Submitted:
27 February 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Part
2.1. Instrumentation
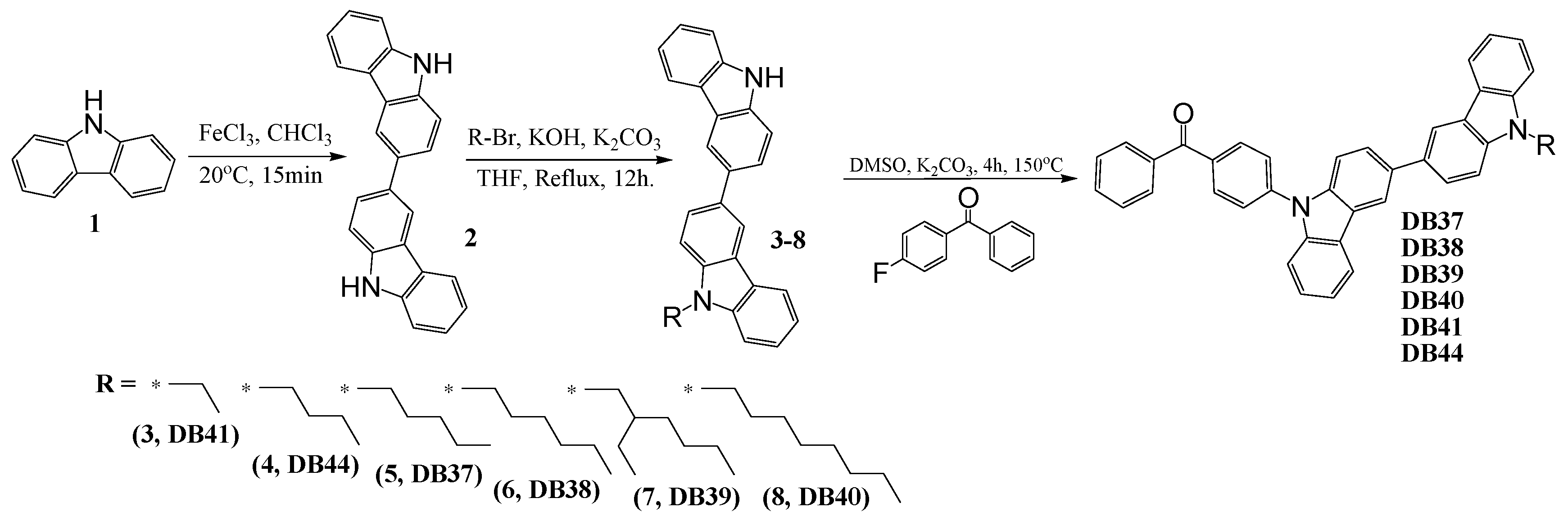
2.2. Synthesis and Structural Analysis
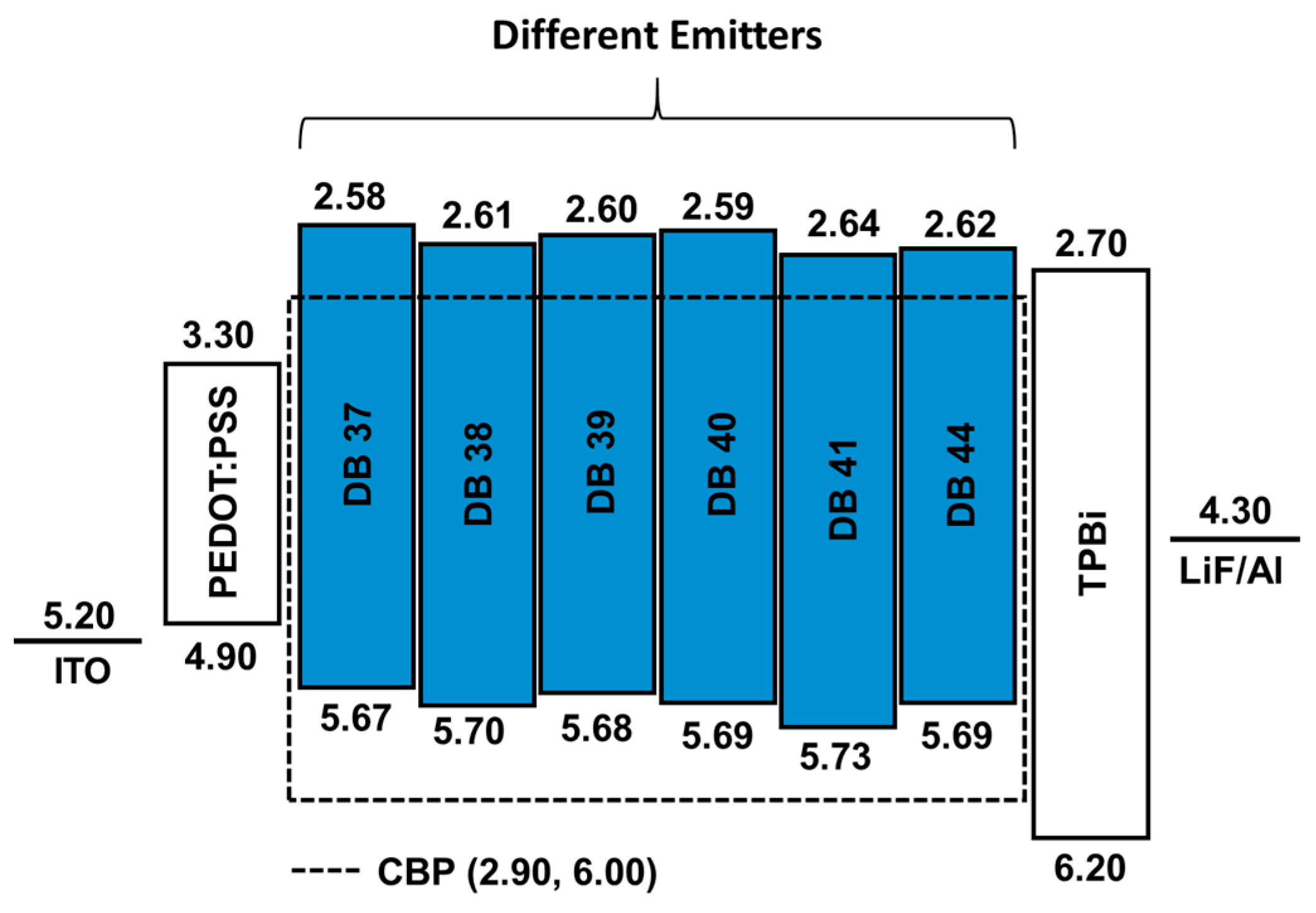
2.3. Fabrication and Characterization of Devices
3. Results and Discussions
3.1. Synthesis
3.2. Thermal and Morphological Properties
3.3. Electrochemical and Photophysical Properties
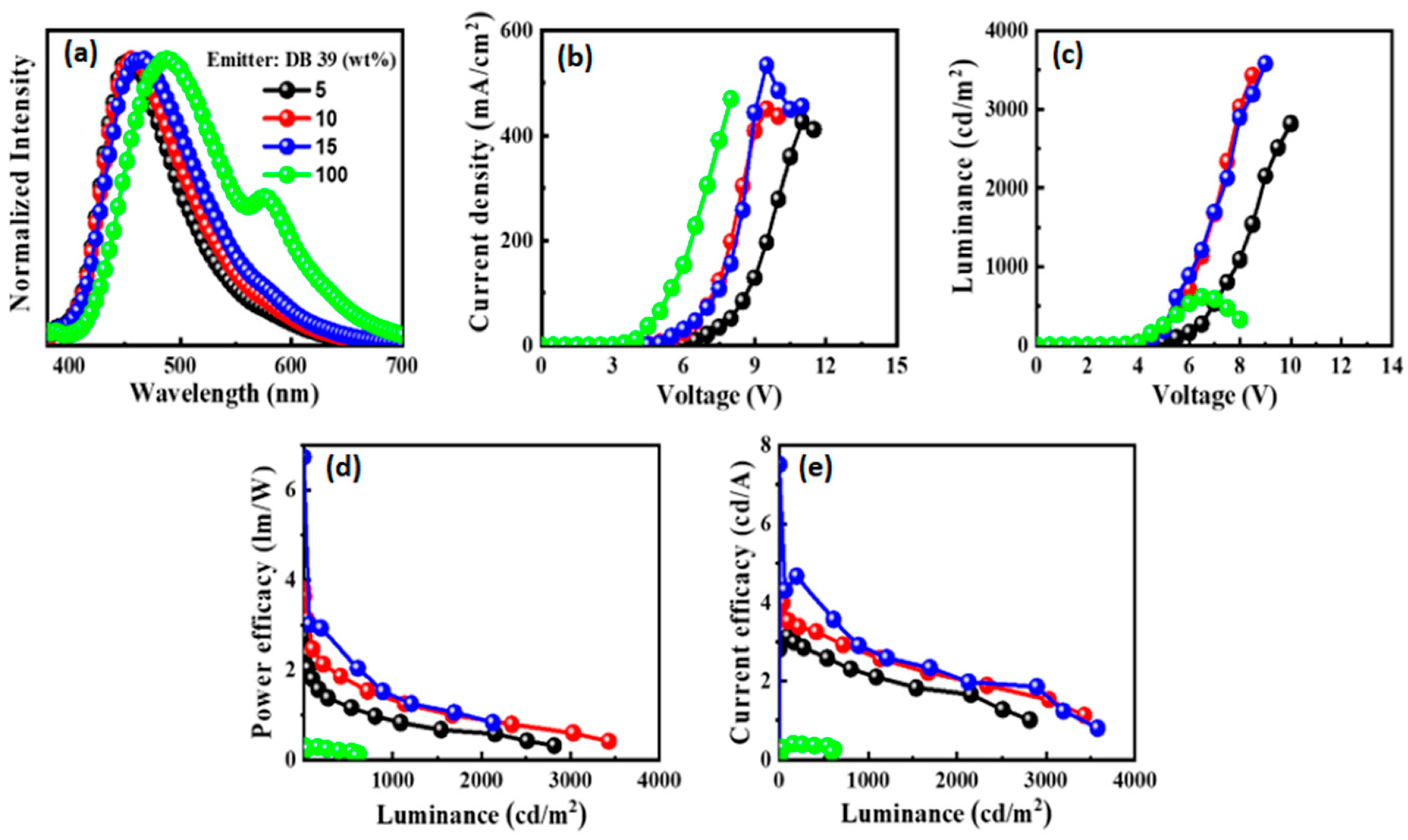
3.4. Electroluminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Advanced Materials 2021, 33. [Google Scholar] [CrossRef]
- Luo, Y.-J.; Lu, Z.-Y.; Huang, Y. Triplet Fusion Delayed Fluorescence Materials for OLEDs. Chinese Chemical Letters 2016, 27, 1223–1230. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, Q.; Liu, J.; Huang, Y.; Zhou, J.; Zhao, S.; Lu, Z. A Red-Emissive Sextuple Hydrogen-Bonding Self-Assembly Molecular Duplex Bearing Perylene Diimide Fluorophores for Warm-White Organic Light-Emitting Diode Application. Chin J Chem 2016, 34, 387–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Ye, K.; Zhang, H. Synthesis, Structure and Properties of a Novel Benzothiazole-Based Diboron-Bridged π-Conjugated Ladder. Acta Chimi Sin 2016, 74, 179. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Ren, Z.; Xie, G.; Li, Q.; Li, Z. Synthesis of Solution Processable Blue AIEgens and the Device Performance. Acta Chimi Sin 2016, 74, 865. [Google Scholar] [CrossRef]
- Im, Y.; Byun, S.Y.; Kim, J.H.; Lee, D.R.; Oh, C.S.; Yook, K.S.; Lee, J.Y. Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes. Adv Funct Mater 2017, 27. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem Rev 2017, 117, 6467–6499. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Han, M.-K.; Noh, Y.-Y. Flexible OLEDs and Organic Electronics. Semicond Sci Technol 2011, 26, 030301. [Google Scholar] [CrossRef]
- Jeong, E.G.; Kwon, J.H.; Kang, K.S.; Jeong, S.Y.; Choi, K.C. A Review of Highly Reliable Flexible Encapsulation Technologies towards Rollable and Foldable OLEDs. Journal of Information Display 2020, 21, 19–32. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lüssem, B.; Leo, K. White Organic Light-Emitting Diodes with Fluorescent Tube Efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Giebink, N.C.; Kanno, H.; Ma, B.; Thompson, M.E.; Forrest, S.R. Management of Singlet and Triplet Excitons for Efficient White Organic Light-Emitting Devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Xu, Y.; Liang, B.; Wei, J.; Li, C.; Mu, X.; Ye, K.; Wang, Y. Purely Organic Phosphorescence Emitter-Based Efficient Electroluminescence Devices. J Phys Chem Lett 2019, 10, 5983–5988. