Submitted:
06 March 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
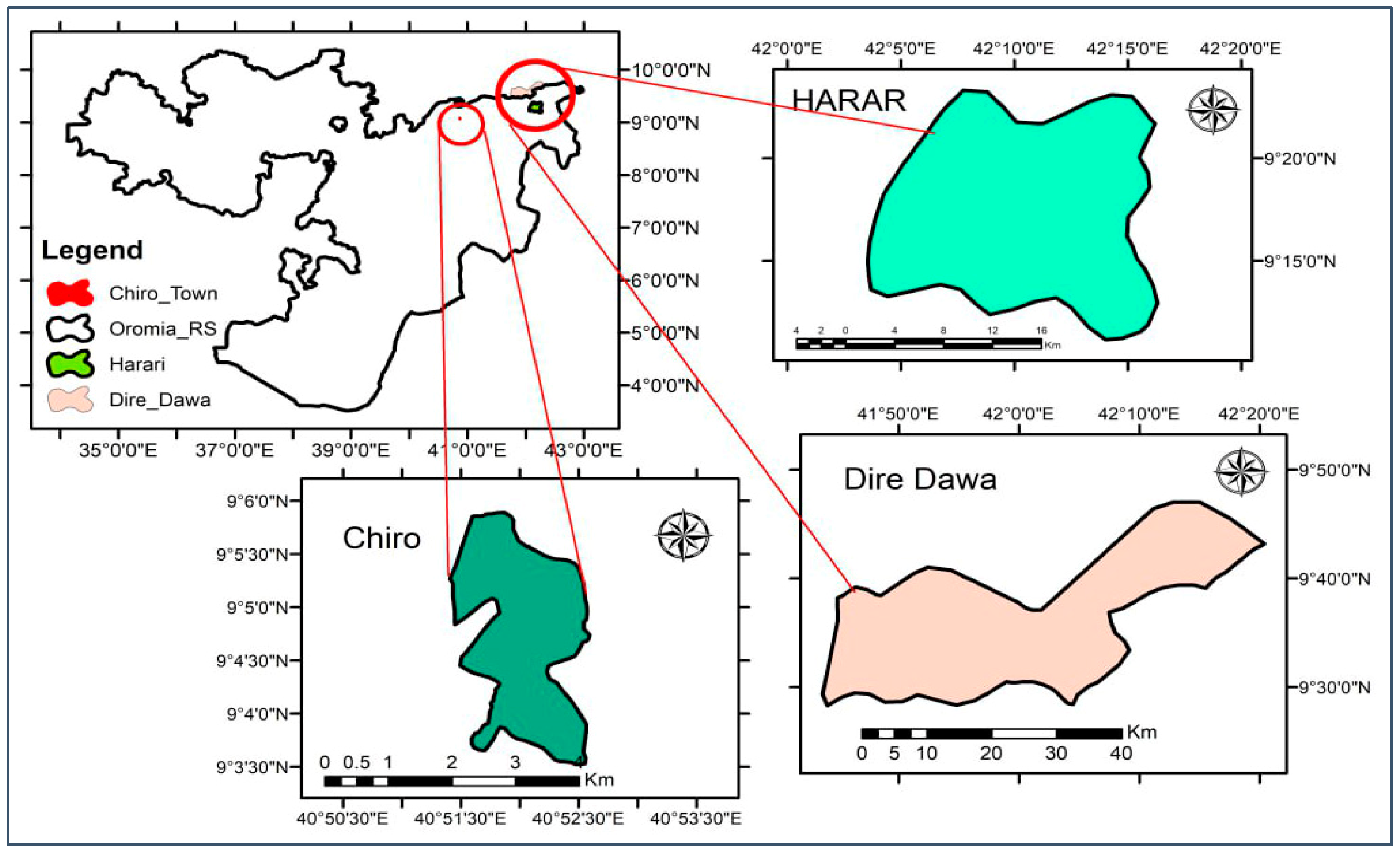
2.1. Study Site Selection
2.2. Milk Sample Collection
2.3. Analysis of Aflatoxin M1 Using HPLC
2.3.1. Milk Sample Extraction
2.3.2. Sample Cleanup and HPLC Condition
2.4. Data Validation
2.5. Data Analysis
3. Results
3.1. Method Validation
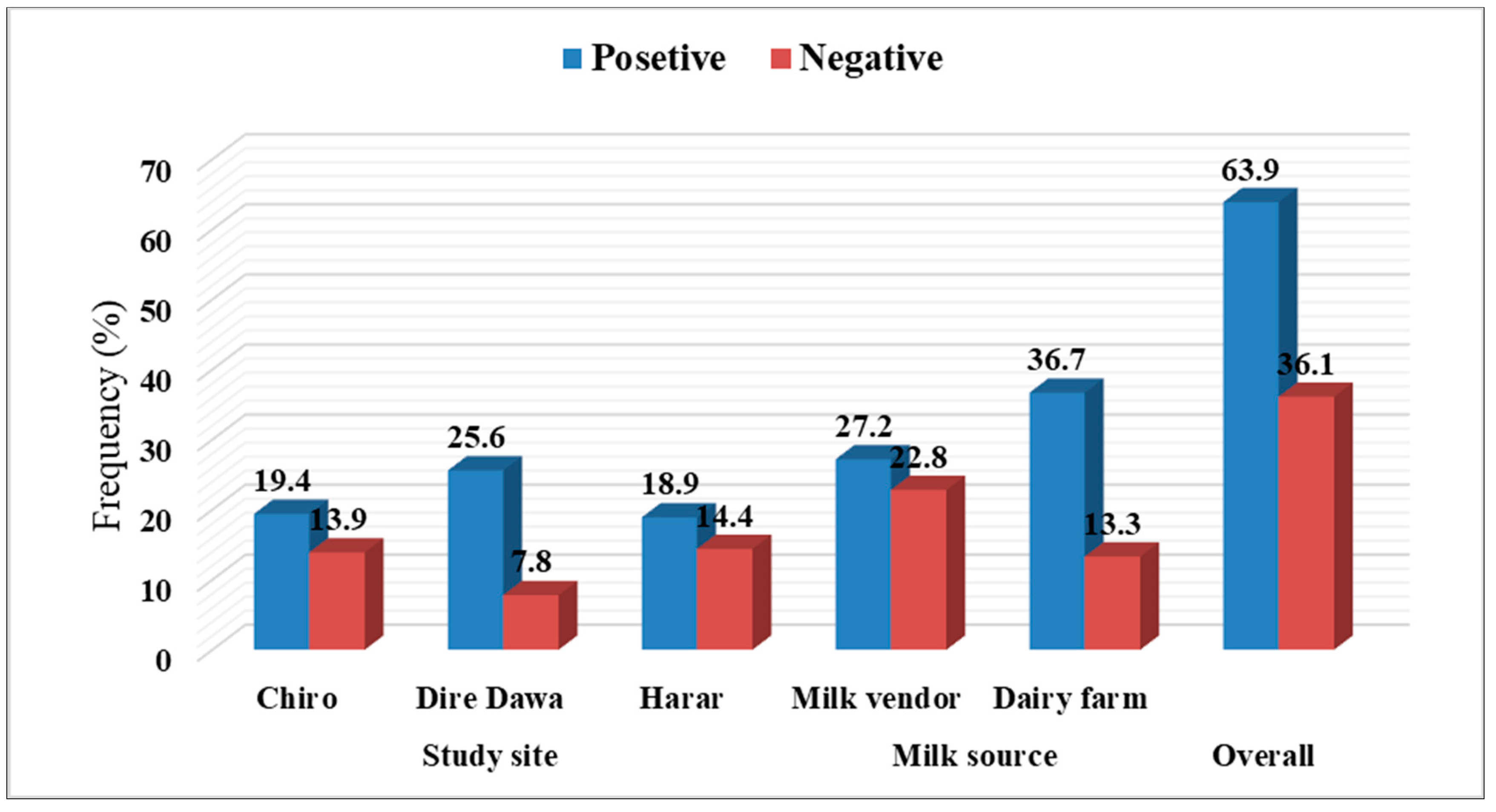
3.2. Occurrence of Aflatoxin M1 in Raw Milk across Study Sites and Milk Sources
| Categories | N+ (%) | LOD-0.05 (%) | 0.051-0.5 (%) | >0.5 (%) | |
|---|---|---|---|---|---|
| Study Sites | |||||
| Chiro town | 35 (30.43) a | 26 (22.61) | 5 (4.35) | 4 (3.48) a | |
| Dire Dawa city | 46 (40.00) b | 20 (17.39) | 9 (7.83) | 17 (14.78) b | |
| Harar city | 34 (29.57) a | 24 (20.87) | 1 (0.87) | 9 (7.83) a | |
| P-value | * | ns | ns | * | |
| Milk Source | |||||
| Milk vendor | 49 (42.61) | 29 (25.22) | 7 (6.09) | 13 (11.30) | |
| Dairy farm | 66 (57.39) | 41 (35.65) | 8 (6.96) | 17 (14.78) | |
| P-value | * | * | ns | ns | |
| Total | 115 (100) | 70 (60.87) | 15 (13.04) | 30 (26.08) | |
3.3. Level of Aflatoxin M1 in Milk across Study Sites and Milk Sources
| Category | Mean ±SD (µg/L) |
Range (µg/L) |
%> ESA/EU (N=115) | %> FDA (N=115) | |
|---|---|---|---|---|---|
| Study Sites | |||||
| Chiro town | 0.055±0.13 a | LOD – 0.545 | 9 (7.83) a | 4 (3.48) a | |
| Dire Dawa city | 0.344±0.72 b | LOD – 3.850 | 26 (22.61) b | 17 (14.78) b | |
| Harar city | 0.140±0.33 a | LOD – 1.550 | 10 (8.7) a | 9 (7.83) a | |
| P-value | ** | ** | * | ||
| Milk Source | |||||
| Milk vendor | 0.107±0.21 | LOD – 2.701 | 20 (17.39) | 13 (11.30) | |
| Dairy farm | 0.252±0.64 | LOD – 1.037 | 25 (21.74) | 17 (14.78) | |
| P-value | * | ns | ns | ||
| Overall | 0.179±0.48 | LOD – 3.85 | 45 (39.13) | 30 (26.08) | |
| Study site*Milk source | ns | - | - | - | |
4. Discussion
| Countries | Methods | N | + (%) | >EU (%) | >FDA (%) | Mean | Ref. |
|---|---|---|---|---|---|---|---|
| Ethiopia | HPLC | 180 | 63.9 | 39.13 | 26.08 | 0.179±0.48 µg/L | This study |
| Sudan | HPLC | 44 | 95.45 | 100 | 83.33 | 2.070 µg/L | [63] |
| Iran | HPLC | 204 | 80.3 | 56.7 | - | 0.660 µg/L | [70] |
| Egypt | HPLC | 10 | 6 | - | - | 0.061 ng/L | [71] |
| Ethiopia | HPLC | 42 | 93 | 86 | - | 0.029 µg/L | [28] |
| Yemen | HPLC | 38 | 68.42 | 36.84 | - | 0.183 µg/L | [50] |
| Pakistan | HPLC | 38 | 64.2 | 25 | - | 0.082 µg/L | [65] |
| Algeria | HPLC | 84 | 46.42 | - | 1.19 | 156.71 ng/L | [67] |
| Lebanon | HPLC | 701 | 58.8 | 28.0 | - | 0.035±0.051 µg/L | [66] |
| Kenya | HPLC | 96 | 100 | 66.4 | 7.5 | 290.3±66.3 ng/L | [64] |
