Submitted:
06 March 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
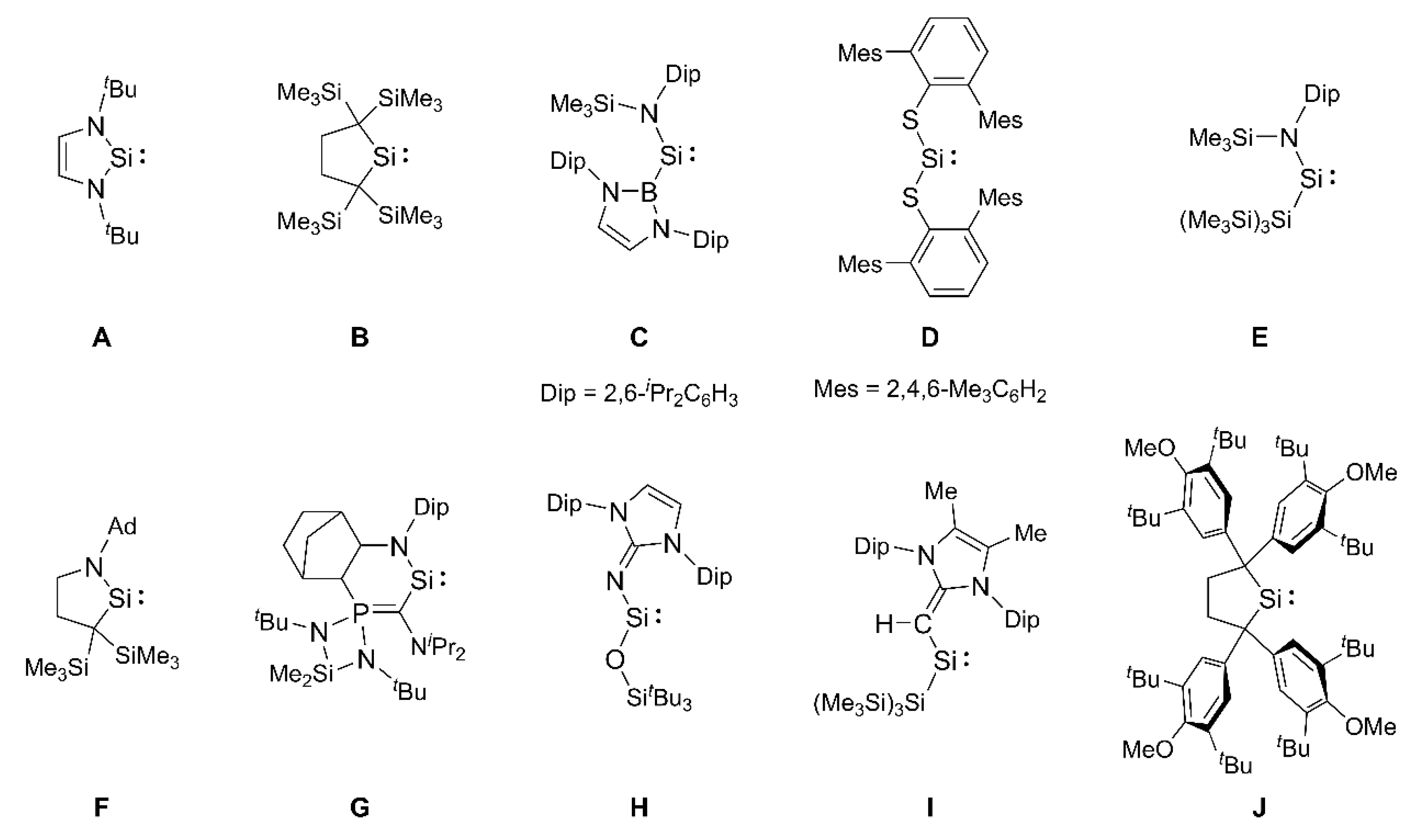
1. Introduction
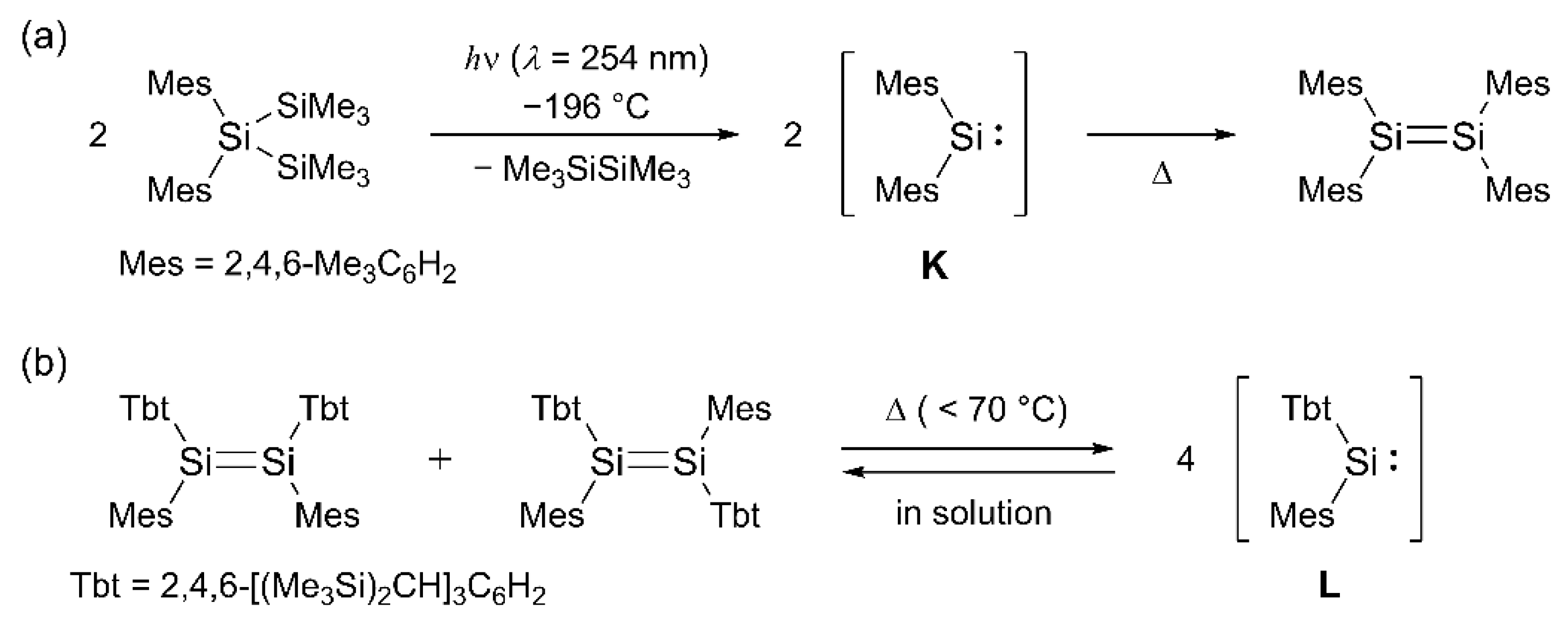
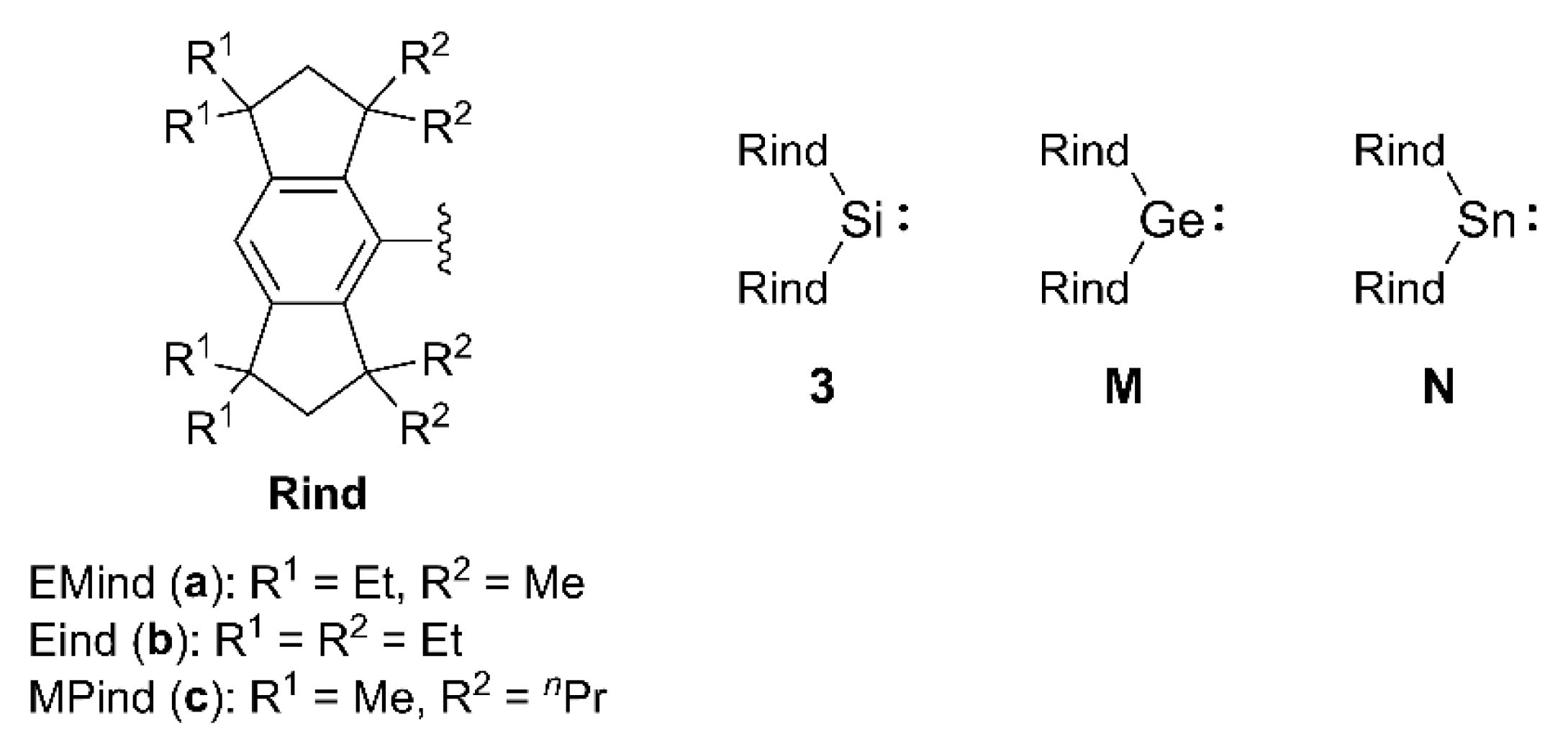
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
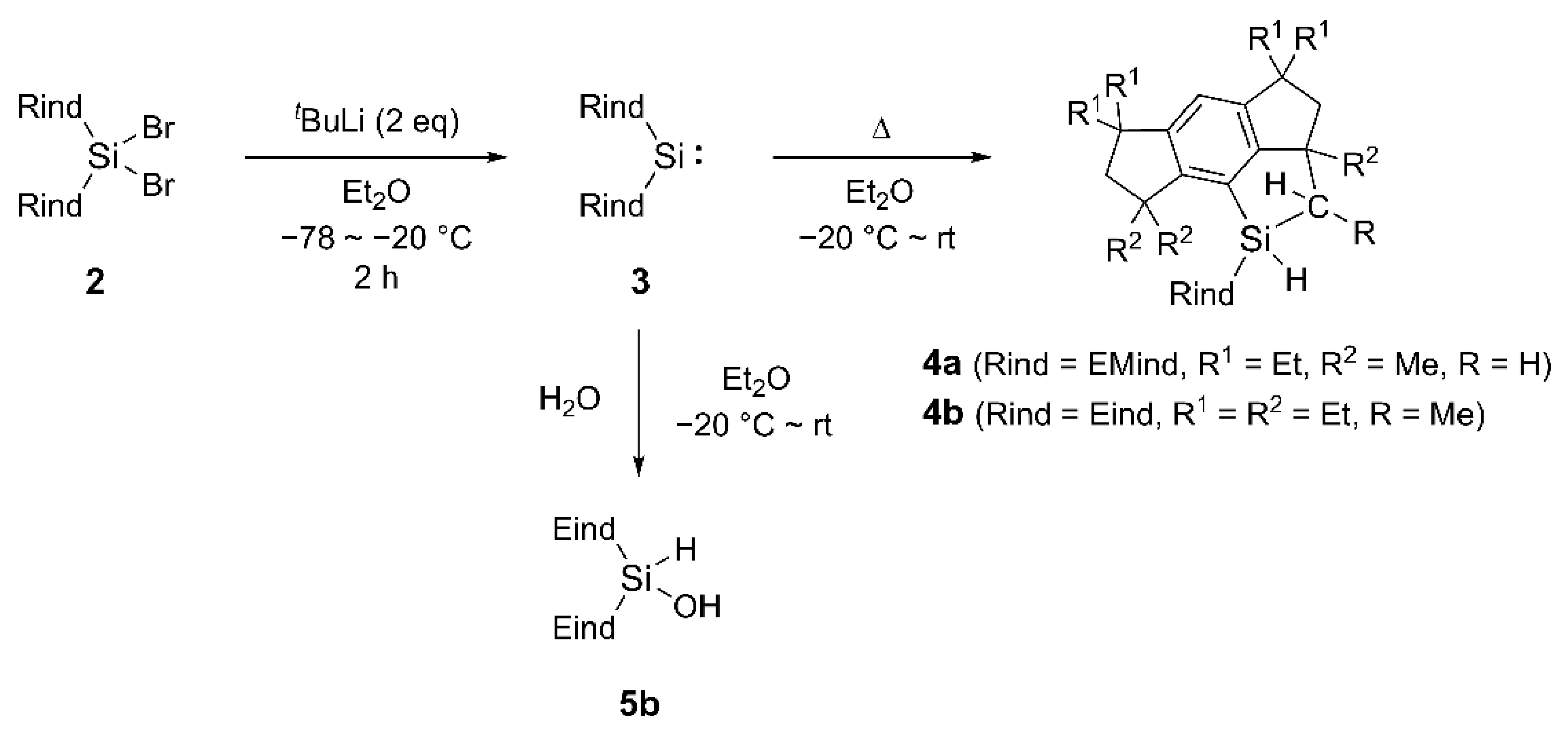
3.1.1. Synthesis of (Eind)2SiH2 (1b)
3.1.2. Synthesis of (Eind)2SiBr2 (2b)
3.1.3. Reduction of (EMind)2SiBr2 (2a) for UV-Vis Measurement of 3a
3.1.4. Reduction of (Eind)2SiBr2 (2b) for UV-Vis Measurement of 3b
3.1.5. Reduction of (Eind)2SiBr2 (2b) for XRD Measurement of Crystal-A
3.1.6. Reduction of (Eind)2SiBr2 (2b) for 29Si NMR Measurement of 3b
3.1.7. Reduction of (EMind)2SiBr2 (2a) for Thermal Reaction of 3a
3.1.8. Reduction of (Eind)2SiBr2 (2b) for Thermal Reaction of 3b
3.1.9. Hydrolysis of 3b for Synthesis of 5b
3.2. X-ray Crystallographic Studies of Crystal-A, Crystal-B, and 5b.
3.2.1. Crystal-A (Co-Crystal of 3b and 4b)
3.2.2. Crystal-B (4b)
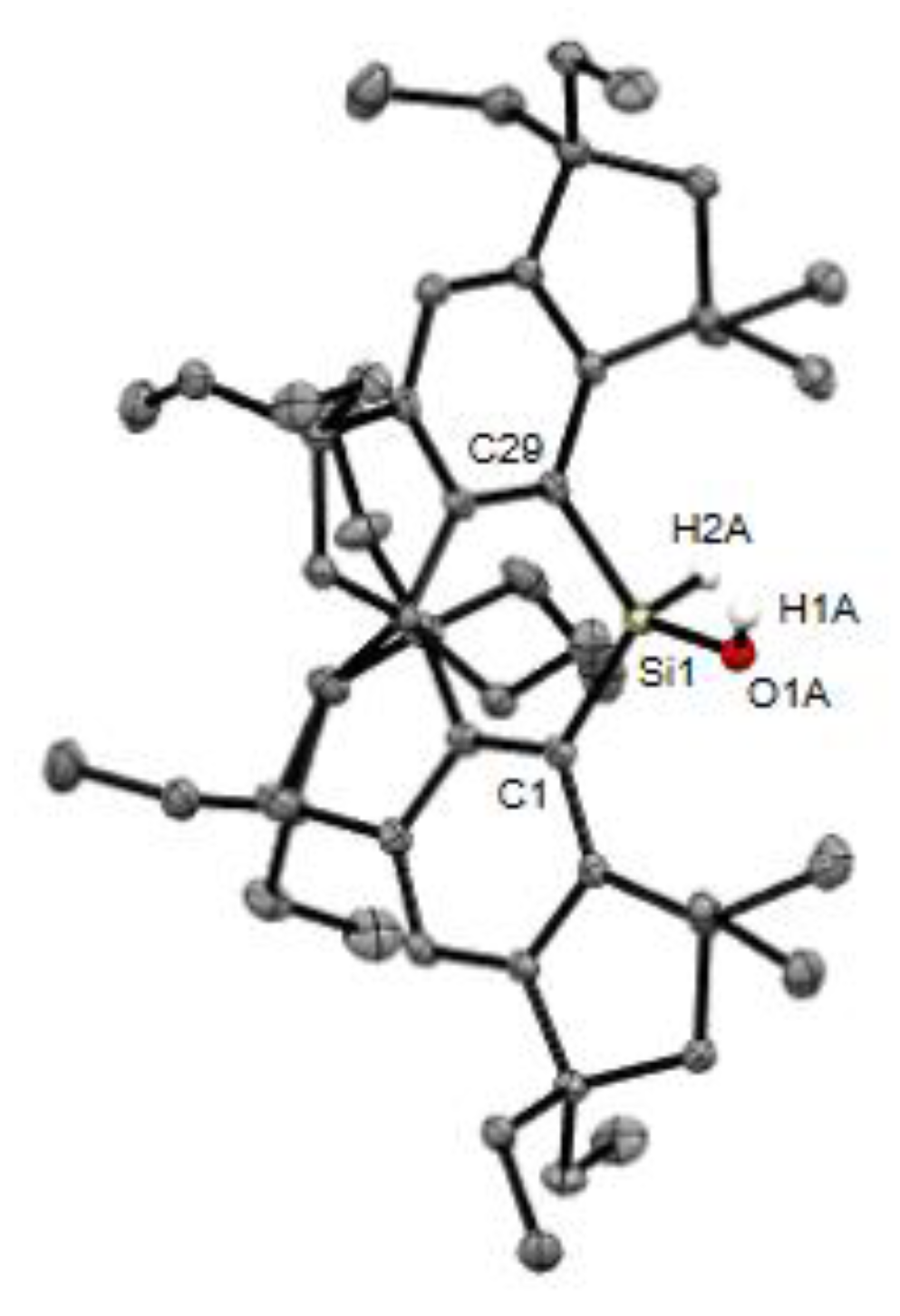
3.2.3. (Eind)2SiH(OH) (5b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jutzi, P.; Holtmann, U.; Kanne, D.; Krüger, C.; Blom, R.; Gleiter, R.; Hyla-Kryspin, I. Decamethylsilicocene—The first stable silicon(II) compound: Synthesis, structure, and bonding. Chem. Ber. 1989, 122, 1629–1639. [CrossRef]
- Wang, L.; Li, Y.; Li, Z.; Kira, M. Isolable silylenes and their diverse reactivity. Coord. Chem. Rev. 2022, 457, 214413. [CrossRef]
- Shan, C.; Yao, S.; Driess, M. Where silylene–silicon centres matter in the activation of small molecules. Chem. Soc. Rev. 2020, 49, 6733–6754. [CrossRef]
- Fujimori, S.; Inoue, S. Small Molecule Activation by Two-Coordinate Silylenes. Chem. Eur. J. 2020, 3131–3142. [CrossRef]
- Zhou, Y.-P.; Driess, M. Isolable Silylene Ligands Can Boost Efficiencies and Selectivities in Metal-Mediated Catalysis. Angew. Chem. Int. Ed. 2019, 58, 3715–3728. [CrossRef]
- Weetman, C.; Inoue, S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem 2018, 10, 4213–4228. [CrossRef]
- Chu, T.; Nikonov, G.I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. [CrossRef]
