1. Introduction
Polyglutamine (polyQ) diseases include nine neurodegenerative diseases with spinocerebellar ataxia type 3 (SCA3) being one of the most common [
1,
2]. SCA3 is caused by an abnormal expansion of CAG repeats in the coding region of the disease gene
ATXN3, generating elongated polyQ tracts in the ataxin-3 (ATXN3) protein [
2]. An important characteristic of SCA3 is the formation of protein aggregates in neurons of several brain regions [
3]. The expansion of the polyQ repeat causes misfolding of the affected protein ATXN3, resulting in polyQ-containing aggregates that are associated with disease progression [
3]. Affected brain regions of SCA3 are cerebellum, substantia nigra, striatum, thalamus, pontine nuclei and other areas of the central nervous system [
4]. PolyQ-containing aggregates do not exist in isolation but sequester other proteins such as chaperones and proteins of the ubiquitin-proteasome-system [
5]. Additionally, Sittler and colleagues identified aggregates as nuclear accumulations positive for ATXN3, ubiquitin and the autophagic protein p62/sequestosome-1 in
post-mortem brains of SCA3 patients [
6]. Moreover, further autophagic proteins like LC3, ATG5, ATG12 or ATG16L has been shown to be recruited into aggregates of polyQ proteins like ATXN3 [
7]. Autophagy, a process of cellular degradation and recycling, could play a significant role in clearance of protein aggregates in polyQ diseases [
8,
9]. However, it was shown that polyQ-expanded ATXN3 leads to an impairment of autophagy already in early disease stages of SCA3 [
10]. Previous studies in zebrafish, patient-derived induced pluripotent stem (iPSCs) cells and a transgenic mouse model indicated that an induction of autophagic activity being beneficial for disease progression of SCA3 [
10,
11,
12]. The ubiquitin-binding protein p62, also called sequestosome-1 (SQSTM1), normally functions as a receptor for selective autophagic degradation of ubiquitinated proteins [
9]. Inhibiting autophagy activity leads to accumulation of p62 inclusions [
9]. Since p62 interacts with the autophagic protein LC3, p62 itself is a substrate of autophagy whose levels seem to increase due to autophagy impairment [
9]. To date it is unclear whether aggregation of polyQ-containing proteins is a consequence of autophagy dysregulation or whether autophagy is enhanced as a defense mechanism in polyQ disorders [
9].
In the current study, the autophagy mechanism was studied in cell culture experiments using HEK-293T cells transfected with ATXN3 plasmids of different polyQ lengths, in a SCA3 304Q knock-in (KI) mouse model [
13], and in post-mortem human brain material of SCA3 patients. In cell culture experiments, autophagy was induced by inhibition of the main regulator mTOR using rapamycin treatment [
8]. Furthermore, proteasomal degradation was blocked with the specific inhibitor MG132 and to investigate degradation of the autophagic proteins cycloheximide treatment was performed [
14,
15]. RNA sequencing data from human post-mortem brain material of SCA3 patients and of SCA3 KI mice revealed differential RNA expression of autophagic genes including p62.
Here we show that the receptor for selective autophagy, p62, plays a significant role in aggregate formation developing in the course of SCA3. With SCA3 disease progression, aggregates containing only ATXN3 or p62, as well as co-localized aggregates comprising both proteins, were found. We concluded that, the increased aggregation of p62 and ATXN3 is thought to be caused by an increase of autophagy impairment.
2. Materials and Methods
2.1. Cell Culture and Transfection
HEK-293T cells (ATCC: CRL-3216) were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific, Waltham, USA) supplemented with 10 % fetal bovine serum (Roche Diagnostics GmbH, Mannheim, DE) and 1 % antibiotic-antimycotic (Gibco, Thermo Fisher Scientific) at 37 °C and 5 % CO2. Cells were passaged at 80 – 90 % confluence.
HEK-293T cells were transfected with plasmids encoding V5-tagged ATXN3 and different polyQ repeat length (V5ATXN3-15Q, V5ATXN3-77Q or V5ATXN3-148Q). Transfection was performed using the Attractene transfection reagent (Qiagen, Hilden, DE) following the standard transfection protocol. Briefly, 24 h before transfection, 400 000 cells per well were seeded on a six-well tissue culture plate in DMEM medium. Cells were transfected with 1.2 µg plasmid DNA and incubated 72 h at 37°C and 5 % CO2.
