1. Introduction
Patients with transfusion dependent Thalassaemia (TDT), one of the commonest heritable blood disorders are now enjoying increased life expectancy in countries with access to with safe blood transfusion and iron chelation.(1) Despite the longevity, complications related iron overload and anaemia are not uncommon in these populations. Heart disease, primarily related to iron overload remains the leading cause of death in them. (2) Heart disease not directly related iron induced cardiomyopathy is also see in thalassaemia syndromes; notably pulmonary hypertension. (PHT) PHT is seen more commonly in the less transfused form of thalassaemia referred to as non- transfused depended thalassaemia, (NTDT).(3,4) Unusual cardiac complications reported in these patients include myocardial infarctions often without minimal or no evidence of plaque disease.(5,6)
Systemic hypertension has never been reported in any patient cohorts to the best of our knowledge. On the contrary, previous studies and clinical experience suggest blood pressure to be on the lower side in these patients.(7)
In this back drop we report the cases of two patients with TDT who had systemic hypertension.
2. Case Presentation
Patient 1
26-year-old female with beta thalassaemia major diagnosed at the age of 6 months. Transfusion frequency which was every four weeks has increased to two weekly since 2019. At the time of assessment, she had received over 350 transfusions life time. In total. Over the last 12 months period her mean pre-transfusion Hb was 8.1 g/dl. Her chelation had started at the age of 2 years with desferrioxamine sub cutaneous infusion. Over the last 10 years she is on dual chelator combination of DFO and defarsirox at a dose of 40mg/kg/day. DFO compliance was variable and the effective dose at the point of assessment was 18mg/kg/day. Her last serum ferritin level was 4354ng/ml and the highest ever ferritin was 10602ng/ml in 2017. She had attained menarche at the age of 15 years only after induction with low dose oestrogen. She was diagnosed with diabetes mellitus in 2011 when she was 14 years of age. She is currently managed with ore mixed insulin twice a day regimen and her overall control is very variable and not very satisfactory. However, at the last annual assessment she did not have ophthalmic, neurological or renal complications related to diabetes mellitus. Her BMI and waist hip ratio were 17 kg/m2, 0.9
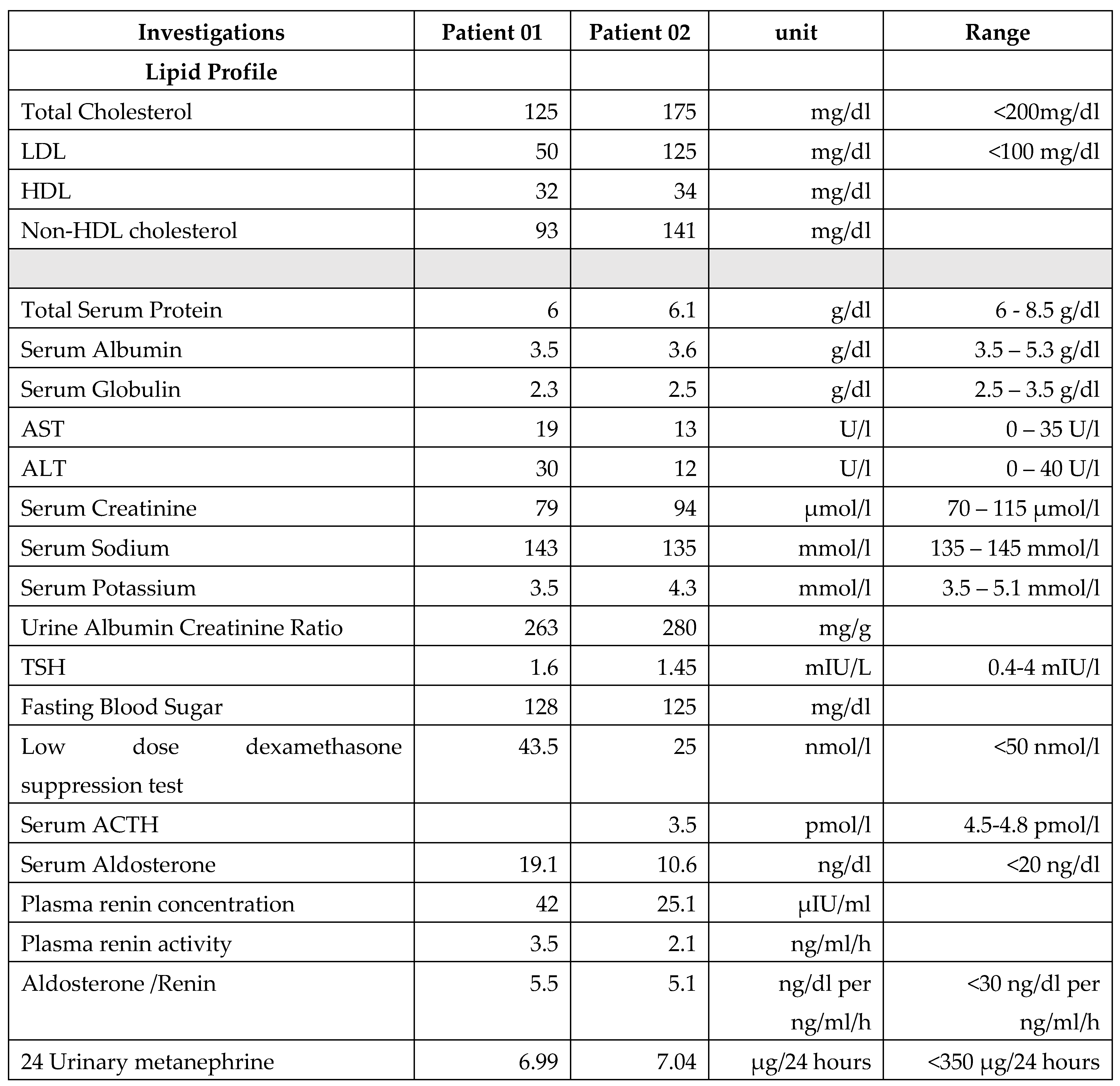
Hypertension was first noted when she was 20years of age. Multiple reading over period over one month was high and she was commenced on Captopril 25mb bd. As this did not sufficiently control the blood pressure bisoprolol 2.5mg bid was added. Her parents were not hypertensive. Investigations related to hypertension are given in
Table 1.