- Hadlington, T.J.; Driess, M.; Jones, C. Low-valent group 14 element hydride chemistry: towards catalysis. Chem. Soc. Rev. 2018, 47, 4176–4197. [CrossRef]
- Tacke, R.; Ribbeck, T. Bis(amidinato)- and bis(guanidinato)silylenes and silylenes with one sterically demanding amidinato or guanidinato ligand: synthesis and reactivity. Dalton Trans. 2017, 46, 13628–13659. [CrossRef]
- Lee, V.Y. Organosilicon Compounds: Theory and Experiment (Synthesis); Elsevier: London, UK, 2017.
- Yadav, S.; Saha, S.; Sen, S.S. Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis. ChemCatChem 2016, 8, 486–501. [CrossRef]
- Scheschkewitz, D. Functional Molecular Silicon Compounds II: Low Oxidation States; Springer: Basel, Switzerland, 2014.
- Asay, M.; Jones, C.; Driess, M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. [CrossRef]
- Lee, V.Y.; Sekiguchi, A. Organometallic Compounds of Low-Coordinate Si, Ge, Sn and Pb; Wiley: West Sussex, UK, 2010.
- Kira, M. An isolable dialkylsilylene and its derivatives. A step toward comprehension of heavy unsaturated bonds. Chem. Commun. 2010, 46, 2893–2903. [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable heavier carbene analogues. Chem. Rev. 2009, 109, 3479–3511. [CrossRef]
- Denk, M.; Lennon, R.; Hayashi, R.; West, R.; Belyakov, A.V.; Verne, H.P.; Haaland, A.; Wagner, M.; Metzler, N. Synthesis and Structure of a Stable Silylene. J. Am. Chem. Soc. 1994, 116, 2691–2692. [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. The First Isolable Dialkylsilylene. J. Am. Chem. Soc. 1999, 121, 9722–9723. [CrossRef]
- Protchenko, A.V.; Birjkumar, K.H.; Dange, D.; Schwarz, A.D.; Vidovic, D.; Jones, C.; Kaltsoyannis, N.; Mountford, P.; Aldridge, S. A Stable Two-Coordinate Acyclic Silylene. J. Am. Chem. Soc. 2012, 134, 6500–6503. [CrossRef]
- Rekken, B.D.; Brown, T.M.; Fettinger, J.C.; Tuononen, H.M.; Power, P.P. Isolation of a Stable, Acyclic, Two-Coordinate Silylene. J. Am. Chem. Soc. 2012, 134, 6504–6507. [CrossRef]
- Protchenko, A.V.; Schwarz, A.D.; Blake, M.P.; Jones, C.; Kaltsoyannis, N.; Mountford, P.; Aldridge, S. A Generic One-Pot Route to Acyclic Two-Coordinate Silylenes from Silicon(IV) Precursors: Synthesis and Structural Characterization of a Silylsilylene. Angew. Chem. Int. Ed. 2013, 52, 568 –571. [CrossRef]