2.2. Pharmacological Treatments and Harvest of HEK-293T Cells
To induce autophagy in HEK-293T cells transfected with ATXN3 plasmids, cells were treated with 100 nM rapamycin for 6 h prior to harvesting. For inhibition of proteasomal degradation, cells were treated with 10 µM MG132 for 6 h prior to harvesting. HEK-293T cells transfected with ATXN3 and were treated with 10 µM cycloheximide for 0 h or 1 h, 5 h and 12 h prior to harvesting to inhibit protein translation. For all treatment conditions dimethyl sulfoxide (DMSO) was used as treatment control.
After incubation, treated HEK-293T cells were harvested using cold DPBS (Thermo Fisher Scientific). A cell pellet was generated by centrifuging the harvested cells at 350 g for 5 min. Cells were lysed in RIPA buffer (50 mM Tris pH 7.45; 150 mM NaCl; 0.1 % (w/v) SDS; 0.5 % (w/v) sodium deoxycholate; 1 % (v/v) Triton X-100;) supplemented with cOmplete protease inhibitor cocktail (Merck KGaA, Darmstadt, D) on ice for 30 min with vortexing every 10 min.
2.3. Sample Preparation for Western Blot, Filter Trap and DD-AGE Analysis
For Western Blot, Filter Trap and
Denaturing Detergent-Agarose Gel Electrophoresis (DD-AGE) analysis, protein extracts from cell culture experiments and animal tissue were used. Brain tissue samples (cerebellum) of 3- and 18-months old male and female wildtype (WT), heterozygous SCA3 (WT/304Q) and homozygous SCA3 (304Q/304Q) KI mice were obtained from a previous study [
13].
Prior to Western Blot analysis samples were mixed with 25 % (v/v) 4X LDS-buffer (2.5 M Tris pH 8.5; 50 % glycerol; 2.5 % phenol red; 2.1 mM EDTA; 294 mM LDS) and 100 mM DTT to a final protein concentration of 30 µg. Samples were heat denatured for 10 min at 70°C and 600 rpm in a thermoshaker.
In order to perform a Filter Trap analysis, samples were mixed with 1X DPBS, 2 % (w/v) SDS and 50 mM DTT to a final protein concentration of 1 µg and afterwards heated for 5 min at 95°C.
DD-AGE samples were mixed with 25 % (v/v) 4X DD-AGE buffer containing 2X TAE buffer (40 mM Tris; 20 mM acetic acid; 1mM EDTA 0.1 % SDS) and 50 % (v/v) glycerol, 8 % (w/v) SDS, and 0.01 g Orange G leading to a final protein concentration of 25 µg.
2.4. Western Blot Analysis
Western Blot analysis was performed according to standard procedures [
16]. Reduced proteins were separated according to their size by SDS-PAGE using a Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Hercules, CA, USA). For stacking gel 6 % (w/v) acrylamide/bis-acrylamide (29:1) and for separating gel 12 % (w/v) acrylamide/bis-acrylamide (29:1) was used. SDS-PAGE was performed using a 1X MOPS running buffer (50 mM MOPS, 50 mM Tris, 0.1% (w/v) SDS, 1 mM EDTA at 100 V and 250 mA.
For protein transfer, a nitrocellulose membrane with 0.2 µm pore size was used (Amersham Protran Premium, Cytiva, Marlborough, MA, USA). A wet transfer was performed using 1X Bicine/Bis-Tris transfer buffer (25 mM Bicine, 25 mM Bis-Tris, 1 mM EDTA) containing 15 % (v/v) methanol. Transfer was carried out at 80 V and 250 mA for 2 h.