| Ethiopia | ELISA | 45 | 71 | 58 | 42 | 0.054 µg/L | [62] |
| Ethiopia | ELISA | 110 | 100 | 97.8 | 26.3 | 4.980 µg/L | [27] |
| Ethiopia | ELISA | 108 | 100 | 96 | 82 | 0.69±0.505 µg/L | [31] |
| Ethiopia | ELISA | 100 | 99 | 41 | - | 0.47±0.73 µg/L | [61] |
| Kenya | ELISA | 72 | 37.5 | 26.4 | - | - | [21] |
| Malawi | VICAM | 112 | 100 | 98 | 22 | 0.55±0.18 µg/L | [22] |
| Pakistan | ELISA | 340 | 86.66 | - | 34.45 | 0.520 µg/L | [48] |
| Kenya | ELISA | 96 | - | - | - | 627.5±238.19 ng/L | [64] |
| Kenya | ELISA | 150 | 100 | 58 | - | - | [20] |
| Kenya | ELISA | 84 | 98.8 | 64 | - | 83.66±64.68 ng/L | [19] |
| Pakistan | HPLC | 28 | 64.2 | 25 | - | 82.4 ± 7.8 ng/L | [65] |
| Algeria | ELISA | 84 | 46.42 | - | 1.19 | 71.92±28.48 ng/L | [67] |
| Iran | ELISA | 180 | 77.2 | 22.7 | - | 56.32 ± 74.37 ng/L | [56] |
| Sudan | VICAM | 25 | 92 | - | - | - | [23] |
| Rwanda | VICAM | 170 | - | 91.8 | 38.8 | 0.89±1.64 µg/L | [24] |
| Tanzania | HPLC | - | 30.7 | 27.9 | - | - | [26] |
| Tanzania | HPLC | 37 | 83.8 | 100 | 16.1 | - | [25] |
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to Fork Risk Assessment of Mycotoxins under Climate Change Influences - Recent Developments. Trends Food Sci. Technol. 2022, 126, 126–141. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors Influencing Production and Control Strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.Y.; Aljaafari, M.N.; Al Ali, H.; Eid, K.; Al Sallagi, M.; Cheng, W.H.; Abushelaibi, A.; Lim, S.H.E.; Loh, J.Y.; Lai, K.S. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 1–16. [Google Scholar] [CrossRef]
- Klingelhofer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.K.; Brüggmann, D.; Groneberg, D.A.; Klingelhöfer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.K.; Brüggmann, D.; Groneberg, D.A. Aflatoxin – Publication Analysis of a Global Health Threat. Food Control 2018, 89, 280–290. [Google Scholar] [CrossRef]
- Ramesh, J.; Sarathchandra, G.; Sureshkumar, V. Analysis of Feed Samples for Aflatoxin B1 Contamination by HPTLC: A Validated Method. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 373–377. [Google Scholar]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Ahmad Faizal, A.R. Aflatoxin M1 in Milk and Dairy Products, Occurrence and Recent Challenges: A Review. Trends Food Sci. Technol. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- FAO. Agricultural Production Statistics 2000–2021. Faostat Analytical Brief 60. Food and Agriculture Organization of the United Nations Rome, Italy 2022. [CrossRef]