- Kosai, T.; Ishida, S.; Iwamoto, T. A Two-Coordinate Cyclic (Alkyl)(amino)silylene: Balancing Thermal Stability and Reactivity. Angew. Chem. Int. Ed. 2016, 55, 15554–15558. [CrossRef]
- Alvarado-Beltran, I.; Baceiredo, A.; Saffon-Merceron, N.; Branchadell, V.; Kato, T. Cyclic Amino(Ylide) Silylene: A Stable Heterocyclic Silylene with Strongly Electron-Donating Character, Angew. Chem. Int. Ed. 2016, 55, 16141–16144. [CrossRef]
- Wendel, D.; Reiter, D.; Porzelt, A.; Altmann, P.J.; Inoue, S.; Rieger, B. Silicon and Oxygen’s Bond of Affection: An Acyclic Three-Coordinate Silanone and Its Transformation to an Iminosiloxysilylene. J. Am. Chem. Soc. 2017, 139, 17193–17198. [CrossRef]
- Roy, M.M.D.; Ferguson, M.J.; McDonald, R.; Zhou, Y.; Rivard, E. A vinyl silylsilylene and its activation of strong homo- and heteroatomic bonds. Chem. Sci. 2019, 10, 6476–6481. [CrossRef]
- Kobayashi, R.; Ishida, S.; Iwamoto, T. An Isolable Silicon Analogue of a Ketone that Contains an Unperturbed Si=O Double Bond. Angew. Chem. Int. Ed. 2019, 58, 9425–9428. [CrossRef]
- West, R.; Fink, M. J.; Michl, J. Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double Bond. Science 1981, 214, 1343–1344. [CrossRef]
- Tokitoh, N.; Suzuki, H.; Okazaki, R. Synthesis, Structure, and Reactivity of Extremely Hindered Disilenes: The First Example of Thermal Dissociation of a Disilene into a Silylene. J. Am. Chem. Soc. 1993, 115, 10428–10429. [CrossRef]
- Matsuo, T.; Hayakawa, N. π-Electron systems containing Si=Si double bonds. Sci. Technol. Adv. Mater. 2018, 19, 108–129. [CrossRef]
- Matsuo, T.; Tamao, K. Fused-Ring Bulky “Rind” Groups Producing New Possibilities in Elemento-Organic Chemistry. Bull. Chem. Soc. Jpn. 2015, 88, 1201–1229. [CrossRef]
- Matsuo, T.; Suzuki, K.; Fukawa, T.; Li, B.; Ito, M.; Shoji, Y.; Otani, T.; Li, L.; Kobayashi, M.; Hachiya, M.; Tahara, Y.; Hashizume, D.; Fukunaga, T.; Fukazawa, A.; Li, Y.; Tsuji, H.; Tamao, K. Synthesis and Structures of a Series of Bulky “Rind-Br” Based on a Rigid Fused-Ring s-Hydrindacene Skeleton. Bull. Chem. Soc. Jpn. 2011, 84, 1178–1191. [CrossRef]