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yue, L.; Yu, Y.; Liu, B.; Dang, J.; Sun, Y.; Zhou, G.; Wu, Z.; Wong, W. Strategically Formulating Aggregation-Induced Emission-Active Phosphorescent Emitters by Restricting the Coordination Skeletal Deformation of Pt(II) Complexes Containing Two Independent Monodentate Ligands. Adv Opt Mater 2020, 8. [Google Scholar] [CrossRef]
- Rajamalli, P.; Senthilkumar, N.; Huang, P.-Y.; Ren-Wu, C.-C.; Lin, H.-W.; Cheng, C.-H. New Molecular Design Concurrently Providing Superior Pure Blue, Thermally Activated Delayed Fluorescence and Optical Out-Coupling Efficiencies. J Am Chem Soc 2017, 139, 10948–10951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, C. Blue Fluorescent Emitters: Design Tactics and Applications in Organic Light-Emitting Diodes. Chem Soc Rev 2013, 42, 4963. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lu, T.; Cheng, Z.; Chang, Y.; Liu, H.; Wang, J.; Wan, L.; Lv, Y.; Lu, P. Rational Molecular Design of Phenanthroimidazole-Based Fluorescent Materials towards High-Efficiency Non-Doped Deep Blue OLEDs. J Mater Chem C Mater 2022, 10, 14186–14193. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent Progress in Metal–Organic Complexes for Optoelectronic Applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- de Leeuw, D.M.; Simenon, M.M.J.; Brown, A.R.; Einerhand, R.E.F. Stability of N-Type Doped Conducting Polymers and Consequences for Polymeric Microelectronic Devices. Synth Met 1997, 87, 53–59. [Google Scholar] [CrossRef]
- Scholz, S.; Kondakov, D.; Lüssem, B.; Leo, K. Degradation Mechanisms and Reactions in Organic Light-Emitting Devices. Chem Rev 2015, 115, 8449–8503. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, C.; Batagoda, T.; Coburn, C.; Thompson, M.E.; Forrest, S.R. Hot Excited State Management for Long-Lived Blue Phosphorescent Organic Light-Emitting Diodes. Nat Commun 2017, 8, 15566. [Google Scholar] [CrossRef]
- Xing, L.; Zhu, Z.-L.; He, J.; Qiu, Z.; Yang, Z.; Lin, D.; Chen, W.-C.; Yang, Q.; Ji, S.; Huo, Y.; et al. Anthracene-Based Fluorescent Emitters toward Superior-Efficiency Nondoped TTA-OLEDs with Deep Blue Emission and Low Efficiency Roll-Off. Chemical Engineering Journal 2021, 421, 127748. [Google Scholar] [CrossRef]