- Ben Hassouna, K.; Ben Salah-Abbès, J.; Chaieb, K.; Abbès, S.; Ferrer, E.; Martí-Quijal, F.J.; Pallarés, N.; Berrada, H. The Occurrence and Health Risk Assessment of Aflatoxin M1 in Raw Cow Milk Collected from Tunisia during a Hot Lactating Season. Toxins 2023, 15, 1–13. [Google Scholar] [CrossRef]
- CSA. Federal Democratic Republic of Ethiopia Central Statistical Agency Agricultural Sample Survey 2020/21 [2013 E.C.] Volume II Report on Livestock and Livestock Characteristics (Private Peasant Holdings); Addis Ababa, Ethiopia, 2021; Volume 589. [Google Scholar]
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Klarić, M.Š.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment Changes, Aflatoxins, and Health Issues, a Review. Int. J. Environ. Res. Public Health 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- IARC. Mycotoxins and Human Health. IARC Sci. Publ. 2012, 158, 87–104. [Google Scholar]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods 2023, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Nyakundi Ondari, E.; Josiah Eseoghene, I.; Twinomuhwezi, H.; Otuosorochi Amagwula, I.; Morya, S.; Ondari, E.N.; Eseoghene, I.J.; Twinomuhwezi, H.; Amagwula, I.O.; Morya, S.; Nyakundi Ondari, E.; Josiah Eseoghene, I.; Twinomuhwezi, H.; Otuosorochi Amagwula, I.; Morya, S. Fungal Growth and Mycotoxins Production: Types, Toxicities, Control Strategies, and Detoxification. Fungal Reprod. Growth 2022, No. September, 1–20. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of Climate Change on Aspergillus Flavus and Aflatoxin B1 Production. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hell, K.; Mutegi, C. Aflatoxin Control and Prevention Strategies in Key Crops of Sub-Saharan Africa. African J. Microbiol. Res. 2011, 5, 459–466. [Google Scholar]
- Tadele, F.; Demissie, B.; Amsalu, A.; Demelash, H.; Mengist, Z.; Ambelu, A.; Yenew, C. Aflatoxin Contamination of Animal Feeds and Its Predictors among Dairy Farms in Northwest Ethiopia: One Health Approach Implications. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Benkerroum, N. Aflatoxins: Producing-molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- Kagera, I.; Kahenya, P.; Mutua, F.; Anyango, G.; Kyallo, F.; Grace, D.; Lindahl, J. Status of Aflatoxin Contamination in Cow Milk Produced in Smallholder Dairy Farms in Urban and Peri-Urban Areas of Nairobi County: A Case Study of Kasarani Sub County, Kenya. Infect. Ecol. Epidemiol. 2019, 9, 1547095. [Google Scholar] [CrossRef]
- Langat, G.; Tetsuhiro, M.; Gonoi, T.; Matiru, V.; Bii, C. Aflatoxin M1 Contamination of Milk and Its Products in Bomet County, Kenya. Adv. Microbiol. 2016, 06, 528–536. [Google Scholar] [CrossRef]