- Ohno, R.; Numata, Y.; Konaka, S.; Yagura, S.; Kuroda, A.; Harada, M.; Fujita, N.; Hayakawa, N.; Nakai, H.; Rosas-Sánchez, A.; Hashizume, D.; Matsuo, T. Synthesis and Characterization of a Series of Diarylgermylene and Dihalodigermenes Having Fused-Ring Bulky “Rind” Groups. Bull. Chem. Soc. Jpn. 2021, 94, 1931–1939. [CrossRef]
- Suzuki, K.; Numata, Y.; Fujita, N.; Hayakawa, N.; Tanikawa, T.; Hashizume, D.; Tamao, K.; Fueno, H.; Tanaka, K.; Matsuo, T. A stable free tetragermacyclobutadiene incorporating fused-ring bulky EMind groups. Chem. Commun. 2018, 54, 2200–2203. [CrossRef]
- Li, L.; Fukawa, T.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. A stable germanone as the first isolated heavy ketone with a terminal oxygen atom, Nat. Chem. 2012, 4, 361–365. [CrossRef]
- Kuroda, A.; Fujita, N.; Horita, T.; Ota, K.; Rosas-Sánchez, A.; Hoshino, M.; Hashizume, D.; Matsuo, T. Formation and Reactions of Ge=O Double-bonded Species Bearing EMind Groups. Chem. Lett. 2022, 51, 828–831. [CrossRef]
- Numata, Y.; Nishikawa, Y.; Inoue, K.; Ohnishi, H.; Konaka, S.; Tanikawa, T.; Hashizume, D.; Matsuo, T. A Series of Room-Temperature Thermally Stable Bromostannylenes Bearing the Bulky Rind Group: Synthesis, Characterization, and Crystal Structures. Organometallics 2021, 40, 1956–1965. [CrossRef]
- Hayakawa, N.; Morimoto, T.; Takagi, A.; Tanikawa, T.; Hashizume, D.; Matsuo, T. Synthesis and Structures of Sterically Congested Diarylsilanes Bearing Two Bulky Rind Groups. Chem. Lett. 2016, 45, 409–411. [CrossRef]
- Sasamori, T.; Hironaka, K.; Sugiyama, Y.; Takagi, N.; Nagase, S.; Hosoi, Y.; Furukawa, Y.; Tokitoh, N. Synthesis and Reactions of a Stable 1,2-Diaryl-1,2-dibromodisilene: A Precursor for Substituted Disilenes and a 1,2-Diaryldisilyne. J. Am. Chem. Soc. 2008, 130, 13856–13857. [CrossRef]
- Michalczyk, M.J.; Fink, M.J.; DeYoung, D.J.; Carlson, C.W.; Welsh, K.M.; West, R.; Michl, J. Organosilylenes and Their Dimerization to Disilenes. Silicon, Germanium, Tin Lead Compd. 1986, 9, 75−83.