- Chen, J.; Tao, W.; Chen, W.; Xiao, Y.; Wang, K.; Cao, C.; Yu, J.; Li, S.; Geng, F.; Adachi, C.; et al. Red/Near-Infrared Thermally Activated Delayed Fluorescence OLEDs with Near 100 % Internal Quantum Efficiency. Angewandte Chemie International Edition 2019, 58, 14660–14665. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. Carbazole Dendrimers as Solution-Processable Thermally Activated Delayed-Fluorescence Materials. Angewandte Chemie International Edition 2015, 54, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J Am Chem Soc 2012, 134, 14706–14709. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, T.; Wang, Z.; Wang, L.; Zhan, L.; Gong, S.; Zhong, C.; Lu, Z.-H.; Zhang, S.; Yang, C. De Novo Design of Excited-State Intramolecular Proton Transfer Emitters via a Thermally Activated Delayed Fluorescence Channel. J Am Chem Soc 2018, 140, 8877–8886. [Google Scholar] [CrossRef] [PubMed]
- Goushi, K.; Yoshida, K.; Sato, K.; Adachi, C. Organic Light-Emitting Diodes Employing Efficient Reverse Intersystem Crossing for Triplet-to-Singlet State Conversion. Nat Photonics 2012, 6, 253–258. [Google Scholar] [CrossRef]
- Ahn, D.H.; Kim, S.W.; Lee, H.; Ko, I.J.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Highly Efficient Blue Thermally Activated Delayed Fluorescence Emitters Based on Symmetrical and Rigid Oxygen-Bridged Boron Acceptors. Nat Photonics 2019, 13, 540–546. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Cai, X.; Chen, D.; Xie, G.; Liu, K.; Wu, Y.-C.; Lo, C.-C.; Lien, A.; Cao, Y.; et al. Structure–Performance Investigation of Thioxanthone Derivatives for Developing Color Tunable Highly Efficient Thermally Activated Delayed Fluorescence Emitters. ACS Appl Mater Interfaces 2016, 8, 8627–8636. [Google Scholar] [CrossRef]
- Im, Y.; Kim, M.; Cho, Y.J.; Seo, J.-A.; Yook, K.S.; Lee, J.Y. Molecular Design Strategy of Organic Thermally Activated Delayed Fluorescence Emitters. Chemistry of Materials 2017, 29, 1946–1963. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Xie, G.; He, Z.; Gao, K.; Liu, K.; Chen, D.; Cao, Y.; Su, S.-J. “Rate-Limited Effect” of Reverse Intersystem Crossing Process: The Key for Tuning Thermally Activated Delayed Fluorescence Lifetime and Efficiency Roll-off of Organic Light Emitting Diodes. Chem Sci 2016, 7, 4264–4275. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect. Advanced Materials 2016, 28, 2777–2781. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, R.; Zhong, C.; Xie, G.; Ning, W.; Huang, M.; Ni, F.; Dias, F.B.; Yang, C. Achieving 21% External Quantum Efficiency for Nondoped Solution-Processed Sky-Blue Thermally Activated Delayed Fluorescence OLEDs by Means of Multi-(Donor/Acceptor) Emitter with Through-Space/-Bond Charge Transfer. Advanced Science 2020, 7. [Google Scholar] [CrossRef]
- Ma, F.; Ji, H.; Zhang, D.; Xue, K.; Zhang, P.; Qi, Z.; Zhu, H. Adjusting the Photophysical Properties of AIE-Active TADF Emitters from through-Bond to through-Space Charge Transfer for High-Performance Solution-Processed OLEDs. Dyes and Pigments 2021, 188, 109208. [Google Scholar] [CrossRef]
- Rajamalli, P.; Rota Martir, D.; Zysman-Colman, E. Pyridine-Functionalized Carbazole Donor and Benzophenone Acceptor Design for Thermally Activated Delayed Fluorescence Emitters in Blue Organic Light-Emitting Diodes. J Photonics Energy 2018, 8, 1. [Google Scholar] [CrossRef]
- Ma, M.; Li, J.; Liu, D.; Li, D.; Dong, R.; Mei, Y. Low Efficiency Roll-off Thermally Activated Delayed Fluorescence Emitters for Non-Doped OLEDs: Substitution Effect of Thioether and Sulfone Groups. Dyes and Pigments 2021, 194, 109649. [Google Scholar] [CrossRef]
- Wu, L.; Wang, K.; Wang, C.; Fan, X.-C.; Shi, Y.-Z.; Zhang, X.; Zhang, S.-L.; Ye, J.; Zheng, C.-J.; Li, Y.-Q.; et al. Using Fluorene to Lock Electronically Active Moieties in Thermally Activated Delayed Fluorescence Emitters for High-Performance Non-Doped Organic Light-Emitting Diodes with Suppressed Roll-Off. Chem Sci 2021, 12, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Tsou, C.-J.; Park, I.S.; Yasuda, T. Aggregation-Induced Delayed Fluorescence from Phenothiazine-Containing Donor–Acceptor Molecules for High-Efficiency Non-Doped Organic Light-Emitting Diodes. Polym J 2017, 49, 197–202. [Google Scholar] [CrossRef]
- Jing, Y.-Y.; Tao, X.-D.; Yang, M.-X.; Chen, X.-L.; Lu, C.-Z. Triptycene-Imbedded Thermally Activated Delayed Fluorescence Emitters with Excellent Film Morphologies for Applications in Efficient Nondoped and Doped Organic Light-Emitting Devices. Chemical Engineering Journal 2021, 413, 127418. [Google Scholar] [CrossRef]