- Anyango, G.; Mutua, F.; Kagera, I.; Andang’O, P.; Grace, D.; Lindahl, J.F. A Survey of Aflatoxin M1 Contamination in Raw Milk Produced in Urban and Peri-Urban Areas of Kisumu County, Kenya. Infect. Ecol. Epidemiol. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Njombwa, C.A.; Moreira, V.; Williams, C.; Aryana, K.; Matumba, L. Aflatoxin M1 in Raw Cow Milk and Associated Hepatocellular Carcinoma Risk among Dairy Farming Households in Malawi. Mycotoxin Res. 2020, 37, 89–96. [Google Scholar] [CrossRef]
- Fadlalla, A.A.; El-Zubeir, I.E.M.; Elnahas, A. Aflatoxin M1 Contamination in Fluid Milk Products, in Khartoum State, Sudan. Vet. Med. Public Heal. J. 2020, 1, 34–40. [Google Scholar] [CrossRef]
- Nishimwe, K.; Bowers, E.L.; Ayabagabo, J.D.; Habimana, R.; Mutiga, S.; Maier, D.E. Preliminary Sampling of Aflatoxin M1 Contamination in Raw Milk from Dairy Farms Using Feed Ingredients from Rwanda. Mycotoxin Res. 2022, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Munissi, J.J.E.; Nyandoro, S.S. Aflatoxin M1 in Raw Milk and Aflatoxin B1 in Feed from Household Cows in Singida, Tanzania. Food Addit. Contam. Part B Surveill. 2016, 9, 85–90. [Google Scholar] [CrossRef]
- Kitigwa, S.J.; Kimaro, E.G.; Nagagi, Y.P.; Kussaga, J.B.; Suleiman, R.A.; Matemu, A. Occurrence and Associated Risk Factors of Aflatoxin Contamination in Animal Feeds and Raw Milk from Three Agroecological Zones of Tanzania. World Mycotoxin J. 2023, 16, 149–163. [Google Scholar] [CrossRef]
- Dawit, G.; Szonyi, B.; Azage, T.; Hanson, J.; Grace, D.; Gizachew, D.; Szonyi, B.; Tegegne, A.; Hanson, J.; Grace, D. Aflatoxin Contamination of Milk and Dairy Feeds in the Greater Addis Ababa Milk Shed, Ethiopia. Food Control 2016, 59, 773–779. [Google Scholar] [CrossRef]
- Abenet, W. Comparative Study of Aflatoxin M1 in Milk Samples Marketed in Capital City and Rural Place. MSc Thesis, Department of Food and Nutritional Sciences, Addis Ababa University, Addis Ababa, Ethiopia, 2017. [Google Scholar]
- Yohannes, B.; Ayalew, W.; Getachew, A. Analysis to Ascertain the Determination for Aflatoxin Contamination of Milk and Feeds from Gurage Zone, Ethiopia. J. Agric. Res. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Zebib, H.; Abate, D.; Woldegiorgis, A.Z. Aflatoxin M1 in Raw Milk, Pasteurized Milk and Cottage Cheese Collected along Value Chain Actors from Three Regions of Ethiopia. Toxins 2022, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Berhanu, T.; Zewdu, A. Aflatoxin M1 in Milk and Milk Products Marketed by Local and Industrial Producers in Bishoftu Town of Ethiopia. Food Control 2020, 118, 1–10. [Google Scholar] [CrossRef]