- Conlin, R.T.; Netto-Ferreira, J.C.; Zhang, Scaiano, J.C. Kinetic study of dimesitylsilylene by laser flash photolysis. Organometallics 1990, 9, 1332−1334. [CrossRef]
- Moiseev, A.G.; Leigh, W.J. Diphenylsilylene. J. Am. Chem. Soc. 2006, 128, 14442–14443. [CrossRef]
- Hayakawa, N.; Sugahara, T.; Numata, Y.; Kawaai, H.; Yamatani, K.; Nishimura, S.; Goda, S.; Suzuki, Y.; Tanikawa, T.; Nakai, H.; Hashizume, D.; Sasamori, T.; Tokitoh, N.; Matsuo, T. 1,2-Dihalodigermenes bearing bulky Eind groups: synthesis, characterization, and conversion to halogermylenoids, Dalton Trans. 2018, 47, 814−822. [CrossRef]
- Ando, W.; Hamada, Y,; Sekiguchi, A. Reactions of Oxasilacyclopropane. Generation of Silanediyl by Photo and Thermal Induced Cycloelimination. J. Chem. Soc., Chem. Commun., 1983, 952−954. [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.P.; Izmaylov, A.F.; Bloino, J.; Zheng, G.; Sonnenberg, J.L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J.A., Jr.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N.J.; Klene, M.; Knox, J.E.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Zakrzewski, V.G.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Dapprich, S.; Daniels, A.D.; Farkas, Ö; Foresman, J.B.; Ortiz, J.V.; Cioslowski, J.; Fox, D.J. Gaussian 09, Rev. D.01; Gaussian, Inc.: Wallingford, CT, 2010.
- Kosa, M.; Karni, M.; Apeloig, Y. Were Reactions of Triplet Silylenes Observed? J. Am. Chem. Soc. 2013, 135, 9032–9040. [CrossRef]
- Guthardt, R.; Jacob, H.L.; Bruhn, C.; Siemeling, U. A complete series of N-heterocyclic tetrylenes (Si–Pb) with a 1,1’-ferrocenediyl backbone enabled by 1,3,2-diazaborolyl N-substituents. Dalton Trans. 2023, 52, 14380–14389. [CrossRef]
- Ishida, S.; Abe, T.; Hirakawa, F.; Kosai, T.; Sato, K.; Kira, M.; Iwamoto, T. Persistent Dialkylsilanone Generated by Dehydrobromination of Dialkylbromosilanol. Chem. Eur. J. 2015, 21, 15100−15103. [CrossRef]
- Krempner, C.; Martens, K.; Reinke, H. Synthesis and structure of silyl acetonitriles. J. Organomet. Chem. 2007, 692, 5799−5803. [CrossRef]
- CrystalClear; Rigaku/MSC. Inc.: The Woodlands, TX, USA, 2005.