- Tani, K.; Yashima, T.; Miyanaga, K.; Hori, K.; Goto, K.; Tani, F.; Habuka, Y.; Suzuki, K.; Shizu, K.; Kaji, H. Carbazole and Benzophenone Based Twisted Donor–Acceptor Systems as Solution Processable Green Thermally Activated Delayed Fluorescence Organic Light Emitters. Chem Lett 2018, 47, 1236–1239. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Chen, Y.; Chen, L.; Li, H.; Wang, W.; Wang, S.; Tian, H.; Tong, H.; Wang, L. Triazatruxene-Based Thermally Activated Delayed Fluorescence Small Molecules with Aggregation-Induced Emission Properties for Solution-Processable Nondoped OLEDs with Low Efficiency Roll-Off. J Mater Chem C Mater 2019, 7, 9719–9725. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Jiang, C.; Yao, C.; Xi, X. Effective Design Strategy for Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence Emitters Achieving 18% External Quantum Efficiency Pure-Blue OLEDs with Extremely Low Roll-Off. ACS Appl Mater Interfaces 2021, 13, 57713–57724. [Google Scholar] [CrossRef] [PubMed]
- Shizu, K.; Lee, J.; Tanaka, H.; Nomura, H.; Yasuda, T.; Kaji, H.; Adachi, C. Highly Efficient Electroluminescence from Purely Organic Donor–Acceptor Systems. Pure and Applied Chemistry 2015, 87, 627–638. [Google Scholar] [CrossRef]
- Nishimoto, T.; Yasuda, T.; Lee, S.Y.; Kondo, R.; Adachi, C. A Six-Carbazole-Decorated Cyclophosphazene as a Host with High Triplet Energy to Realize Efficient Delayed-Fluorescence OLEDs. Mater. Horiz. 2014, 1, 264–269. [Google Scholar] [CrossRef]
- Huang, B.; Ban, X.; Sun, K.; Ma, Z.; Mei, Y.; Jiang, W.; Lin, B.; Sun, Y. Thermally Activated Delayed Fluorescence Materials Based on Benzophenone Derivative as Emitter for Efficient Solution-Processed Non-Doped Green OLED. Dyes and Pigments 2016, 133, 380–386. [Google Scholar] [CrossRef]
- Liang, J.; Li, C.; Zhuang, X.; Ye, K.; Liu, Y.; Wang, Y. Novel Blue Bipolar Thermally Activated Delayed Fluorescence Material as Host Emitter for High-Efficiency Hybrid Warm-White OLEDs with Stable High Color-Rendering Index. Adv Funct Mater 2018, 28. [Google Scholar] [CrossRef]
- Pocock, I.A.; Alotaibi, A.M.; Jagdev, K.; Prior, C.; Burgess, G.R.; Male, L.; Grainger, R.S. Direct Formation of 4,5-Disubstituted Carbazoles via Regioselective Dilithiation. Chemical Communications 2021, 57, 7252–7255. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Tian, Q.-S.; Zheng, Q.; Tao, X.-C.; Yuan, Y.; Yu, Y.-J.; Li, Y.; Jiang, Z.-Q.; Liao, L.-S. A Sky-Blue Thermally Activated Delayed Fluorescence Emitter Based on Multimodified Carbazole Donor for Efficient Organic Light-Emitting Diodes. Org Electron 2019, 68, 113–120. [Google Scholar] [CrossRef]