- CSA. Population Projections for Ethiopia 2007-2037. In Central Statistical Agency; Federal Democratic Republic of Ethiopia, Central Statistical Ageny: Addis Ababa, Ethiopia, 2013; p. 188. [Google Scholar]
- Alemu, M.M. Urban and Peri-Urban Dairy Cattle Production in Ethiopia: A Review. Online J. Anim. Feed Res. 2019, 9, 173–177. [Google Scholar]
- Shapiro, B.I.; Gebru, G.; Desta, S.; Negassa, A.; Negussie, K.; Aboset, G.; Mecha, H. Ethiopia Livestock Master Plan. ILRI Project Report. International Livestock Research Institute (ILRI): Nairobi, Kenya, 2020. [Google Scholar]
- Tegegn, A.; Baudronb, F.; Wegary, D. Comparative Performance of Five Maize Varieties as Livestock Feed in the Rift Valley of Ethiopia. Acad. Res. J. Agric. Sci. Res. 2017, 5, 366–379. [Google Scholar] [CrossRef]
- Teshome, D.; Fita, L.; Feyissa, F.; Kitaw, G.; Wondatir, Z. Effect of Total Mixed Ration on Dry Matter Intake, Milk Yield and Composition Effect of Total Mixed Ration on Dry Matter Intake, Milk Yield and Composition of Early Lactating Jersey Cows. J. Biol. Agric. Healthc. 2019, 7, 19–24. [Google Scholar]
- Zeleke, M. Ethiopia’s Livestock Systems: Overview and Areas of Inquiry. Feed the Future Innovation Lab for Livestock Systems. Gainesville, FL, USA 2021, p. 54. [CrossRef]
- Ahmedin, A.; Yesihak, Y. Milk Production Performance, Challenges and Opportunities of Dairy Cattle Production in West Hararghe, Oromiya Regional State. Open J. Anim. Sci. 2020, 10, 219–235. [Google Scholar] [CrossRef]
- Brandsma, W.; Mengistu, D.; Kassa, B.; Yohannes, M.; van der Lee, J. The Major Ethiopian Milksheds; Wageningen, Wageningen UR (University & Research Centre) Livestock Research. Livestock Research Report 735, 245 blz, December Lelystad, Netherlands 2012. [CrossRef]
- Mohammed, Y.K.; Waal, H.O. de. Herd Management, Milk Production and Reproduction of Urban Dairy Farms in the Harar Milk Shed. Ethiop. J. Anim. Prod. 2009, 9, 57–75. [Google Scholar]
- Lemma, S.; Dagne, T.; Gashaw, G.; Getu, A. Assessment of Cow’s Milk Hygienic Practices Under Small Scale Farmers in West Hararghe Zone, Oromia National Regional State, Ethiopia. Adv. Life Sci. Technol. 2018, 68, 46–55. [Google Scholar]
- Mengistu, K.; Mohammed, A.; Eyassu, S.; Tarekegn, G.; Estifanos, H.; Yonas, H. The Dairy Value Chain and Factors Affecting Choice of Milk Channels in Harar and Dire Dawa Areas, Eastern Ethiopia. Rev. Agric. Appl. Econ. 2016, 19, 10–18. [Google Scholar] [CrossRef]