Patient 2
30-year-old female with beta thalassaemia major was diagnosed at the age of one year and has been on regular monthly blood transfusions since. At present, she had received over 350 transfusions and her mean pre-transfusion Hb over the last 12 months was 7.5 g/dl. Chelation was commenced with desferrioxamine at the age of 4 years and her current chelator use included DFO together with DFX; which she had been using for over 10 years. The current dosages were 23 mg/kg/day and 40mg/kg/d respectively. Her current serum ferritin level was 1003 ng/ml and her highest ever ferritin was 5550 in 2011. She attained menarche at the age of 16 years spontaneously but as the periods were irregular and finally stopped she had been commenced on HRT. Diabetes mellitus was diagnosed in 2007 at the age of 14 years. Currently she is on pre-mixed insulin bid and pre-lunch soluble insulin regimen. Overall glycaemic control has been deemed unsatisfactory. There were no ophthalmic, neuropathic or renal complications detected at the last assessment done 6 months prior to the current admission. Her BMI and waist hip ratio were 21kg/m2 and 0.8 respectively.
Hypertension was diagnosed 6 months back and due to persistently elevated blood pressure and she was commenced on Loastaren 25 mg bid. Investigations related to hypertension are shown in
Table 1.
As hypertension is not an expected finding in this disease sub group and both patients were of a young age, causes for secondary hypertension were rigorously evaluated.
No obvious cause for secondary cause for hypertension could be identified in either patient. Though both had diabetes mellitus for periods extending over 15 years neither had evidence of microvascular complications.
3. Discussion
The diagnosis of systemic hypertension in both patients described above were ascertained with great veracity as clinical experience or literature in thalassaemia do not speak of hypertension in this disorder. In fact more often than not blood pressure in patients with thalassaemia seems to be in the lower side.(8)
Thalassemia patients have previously been shown to have defects in endothelial relaxation, intimal thickening, abnormal vascular stiffening, and degeneration of elastic arteries. (8) The aforesaid defects are features are linked with premature vascular aging but as yet never kinked with systemic hypertension in patients with thalassaemia. well known complications in beta thalassemia are iron induced cardiomyopathy, pulmonary hypertension, arrhythmias, premature vascular ageing and peripheral artery disease and sudden cardiac death. Over the last 20 years the presence of pulmonary hypertension in beta thalassemia has been an area of special interest. These two patients consisted the only two identifiable patients with elevated systemic blood pressure in a clinic with over 350 adult patients with the disorder suggests that hypertension should not be considered to be a common occurrence. Though our initial observations did not identify a possible secondary cause for hypertension its of interest to suggest that both patients with hypertension had prolonged duration of diabetes clearly linked inadequate iron chelation in childhood and at the present time. Interestingly, neither patient had the body habitus nor criteria that fulfils a diagnosis of metabolic syndrome, and there was no family history of hypertension in either of them.
4. Conclusion
Measurement of blood pressure should not be ignored in patients with thalassaemia. Though rare, systemic hypertension could be identified in these patients. The two patients described here suggested an association with iron overload and diabetes mellitus but if its aetiologically linked could not be proven.
References
- Farmakis D, Giakoumis A, Angastiniotis M, Eleftheriou A. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur J Haematol. 2020, 105, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Akiki N, Hodroj MH, Bou-Fakhredin R, Matli K, Taher AT. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalassemia Reports. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Wood, JC. Pulmonary hypertension in thalassemia: a call to action. Blood 2022, 139, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Kunrada Inthawong, Pimlak Charoenkwan, Suchaya Silvilairat, Adisak Tantiworawit, Arintaya Phrommintikul, Worawut Choeyprasert, Rungrote Natesirinilkul, Chate Siwasomboon, Pannee Visrutaratna, Somdet Srichairatanakool, Nipon Chattipakorn & Torpong Sanguansermsri (2015) Pulmonary hypertension in non-transfusion-dependent thalassemia: Correlation with clinical parameters, liver iron concentration, and non-transferrin-bound iron. Hematology 2015, 20, 610–617. [CrossRef]
- Fridlender ZG, Rund D. Myocardial infarction in a patient with beta-thalassemia major: first report. Am J Hematol. 2004, 75, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Premawardhena A, De Silva S, Rajapaksha M, Ratnamalala V, Nallarajah J, Galappatthy G. Myocardial infarction in patients with severe beta thalassaemia: a case series. Int J Emerg Med. 2023, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Veglio F, Melchio R, Rabbia F, Molino P, Genova GC, Martini G, Schiavone D, Piga A, Chiandussi L. Blood pressure and heart rate in young thalassemia major patients. Am J Hypertens. 1998, 11, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wood JC. Cardiac complications in thalassemia major. Hemoglobin 2009, 33 (Suppl. S1), S81–S6. [Google Scholar]
Table 1.
shows the results.
Table 1.
shows the results.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).