- Liu, F.; Zou, J.; He, Q.; Tang, C.; Xie, L.; Peng, B.; Wei, W.; Cao, Y.; Huang, W. Carbazole End-capped Pyrene Starburst with Enhanced Electrochemical Stability and Device Performance. J Polym Sci A Polym Chem 2010, 48, 4943–4949. [Google Scholar] [CrossRef]
- Sęk, D.; Szlapa-Kula, A.; Siwy, M.; Fabiańczyk, A.; Janeczek, H.; Szalkowski, M.; Maćkowski, S.; Schab-Balcerzak, E. Branched Azomethines Based on Tris(2-Aminoethyl)Amine: Impact of Imine Core Functionalization on Thermal, Electrochemical and Luminescence Properties. Mater Chem Phys 2020, 240, 122246. [Google Scholar] [CrossRef]
- Sebris, A.; Novosjolova, I.; Traskovskis, K.; Kokars, V.; Tetervenoka, N.; Vembris, A.; Turks, M. Photophysical and Electrical Properties of Highly Luminescent 2/6-Triazolyl-Substituted Push–Pull Purines. ACS Omega 2022, 7, 5242–5253. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, W.; Duan, L. Recent Progress in Solution Processable TADF Materials for Organic Light-Emitting Diodes. J Mater Chem C Mater 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Jiang, C.; Yao, C.; Gu, M.; Wang, W. Solution-Processed Aggregation-Induced Delayed Fluorescence (AIDF) Emitters Based on Strong π-Accepting Triazine Cores for Highly Efficient Nondoped OLEDs with Low Efficiency Roll-Off. Org Electron 2019, 65, 170–178. [Google Scholar] [CrossRef]
- Li, Y.; Xie, G.; Gong, S.; Wu, K.; Yang, C. Dendronized Delayed Fluorescence Emitters for Non-Doped, Solution-Processed Organic Light-Emitting Diodes with High Efficiency and Low Efficiency Roll-off Simultaneously: Two Parallel Emissive Channels. Chem Sci 2016, 7, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, J.; Chen, L.; Chen, R.; Jin, J.; Chen, C.; Chen, S.; Xie, G.; Zheng, C.; Huang, W. Thermally Activated Delayed Fluorescence Enantiomers for Solution-Processed Circularly Polarized Electroluminescence. J Mater Chem C Mater 2019, 7, 14511–14516. [Google Scholar] [CrossRef]
- Inoue, S.; Minemawari, H.; Tsutsumi, J.; Chikamatsu, M.; Yamada, T.; Horiuchi, S.; Tanaka, M.; Kumai, R.; Yoneya, M.; Hasegawa, T. Effects of Substituted Alkyl Chain Length on Solution-Processable Layered Organic Semiconductor Crystals. Chemistry of Materials 2015, 27, 3809–3812. [Google Scholar] [CrossRef]
- Vaitkeviciene, V.; Grigalevicius, S.; Grazulevicius, J.V.; Jankauskas, V.; Syromyatnikov, V.G. Hole-Transporting [3,3′]Bicarbazolyl-Based Polymers and Well-Defined Model Compounds. Eur Polym J 2006, 42, 2254–2260. [Google Scholar] [CrossRef]