- Kebede, A.; Balina, A.; Kebede, A.; Tamiru, Y. Review on Aflatoxin and Its Impacts on Livestock. J. Dairy Vet. Sci. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Abibeker, S.A.; Mume, A.A.; Mariye, M.; Desalegn, D.G.; Furgasa, W. Causes of Water Pollution in Chiro River Eastern Oromia, Ethiopia. Int. J. Sci. Res. Publ. 2023, 13, 111–119. [Google Scholar] [CrossRef]
- Arabali, M.; Amare, E.G. A Cross Sectional Study on Prevalence of Cephalopina Titillator Infection in Camel (Camelus Dromedaries) in Dire Dawa Administrative Region, Ethiopia. Adv. Biol. Res. (Rennes). 2015, 9, 225–229. [Google Scholar] [CrossRef]
- Biri, A.; Ketema, K.; Ayele, S.; Lule, D. Analysis of Crop Production Constraints Through Participatory Rural Appraisal in Harari Region, Eastern Ethiopia; Implications for Research and Development. J. Agric. Crop. 2019, 5, 209–217. [Google Scholar] [CrossRef]
- Daniel, W.W.; Cross, C.L. Biostatistics: A Foundation for Analysis in the Health Sciences, 10th ed.; Daniel, W.W., Cross, C.L., Eds.; John Wiley and Sons: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Tahira, I.; Sultana, N.; Munir, A.; Hasan, S.M.; Hanif, N.Q. Occurrence of Aflatoxin M1 in Raw and Processed Milk Consumed in Pakistan. Pak. J. Pharm. Sci. 2019, 32, 1097–1101. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists. AOAC Official Method Washington DC, 935.14 and 992.24. 2005, p 334. [CrossRef]
- Murshed, S. Evaluation and Assessment of Aflatoxin M1 in Milk and Milk Products in Yemen Using High-Performance Liquid Chromatography. J. Food Qual. 2020, 2020, 8. [Google Scholar] [CrossRef]
- EC. European Commission (EU) Document No SANTE/2015/11945. Guidance document on analyttical quality control and method validation procedures for pesticides residue analysis in food and feeds. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/%0AAqcGuidance_SANTE_2015_11945.pdf.
- Kassa, A.; Talema, A.; Ketsela, G. DETERMINATION OF AFLATOXIN M1 IN RAW COW’S MILK BY USING HPLC- FLD, IN INJIBARA TOWN, AWI ZONE, AMHARA, ETHIOPIA, MSc Thesis, Department of Chemistry, College of Natural and Computational Sciences, Injibara University, Injibara, Ethiopia, 2020. [Google Scholar] [CrossRef]
- ESA. Unprocessed Whole/Raw Cow Milk Specification. Ethiopian Standard Agency. 2nd Ed., ES: 3460; 2009. [Google Scholar]
- EU. Commission Regulation (EC) No. 165/2010 of 26 February 2010 Amending Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. Off. J. Eur. Union 2010, 50, 8–12. [Google Scholar]
- FDA. Whole Milk, Lowfat Milk, Skim Milk - Aflatoxin M1. FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1 (accessed on 29 February 2024).