After transferring the proteins, the membrane was blocked for 1 h using 5 % (w/v) milk powder in 1X TBST buffer (10 mM Tris pH 7.5; 150 mM NaCl; 0.1 % (v/v) Tween 20). For protein detection, the membrane was incubated with a primary antibody diluted in TBST buffer overnight at 4°C. Primary antibodies used for Western Blot detection are listed in
Table 1. Subsequently, the membrane was incubated with a fluorescence-coupled secondary antibody for 1 h at RT. Depending on the primary antibody species, IRDye 800CW-labelled goat anti-rabbit (926-32211) or IRDye 800CW-labelled goat anti-mouse (926-32210) secondary antibodies (both from LI-COR Biosciences, Lincoln, NE, USA) were used, diluted 1:5000 in 1X TBST buffer. Immunodetection was performed with the Odyssey FC instrument and Image Studio 4.0 software (both LI-COR Biosciences).
2.5. Filter Trap Analysis
Filter Trap analysis was performed according to standard protocols using the Minifold II Slot-Blot System (Schleicher & Schuell, Düren, DE) [
16]. Nitrocellulose membrane with 0.45 µm pore size (Amersham Protran, Cytiva) were used. The membrane was first equilibrated two times with equilibration buffer (1X DPBS; 0.1 % (w/v) SDS). Afterwards, the samples were loaded onto the membrane applying a vacuum. The membrane was washed twice with 1X DPBS. Blocking and immunodetection were performed according to Western Blot analysis. As a primary antibody against ATXN3, Ataxin-3 Monoclonal Antibody (clone 2SCA-1H9) was used (MA3-082, Thermo Fisher Scientific) with a dilution of 1:2500 in 1X TBST. For fluorescence detection the IRDye 800CW-labbeled goat anti-mouse (926-32210) secondary antibody (LI-COR Biosciences) with a 1:5000 dilution in 1X TBST was used. Immunodetection was performed with the Odyssey FC instrument and Image Studio 4.0 software (both LI-COR Biosciences).
2.6. DD-AGE Analysis
The DD-AGE analysis was performed to detect p62- and ATXN3-aggregates according to the SDD-AGE protocol of Halfmann [
17]. Modifications of the protocol are mentioned below.
First, an agarose gel with 1 % (w/v) agarose in 50 mL 1X TAE buffer (40 mM Tris; 20 mM acetic acid; 1 mM EDTA) and 0.1 % (w/v) SDS was poured in a PerfectBlueTM Gel System Mini S from Peqlab. A 1X TAE running buffer was used containing 0.1 % (w/v) SDS. Gel electrophoresis was performed at 40 V for approximately 1.5 h until the running front migrated 4 cm. Transfer of the proteins onto the nitrocellulose membrane was performed according to Western Blot analysis except for the Bicine/Bis-Tris transfer buffer containing 10 % methanol. Blocking of the membrane and immune detection was identical to Western Blot analysis. For p62 detection, the primary antibody 5114S (Cell Signaling Technology) was used in a 1:1000 dilution in 1X TBST. ATXN3 was detected with the Ataxin-3 Monoclonal Antibody (clone 2SCA-1H9, MA3-082, Thermo Fisher Scientific) diluted 1:2500 in TBST.
2.7. Immunohistochemistry Staining
Immunohistochemistry (IHC) staining was performed to stain the autophagic proteins p62 and BECN1 as well as the SCA3 disease protein ATXN3 in brain slices of wildtype (WT/WT) and homozygous 304Q-KI (304Q/304Q) mice. First, brain slices were deparaffinized and washed 3-times for 5 min with 1X PBS. Then, a 3 min microwave treatment for epitope retrieval was performed using 1X citric acid and 1X sodium citrate. Brain slices were washed again 3-times with 1X PBS for 5 min each. Afterwards, brain slices were blocked with 5 % (v/v) normal goat serum and 0.3 % (v/v) Triton X-100 in 1X PBS (770 mM NaCl; 319 mM NaH₂PO₄ * H₂O; 99.7 mM Na₂HPO₄ * 2 H₂O) and washed with 1X PBS prior to immunostaining. Primary antibodies were diluted in 1X PBS with 15 % (v/v) normal goat serum and incubated overnight at 4°C in a humidity chamber on the slices. Primary antibodies used for staining are listed in
Table 2. Brain slices were washed 3-times with 1X PBS for 5 min each. Secondary antibodies were also diluted in 1X PBS with 15 % (v/v) normal goat serum and incubated for 1 h at RT. Depending on the species, the goat-anti-rabbit IgG (H+L) diluted 1:250 or the goat-anti-mouse IgG (H+L) diluted 1:200 (both from Vector Laboratories) was used. Staining was carried out incubating an Avidin–Biotin Complex (ABC) (Vectastain ABC KIT, Elite PK-6100 Standard; Vector Laboratories) for 2 h on the brain slices and using 3,3'-Diaminobenzidine (Sigma-Aldrich) staining. The reaction was stopped by adding water. Brain slices were dehydrated after staining and sealed with the Leica CV ultra-mounting medium (Leica Biosystems, Nussloch, DE), coverslips and nail polish.