- Gautam, P.; Shahnawaz; Siddiqui, I; Blazevicius, D.; Krucaite, G.; Tavgeniene, D.; Jou, J.-H.; Grigalevicius, S. Bifunctional Bicarbazole-Benzophenone-Based Twisted Donor–Acceptor–Donor Derivatives for Deep-Blue and Green OLEDs. Nanomaterials 2023, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Blazevicius, D.; Siddiqui, I.; Gautam, P.; Krucaite, G.; Tavgeniene, D.; Nagar, M.R.; Kumar, K.; Banik, S.; Jou, J.-H.; Grigalevicius, S. Bicarbazole-Benzophenone-Based Twisted Donor-Acceptor-Donor Derivatives as Blue Emitters for Highly Efficient Fluorescent Organic Light-Emitting Diodes. Nanomaterials 2024, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- De Sa Pereira, D.; Data, P.; Monkman, A.P. Methods of Analysis of Organic Light Emitting Diodes. Display 2017, 2, 323–337. [Google Scholar]
- Zhao, L.; Liu, Y.; Wang, S.; Tao, Y.; Wang, F.; Zhang, X.; Huang, W. Novel Hyperbranched Polymers as Host Materials for Green Thermally Activated Delayed Fluorescence OLEDs. Chinese Journal of Polymer Science 2017, 35, 490–502. [Google Scholar] [CrossRef]
- Sworakowski, J. How Accurate Are Energies of HOMO and LUMO Levels in Small-Molecule Organic Semiconductors Determined from Cyclic Voltammetry or Optical Spectroscopy? Synth Met 2018, 235, 125–130. [Google Scholar] [CrossRef]
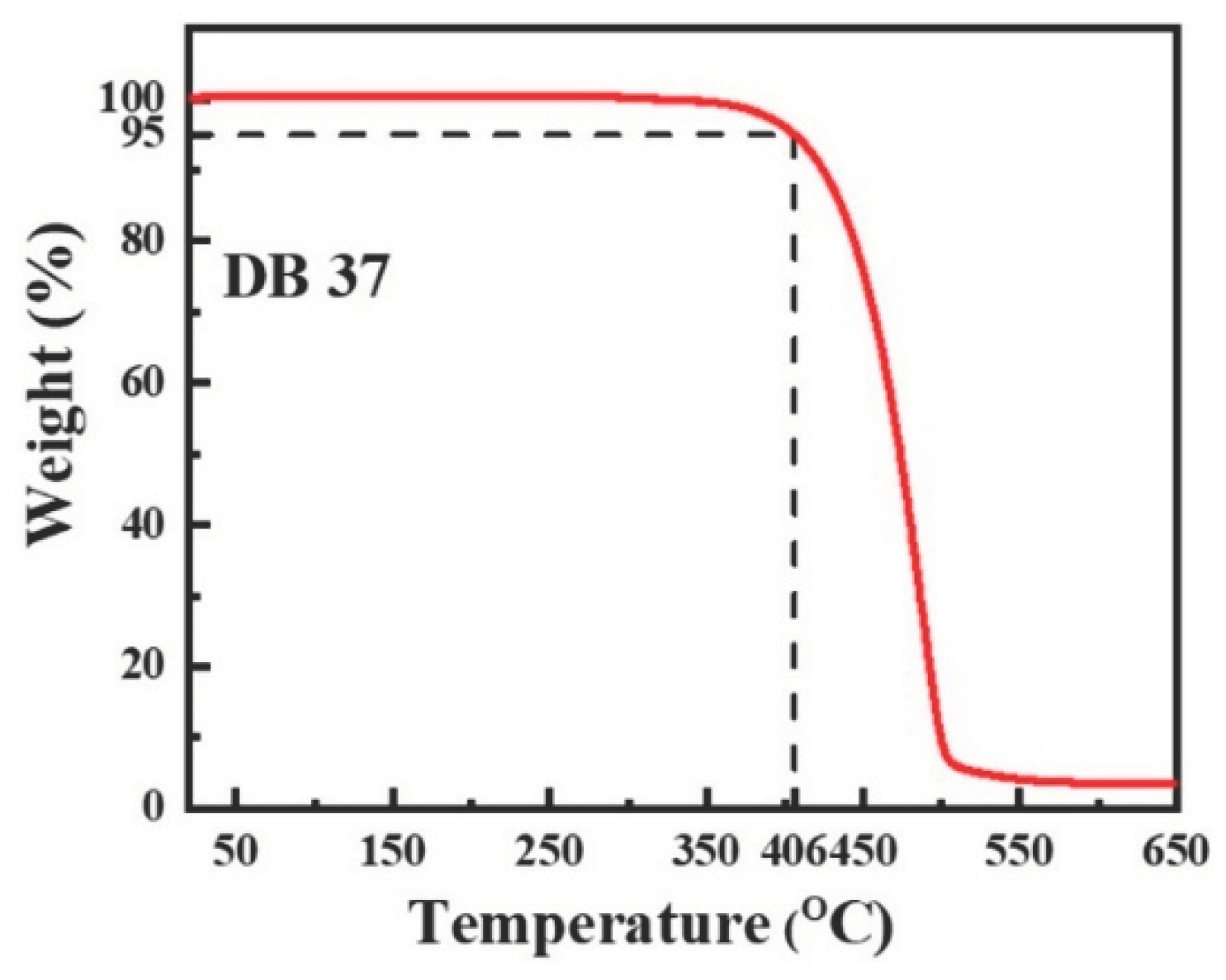
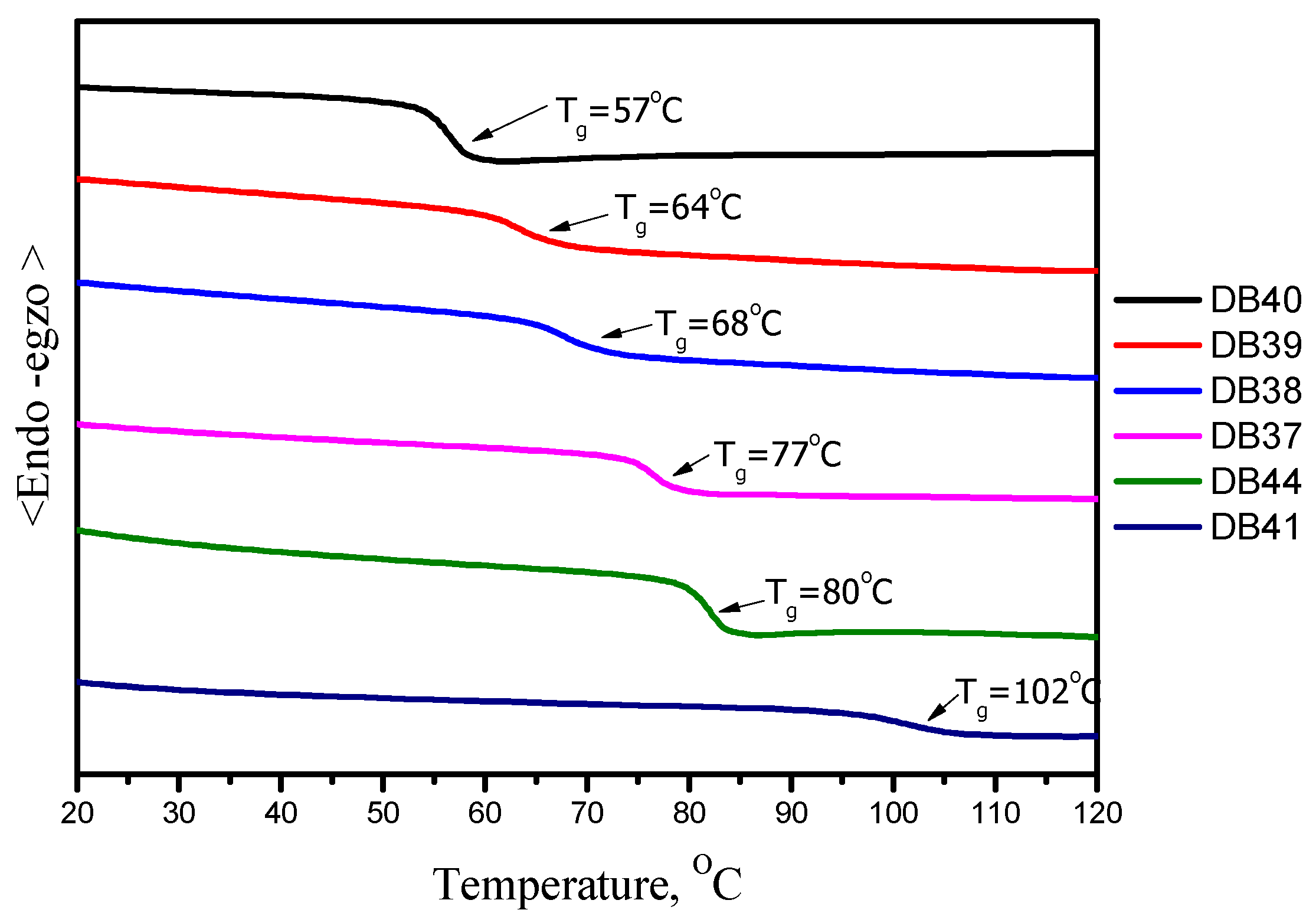
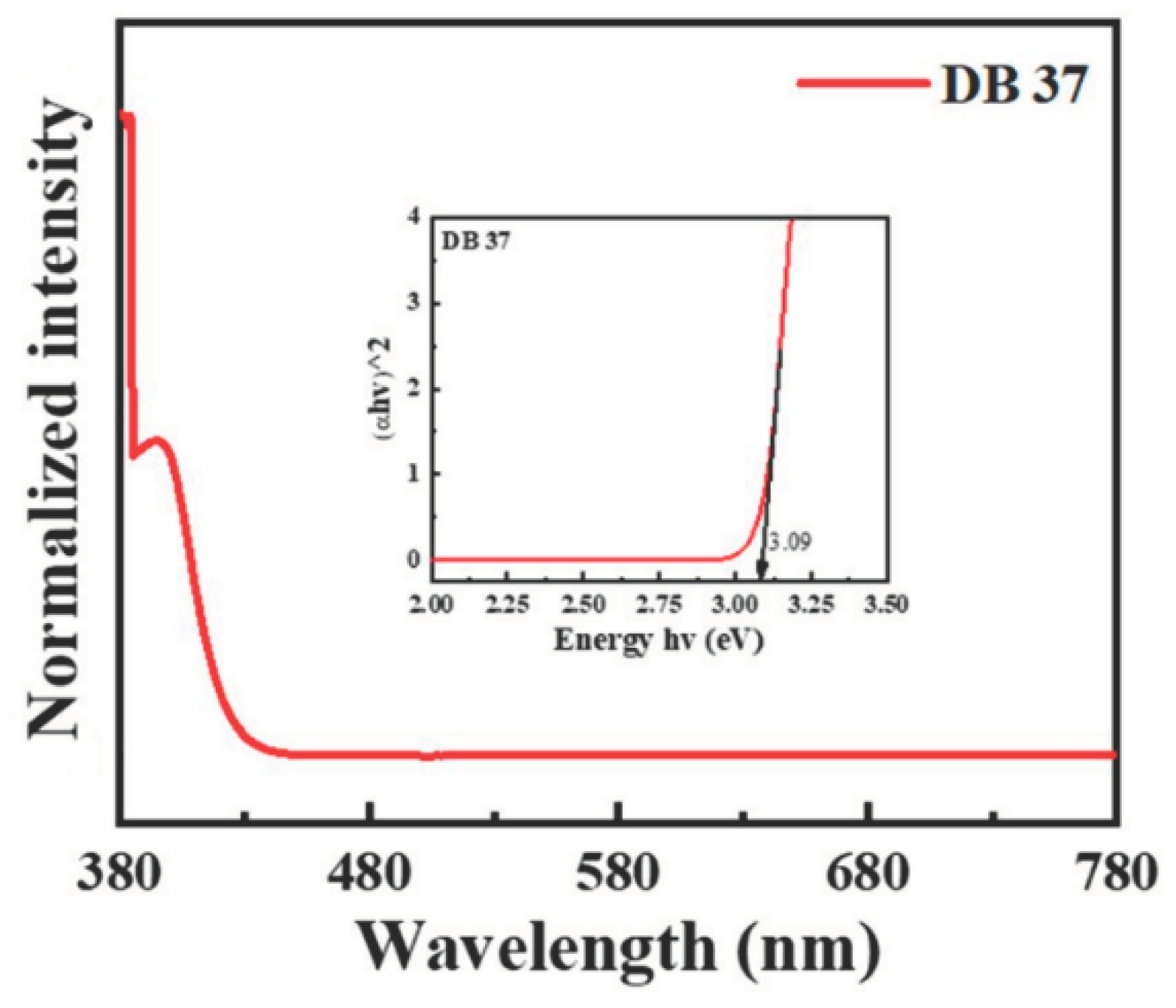
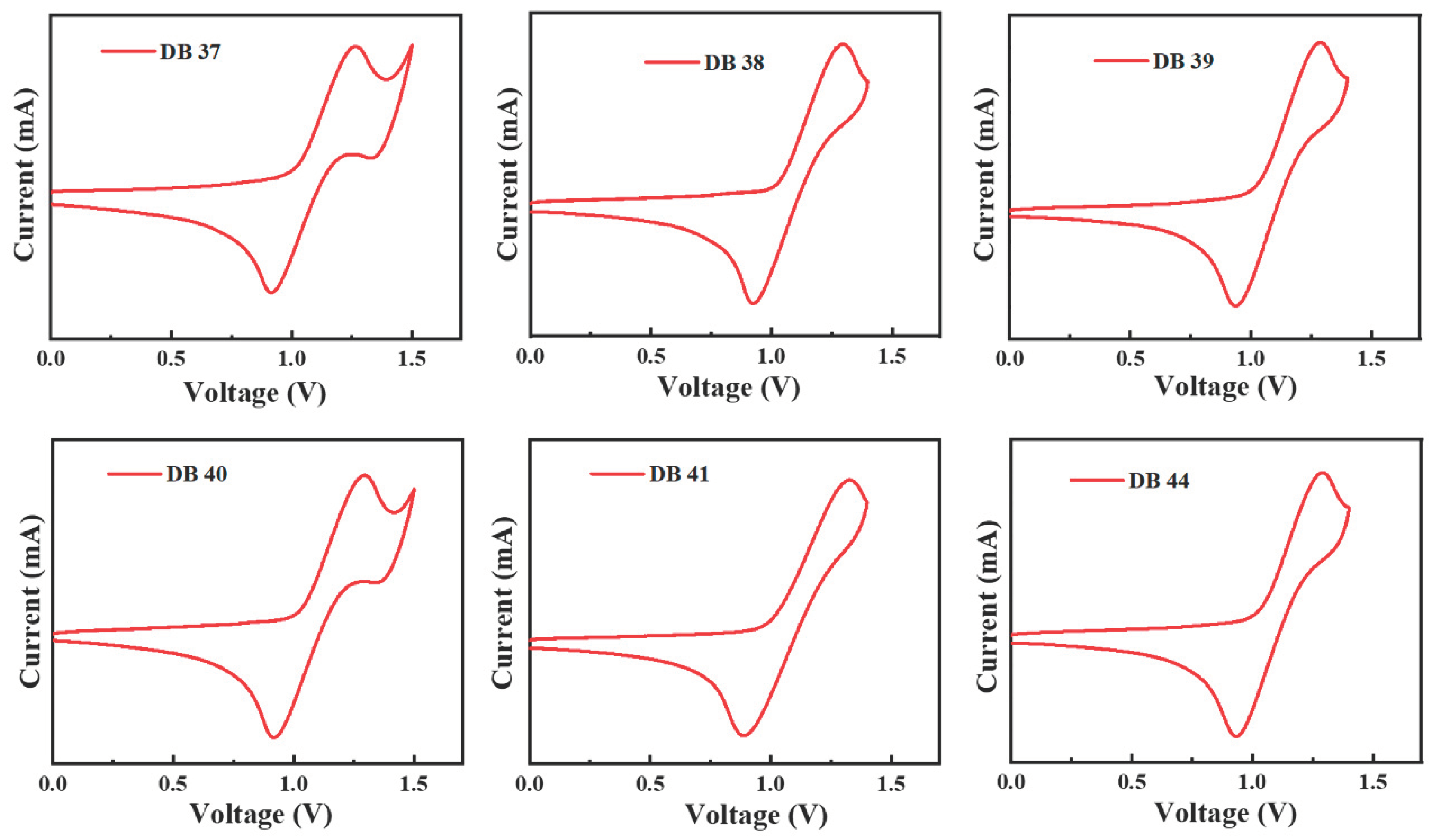
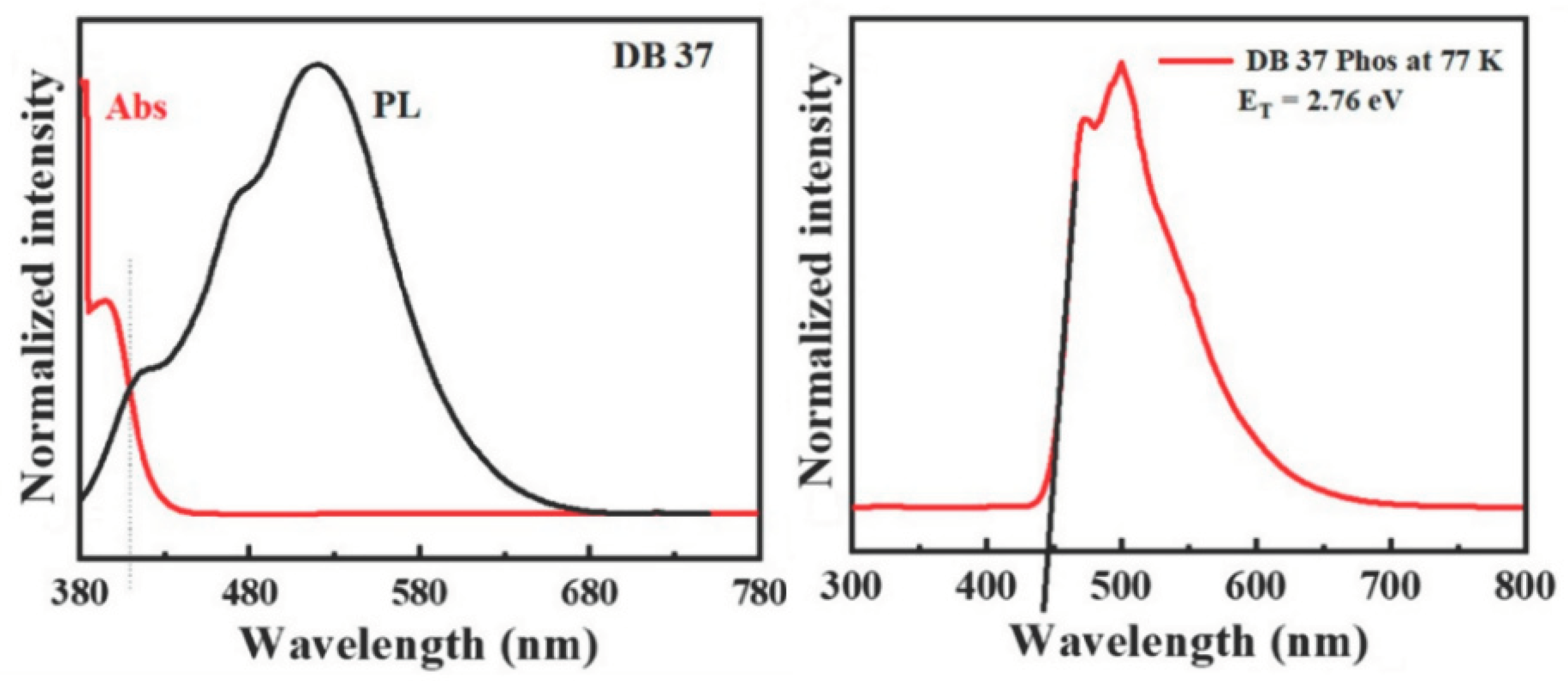
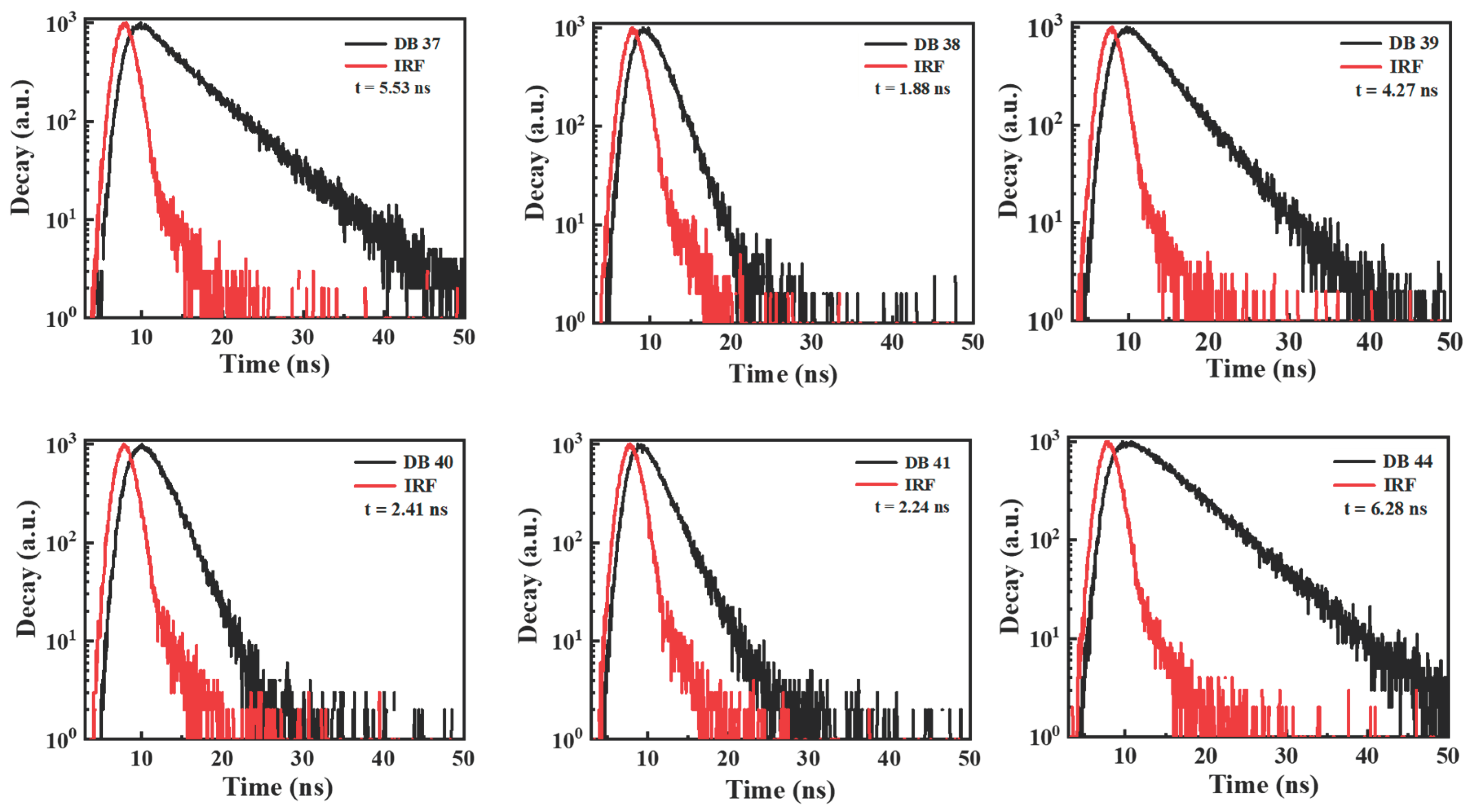
| Emitter | λem (nm) | λem (nm) | Φ (%) | Homo (eV) | Lumo (eV) | Eg (eV) | Decay (ns) | S1 (eV) | T1 (eV) | ΔEST | Td (oC) | Tg (oC) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB37 | 384.5, 395.6 | 509 | 65.5 | -5.67 | -2.58 | 3.09 | 5.53 | 3.04 | 2.76 | 0.28 | 406 | 102 |
| DB38 | 383.5, 400 | 510 | 45.3 | -5.70 | -2.61 | 3.09 | 1.88 | 2.94 | 2.89 | 0.05 | 398 | 80 |
| DB39 | 382.7, 400 | 528 | 75.5 | -5.68 | -2.60 | 3.08 | 4.27 | 3.10 | 2.81 | 0.29 | 383 | 77 |
| DB40 | 384.5, 408.2 | 513 | 52.5 | -5.69 | -2.59 | 3.10 | 2.41 | 3.06 | 2.80 | 0.26 | 397 | 68 |
| DB41 | 381.0, 399.7 | 528 | 62.5 | -5.73 | -2.64 | 3.09 | 2.24 | 3.22 | 2.80 | 0.42 | 374 | 64 |
| DB44 | 383.9, 398.6 | 529 | 68.5 | -5.69 | -2.62 | 3.07 | 6.28 | 3.18 | 2.82 | 0.15 | 389 | 57 |