- CrysAlisPro; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.: Caro, L.D.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: a suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609−613. [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [CrossRef]
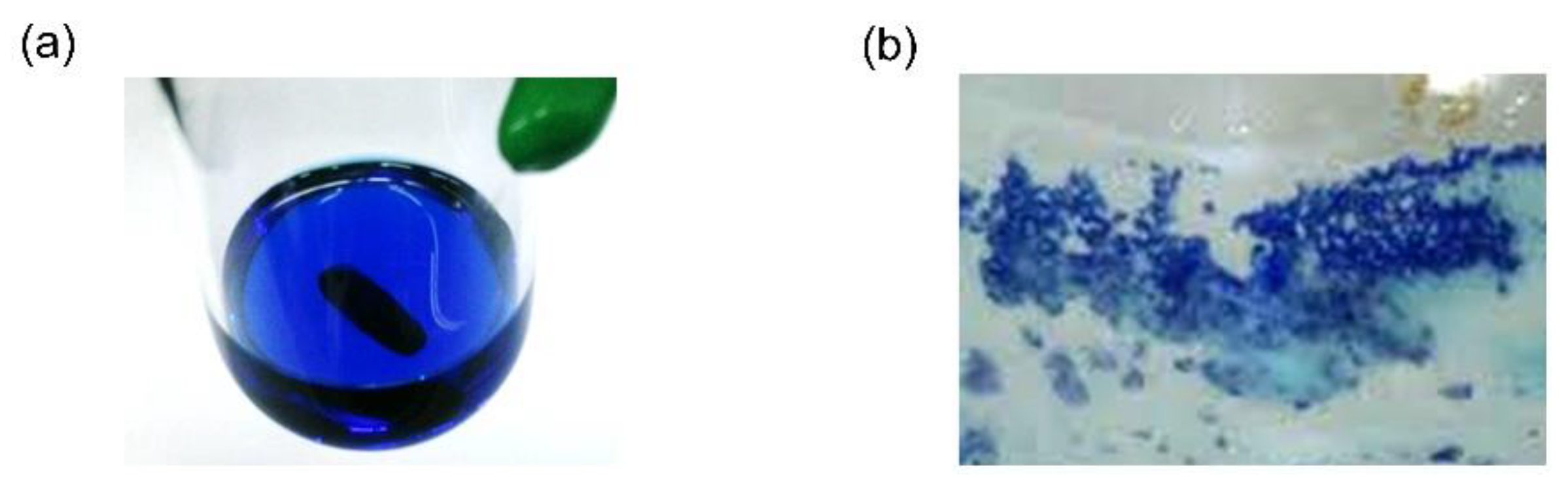
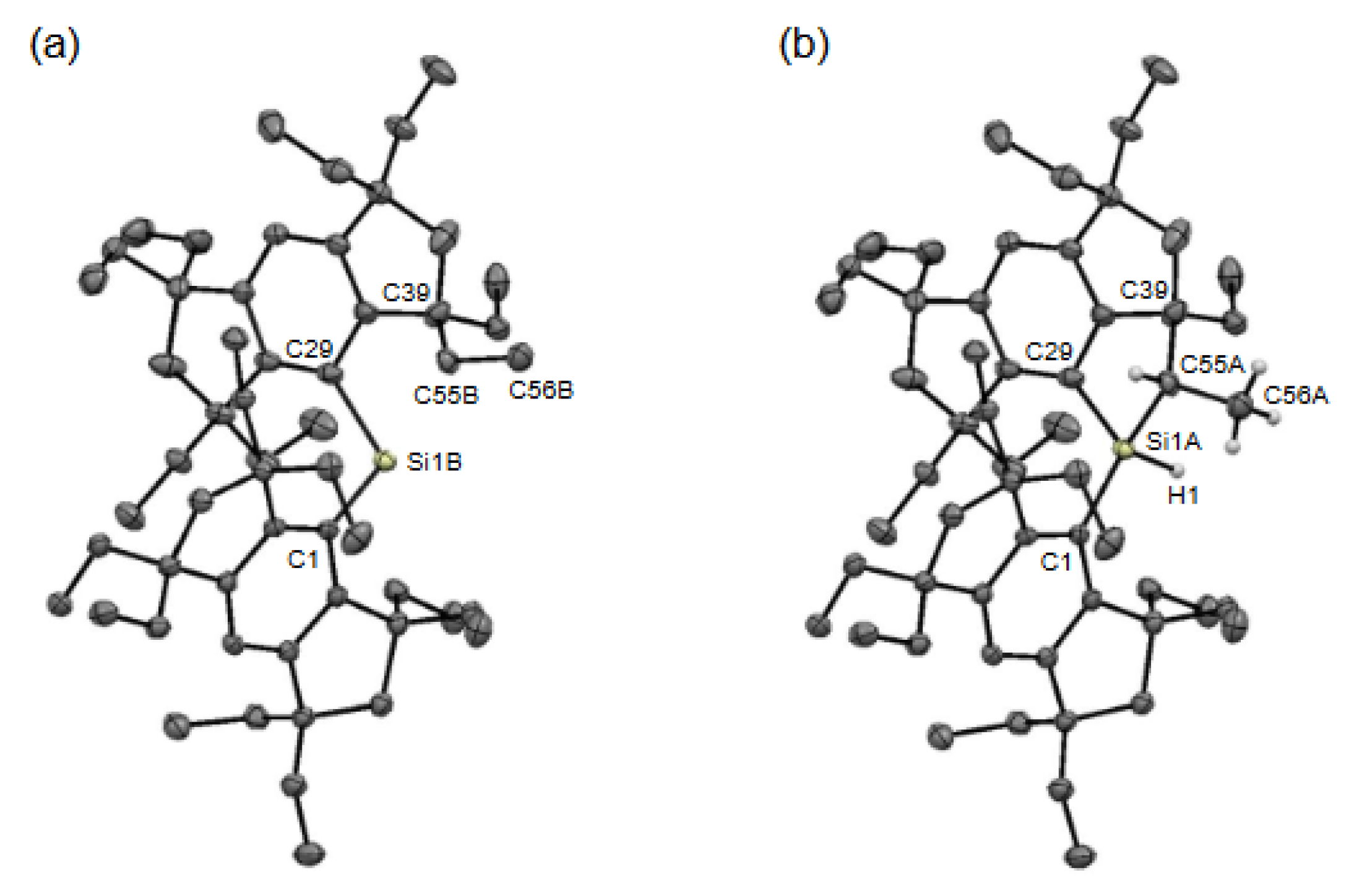
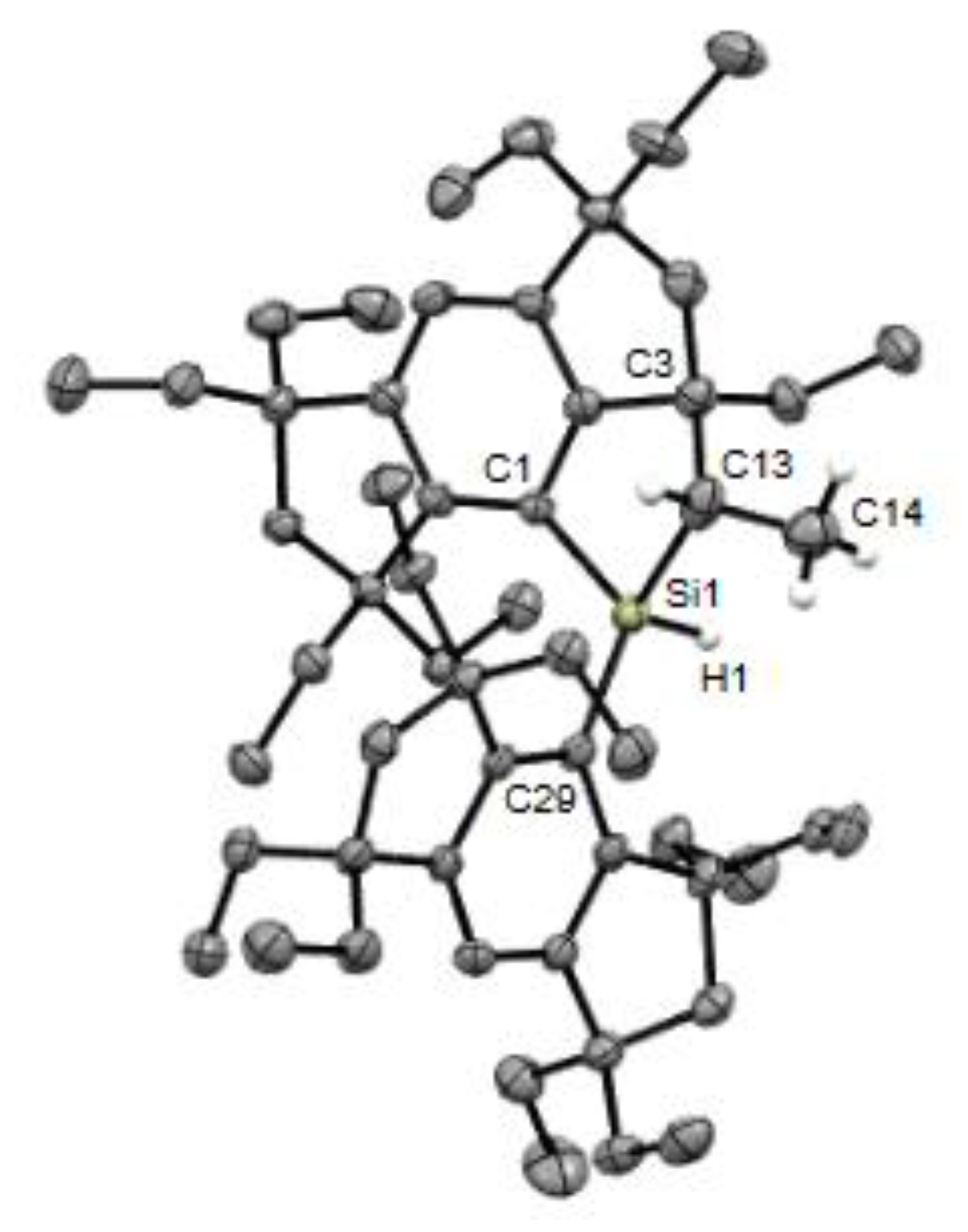
