[38] | Pos: AA; Neg: CK, Igκ, Igλ [35] Pos: CK; Neg: AA, TTR, Igκ, Igλ [36,37] |
NE |
| Pituitary gland [38,39,40,41,42,43,44] | L | Prolactinoma | Pos: PRL; Neg: CK, vimentin, GFAP, GH, FSH, LH, TSH, ACTH, β-A4 [39,41,42] | NE |
| Breast [45]* | L | Mammary tumor | Pos: α-casein, Lac; Neg: AA, TTR, CK, Igκ, Igλ | NE |
| Prostatic-CA | Pulmonary-CA | CTAVN-CA | STAD | |
| Congophilia | Strong | Moderate–strong | Weak–moderate | Strong |
| Strength of the AGBR | Strong | Strong | Weak–moderate | Strong |
| Macrophages | Positive | Positive | Negative–positive | Positive |
| Presence of CPs | Positive | Positive | Negative | Negative |
| Presence of AAPs | Positive | Positive | Negative | Positive |
| p62-IR | Positive (focal) | Positive (focal) | Negative–Positive (focal) | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