| Emitter | Concentration (wt%) | Turn-on Voltage (Von)a | Power efficacy (lm/W) | Current efficacy (cd/A) | EQE (%) | CIExy | LMax (cd/m2) |
|---|---|---|---|---|---|---|---|
| @100 cd/m2/@1,000 cd/m2/max | @100cd/m2 / @1000cd/m2 | ||||||
| DB37 | 5.0 | 4.0 | 2.1/1.1/3.4 | 3.4/2.4/3.9 | 2.1/1.6/2.1 | (0.17, 0.22) / (0.17, 0.30) | 3449 |
| 10 | 3.5 | 2.5/1.3/3.4 | 3.5/2.5/3.8 | 1.8/1.5/1.9 | (0.18, 0.26) / (0.18, 0.23) | 3658 | |
| 15 | 3.4 | 2.8/1.5/3.6 | 3.7/2.7/4.0 | 1.7/1.5/1.8 | (0.19, 0.28) / (0.18, 0.25) | 3464 | |
| 100 | 3.1 | 0.3/ - / - | 0.3/ - / - | 0.1 / - / - | (0.24, 0.40) / - | 616 | |
| DB38 | 5.0 | 3.9 | 1.9/1.0/3.4 | 3.1/2.2/3.8 | 1.9/1.5/1.9 | (0.18, 0.22) / (0.17, 0.20) | 2801 |
| 10 | 3.5 | 2.7/1.4/2.9 | 3.7/2.7/3.8 | 2.0/1.6/1.9 | (0.18, 0.25) / (0.18, 0.22) | 3430 | |
| 15 | 3.4 | 2.8/1.5/3.5 | 3.6/2.8/4.2 | 1.7/1.6/1.8 | (0.19, 0.27) / (0.18, 0.24) | 3555 | |
| 100 | 3.2 | 0.2/ - / - | 0.3/ - / - | 0.1/ - / - | (0.22, 0.38) / - | 618 | |
| DB39 | 5.0 | 4.0 | 1.8/0.9/3.3 | 3.1/2.2/3.7 | 2.0/1.6/2.1 | (0.18, 0.20) / (0.17, 0.18) | 2818 |
| 10 | 3.5 | 2.5/1.3/4.4 | 3.5/2.7/4.9 | 2.0/1.8/2.2 | (0.18, 0.23) / (0.18, 0.21) | 3430 | |
| 15 | 3.9 | 3.0/1.4/4.1 | 4.4/2.8/5.7 | 2.2/1.6/2.7 | (0.19, 0.27) / (0.19, 0.24) | 3581 | |
| 100 | 3.4 | 0.3/ - / - | 0.4/ - / - | 0.4/ - / - | (0.24, 0.39) / - | 615 | |
| DB40 | 5.0 | 4.2 | 2.0/1.1/2.1 | 3.4/2.4/3.4 | 2.2/1.6/2.3 | (0.17, 0.22) / (0.17, 0.20) | 3166 |
| 10 | 3.5 | 2.8/1.5/2.9 | 3.8/2.8/3.7 | 2.0/1.7/2.0 | (0.18, 0.25) / (0.18, 0.22) | 3840 | |
| 15 | 3.3 | 2.8/1.6/2/8 | 3.6/2.8/3.6 | 1.8/1.6/1.8 | (0.18, 0.27) / (0.18, 0.24) | 3950 | |
| 100 | 3.2 | 0.2/ - / - | 0.3/ - / - | 0.1 / - / - | (0.22, 0.38) / - | 685 | |
| DB41 | 5.0 | 4.4 | 1.6/0.8/1.9 | 2.9/1.9/3.1 | 1.9/1.3/2.0 | (0.18, 0.21) / (0.18, 0.19) | 2687 |
| 10 | 3.8 | 2.4/1.1/2.4 | 3.6/2.5/3.6 | 2.0/1.5/2.0 | (0.19, 0.26) / (0.19, 0.23) | 3347 | |
| 15 | 3.5 | 2.6/1.3/2.7 | 3.6/2.5/3.6 | 1.7/1.1/1.8 | (0.20, 0.28) / (0.19, 0.24) | 3128 | |
| 100 | 3.1 | 0.2/ - / - | 0.3/ - / - | 0.1/ - / - | (0.30, 0.45) / - | 486 | |
| DB44 | 5.0 | 4.8 | 1.6/0.7/1.6 | 3.3/2.2/3.3 | 2.3/ - /2.3 | (0.17, 0.20) / - | 1718 |
| 10 | 4.0 | 2.1/0.8/2.7 | 3.9/2.3/4.1 | 2.1/1.4/2.3 | (0.18, 0.24) / (0.18, 0.22) | 1283 | |
| 15 | 3.8 | 2.1/0.5/2.3 | 3.6/1.5/3.7 | 1.9/ - /2.0 | (0.19, 0.26) / - | 1275 | |
| 100 | 5.1 | - / - / - | - / - / - | - / - / - | - / - | 55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