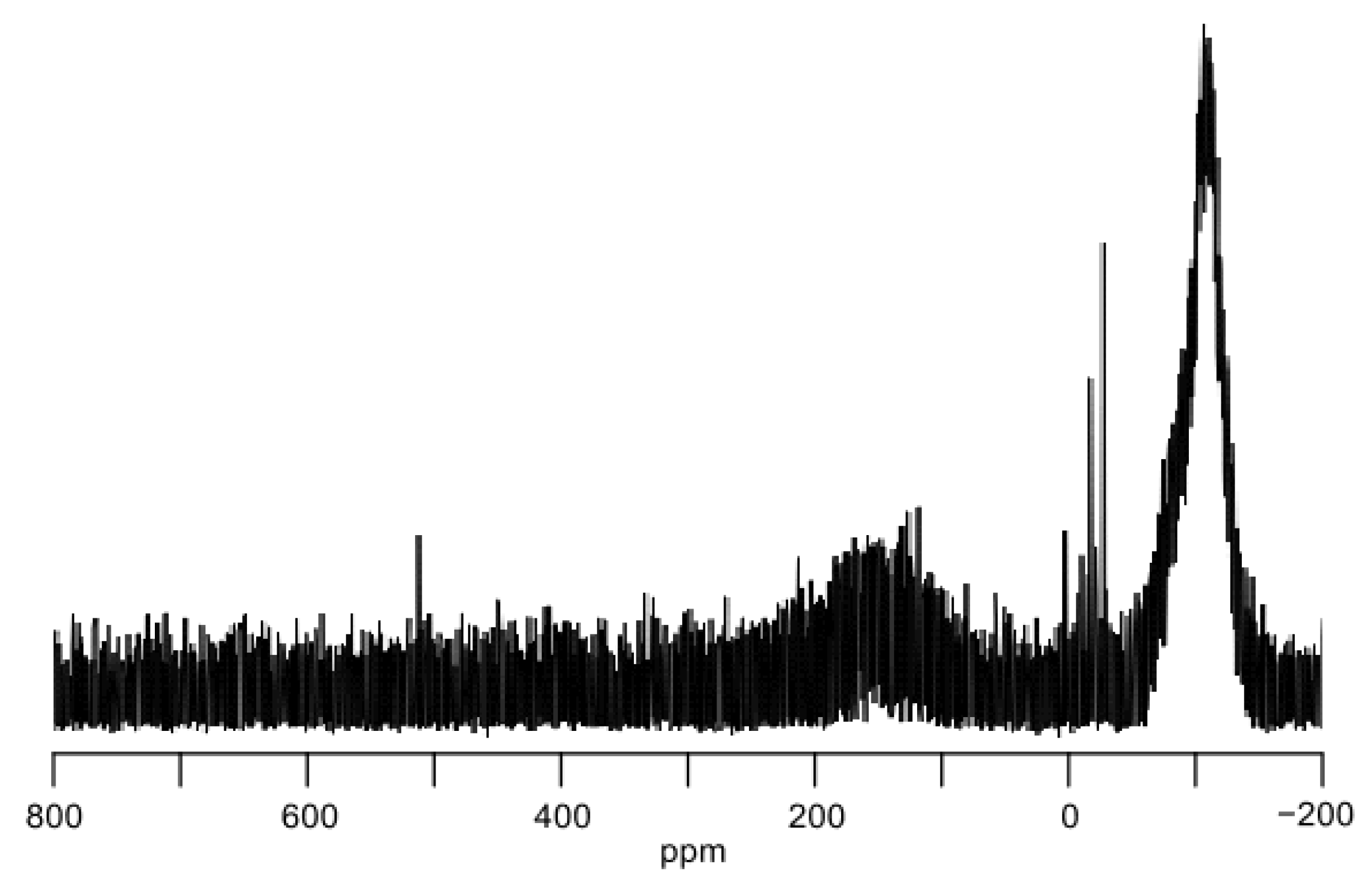
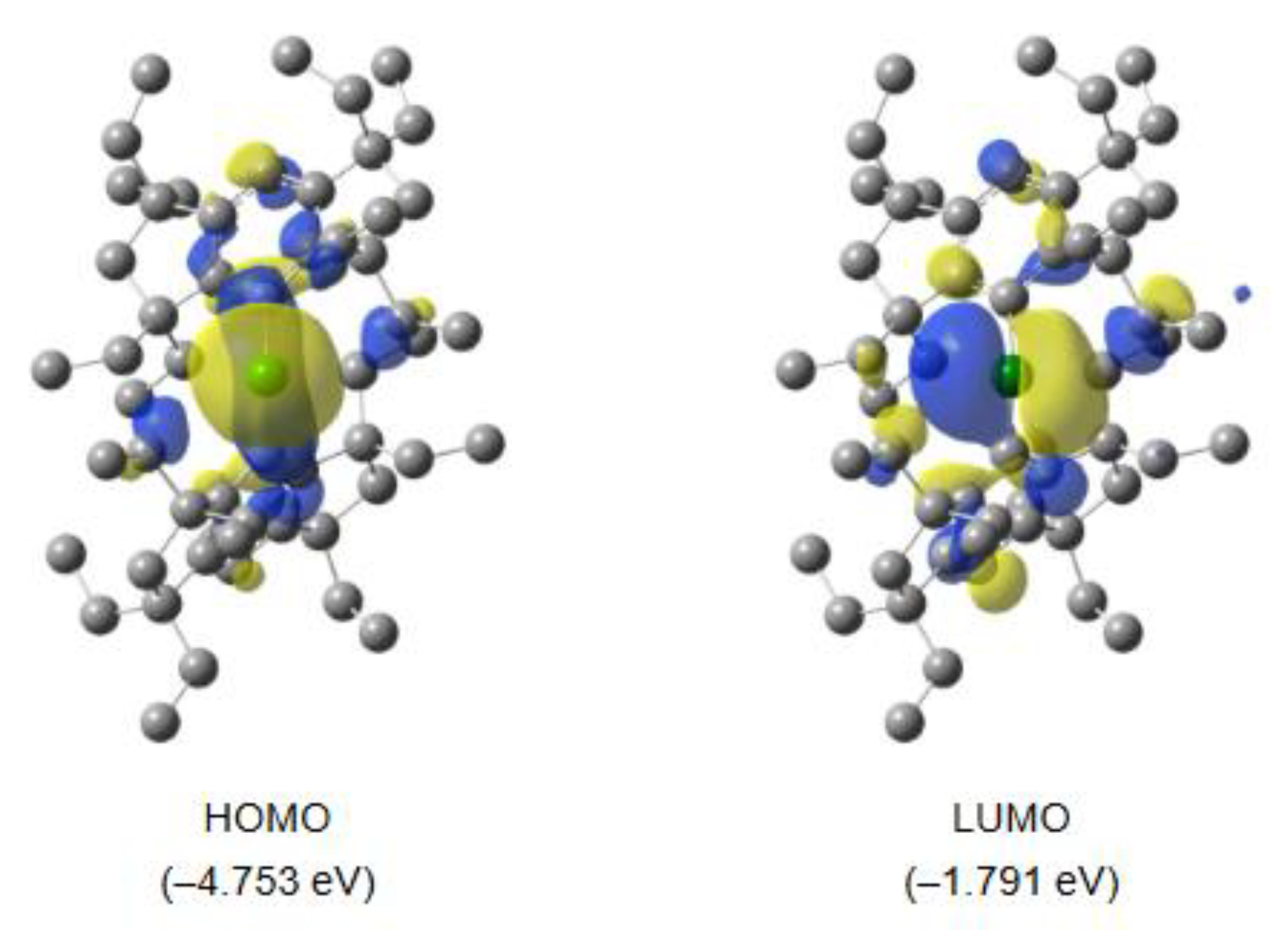
| Compounds | Si−C (Å) | C(sp2)−Si−C(sp2) (°) |
|---|---|---|
| experimental | ||
| 1b [37] | 1.950(4), 1.938(5) | 123.60(17) |
| 2b [37] | 1.934(3) | 116.60(13) |
| 3b (Crystal-A) | 1.941(5), 2.004(5) | 112.3(2) |
| 4b (Crystal-A) | 1.919(2), 1.873(3), | 119.57(12) |
| 1.904(4) | ||
| 4b (Crystal-B) | 1.899(3), 1.909(2), | 122.11(11) |
| 1.964(3) | ||
| 5b | 1.9090(11), 1.9228(12) | 122.05(5) |
| calculations | ||
| 3b (singlet) | 1.9470, 1.9489 | 112.00 |
| 3b (triplet) | 1.8980, 1.8926 | 127.95 |
| 4b | 1.9099, 1.9012, | 119.50 |
| 1.9358 | ||
| 5b | 1.9294, 1.9174 | 121.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