- Rahimzadeh Barzoki, H.; Faraji, H.; Beirami, S.; Keramati, F.Z.; Nayik, G.A.; Izadi Yazdanaabadi, Z.; Mozaffari Nejad, A.S. Seasonal Study of Aflatoxin M1 Contamination in Cow Milk on the Retail Dairy Market in Gorgan, Iran. Dairy 2023, 4, 571–580. [Google Scholar] [CrossRef]
- Hashemi, M. A Survey of Aflatoxin M1 in Cow Milk in Southern Iran. J. Food Drug Anal. 2016, 24, 888–893. [Google Scholar] [CrossRef]
- Sahin, H.Z.; Celik, M.; Kotay, S.; Kabak, B. Aflatoxins in Dairy Cow Feed, Raw Milk and Milk Products from Turkey. Food Addit. Contam. Part B Surveill. 2016, 9, 152–158. [Google Scholar] [CrossRef]
- Robert, O.A.; Nyamete, F.A.; Kabululu, M.L.; Ngungulu, A.W. Aflatoxins Contamination in Animal Feeds and Fresh Milk in Kondoa District, Tanzania. Eur. J. Nutr. Food Saf. 2023, 15, 10–21. [Google Scholar] [CrossRef]
- Zebib, H.; Abate, D.; Woldegiorgis, A.Z. Exposure and Health Risk Assessment of Aflatoxin M1 in Raw Milk and Cottage Cheese in Adults in Ethiopia. Foods 2023, 12, 1–11. [Google Scholar] [CrossRef]
- Admasu, F.T.; Melak, A.; Demissie, B.; Yenew, C.; Legesse, M.; Tefera, T.; Teka, O.; Ermias, S.; Tiruneh, G.M.; Malik, T.; Dejenie, T.A.; Habtie, M.L.; Bekele, T.T.; Feyesa, T.O.; Chanie, E.S.; G/Medhin, M.T.; Malik, T.; Dejenie, T.A. Occurrence and Associated Factors of Aflatoxin M1 in Raw Cow Milk in South Gondar Zone, North West Ethiopia. Food Sci. Nutr. 2021, 9, 6286–6293. [Google Scholar] [CrossRef]
- Rehrahie, M.; Getnet, A.; Fassil, A. Determination of Aflatoxin in Dairy Feeds and Milk in Some Selected Areas of Ethiopia. Food Environ. Saf. 2018, XVII, 286–299. [Google Scholar]
- Elzupir, A.O.; Elhussein, A.M. Determination of Aflatoxin M1 in Dairy Cattle Milk in Khartoum State, Sudan. Food Control 2010, 21, 945–946. [Google Scholar] [CrossRef]
- Kuboka, M.M.; Imungi, J.K.; Njue, L.; Mutua, F.; Grace, D.; Lindahl, J.F. Occurrence of Aflatoxin M1 in Raw Milk Traded in Peri-Urban Nairobi, and the Effect of Boiling and Fermentation. Infect. Ecol. Epidemiol. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Waqas, M.; Latif, S. Incidence of Aflatoxin M1 in Milk and Milk Products from Punjab, Pakistan, and Estimation of Dietary Intake. Dairy 2022, 3, 577–586. [Google Scholar] [CrossRef]
- Daou, R.; Afif, C.; Joubrane, K.; Khabbaz, L.R.; Maroun, R.; Ismail, A.; Khoury, A. El. Occurrence of Aflatoxin M1 in Raw, Pasteurized, UHT Cows’ Milk, and Dairy Products in Lebanon. Food Control 2020, 111, 1–29. [Google Scholar] [CrossRef]
- Mohammedi-Ameur, S.; Dahmane, M.; Brera, C.; Kardjadj, M.; Ben-Mahdi, M.H. Occurrence and Seasonal Variation of Aflatoxin M1 in Raw Cow Milk Collected from Different Regions of Algeria. Vet. World 2020, 13, 433–439. [Google Scholar] [CrossRef]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The Toxic Effects of Aflatoxin B1 and Aflatoxin M1 on Kidney through Regulating L-Proline and Downstream Apoptosis. Biomed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Kirino, Y.; Makita, K.; Grace, D.; Lindahl, J. Survey of Informal Milk Retailers in Nairobi, Kenya and Prevalence of Aflatoxin M1 in Marketed Milk. African J. food, Agric. Nutr. Dev. 2016, 03, 11022–11033. [Google Scholar] [CrossRef]
- Fallah, A.A.; Barani, A.; Nasiri, Z. Aflatoxin M1 in Raw Milk in Qazvin Province, Iran: A Seasonal Study. Food Addit. Contam. Part B Surveill. 2015, 8, 195–198. [Google Scholar] [CrossRef]
- Younis, G.; Ibrahim, D.; Awad, A.; El Bardisy, M.M. Determination of Aflatoxin M1 and Ochratoxin A in Milk and Dairy Products in Supermarkets Located in Mansoura City, Egypt. Adv. Anim. Vet. Sci. 2016, 4, 114–121. [Google Scholar] [CrossRef]
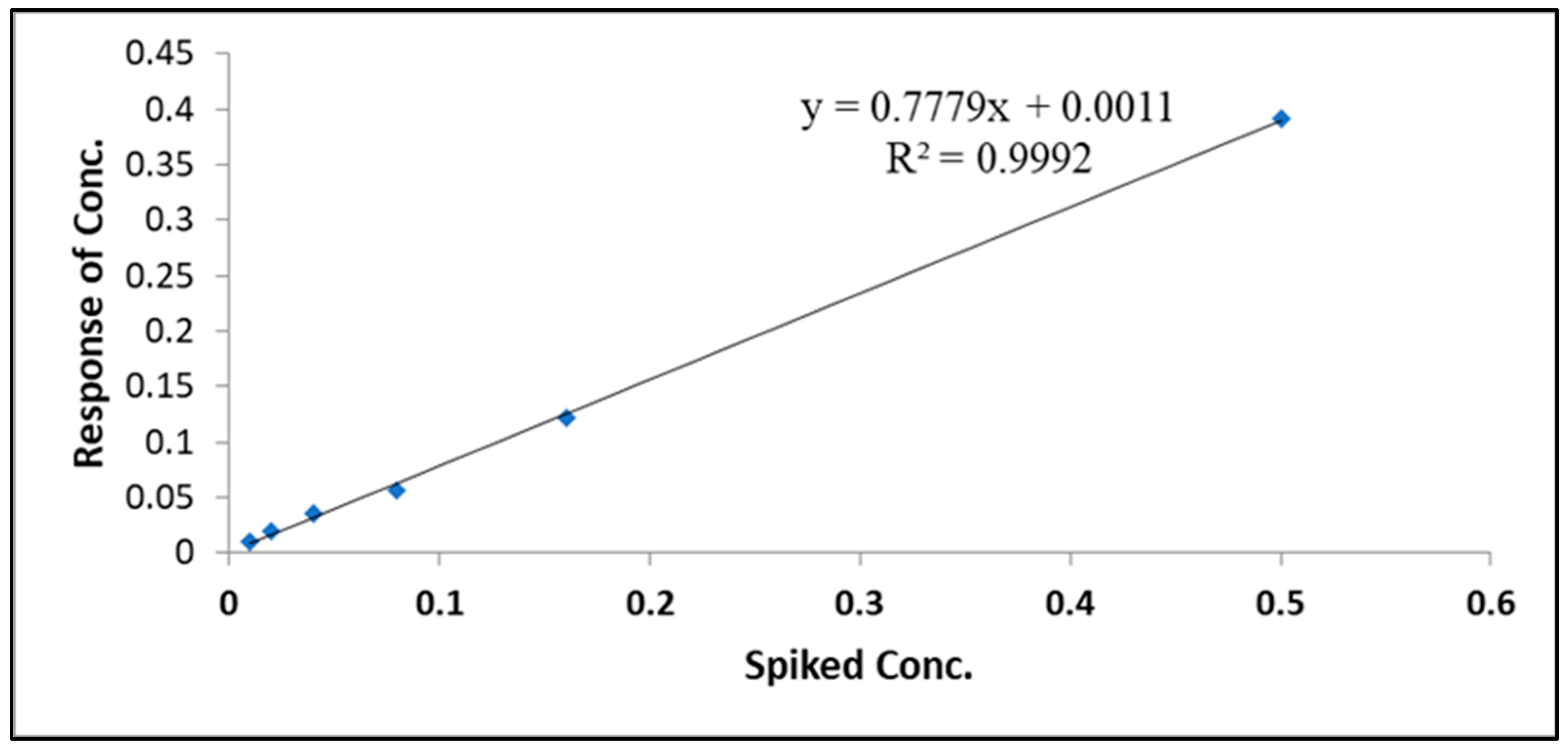
| Aflatoxins | Spiking Level (ppb) | R% | LOD (µg/kg) | LOQ (µg/kg) | RSD% |
|---|---|---|---|---|---|
| AFM1 | 0.01–0.5 | 127.62–91.64 | 0.008 | 0.026 | 1.71 |
| Categories | Mean ±SD (µg/L) | Min. (µg/L) | Max. (µg/L) | P-value | |
|---|---|---|---|---|---|
| Herd Size | |||||
| Small scale (≤5 cows) | 0.029±0.089 a | LOD | 0.513 | 0.01 | |
| Medium scale (6-15) | 0.028±0.030 a | LOD | 0.109 | ||
| Large scale (≥16) | 0.720±0.975 b | 0.017 | 3.850 | ||
| Milk Produced/Farm (L) | |||||
| Small scale (≤20) | 0.017±0.031 a | LOD | 0.109 | 0.01 | |
| Medium scale (20-40) | 0.023±0.026 a | LOD | 0.126 | ||
| Large scale (≥41) | 0.763±0.974 b | 0.021 | 3.850 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