2.8. Immunofluorescence Staining
Immunofluorescence (IF) staining was performed to stain the autophagic protein p62 and ATXN3 on brain slices of WT/WT and 304Q/304Q mice. Deparaffinization, microwave treatment and blocking were identical with IHC staining. After blocking, the brain slices were washed 3-times for 10 min with 1X PBS. Primary antibody against ATXN3 (clone 1H9, MAB5360, Merck) was diluted 1:500 and mixed with an antibody against p62 (5114S, Cell Signaling Technology) that was diluted 1:250 in 1X PBS and 3 % normal goat serum. Primary antibodies were incubated overnight at 4°C in a humid chamber. Afterwards brain slices were washed 3-times for 10 min each with 1X PBS. Brain slices were incubated with fluorescent secondary antibodies in the following order: goat-anti-mouse-Alexa488 antibody (ab150077, Abcam) was added followed by the goat-anti-rabbit-Alexa555 (A-10667, Thermo Fisher Scientific), both diluted 1:500 in 1X PBS with 3 % (v/v) normal goat serum and each incubated for 1 h at RT. The brain slices were sealed using VECTASHIELD® Antifade Mounting Medium (Vector Laboratories) containing DAPI to stain cell nuclei, coverslips, and nail polish. Stained brain slices were stored at 4°C until microscopy.
2.9. Microscopy
Images of the stained brain slices were taken with the Axioplan 2 Imaging fluorescence microscope containing an Apotome, a Plan-Neofluar 20 x /0.50 objective and the AxioXam MRm camera (Zeiss, Oberkochen, DE). Software used was the AxioVision SE64 Rel. 4.9.1 (Zeiss).
2.10. RNA Sequencing of Human and Mouse Cerebellar RNA
RNA isolation, RNA sequencing protocol and bioinformatic analyses of RNA sequencing were already described in Haas et al., 2021. SCA3 mouse raw sequencing files are available through GEO under accession number: GSE145613. Human RNA-seq data set has been deposited at the European Genome-phenome Archive (EGA) under the accession number: EGAS00001004241.
2.11. Statistical Analysis
Quantification of Western Blot, Filter Trap and DD-AGE results was performed with the LI-COR Image Studio program (Image Studio Lite Ver 5.2; Image Studio Ver 2.1; LI-COR® Odyssey® Fc Imaging System).
Statistical analysis was performed with the GraphPad Prism software 6 using one-way ANOVA, Student’s t-test or Tukey´s multiple comparison test. The data are presented as bar charts showing means ± standard error of the mean (SEM). Statistical significance was indicated by asterisks representing p-values ≤ 0.05 (*), ≤ 0.01 (**), and ≤ 0.001 (***).
4. Discussion
SCA3 is a disease where protein aggregation plays an important role in disease progression, caused by the expansion of polyQ repeats in the mutated protein ATXN3 [
3]. The polyQ-expanded protein forms inclusion that sequester a variety of other proteins [
5,
6,
7]. An important protein found in these aggregates is the receptor for selective autophagic degradation of ubiquitinated proteins p62/ sequestosome-1 [
6,
9]. In this study we demonstrate decreasing p62 expression in the 304Q-KI mouse model of SCA3 with disease progression, which is consisting with previous studies [
10,
28]. This reduced soluble p62 protein expression is probably caused by aggregation of the soluble p62 protein in inclusions. Analysis of
port-mortem brains by Sittler et al. revealed an accumulation of p62-positive aggregates together with misfolded ATXN3 and ubiquitin [
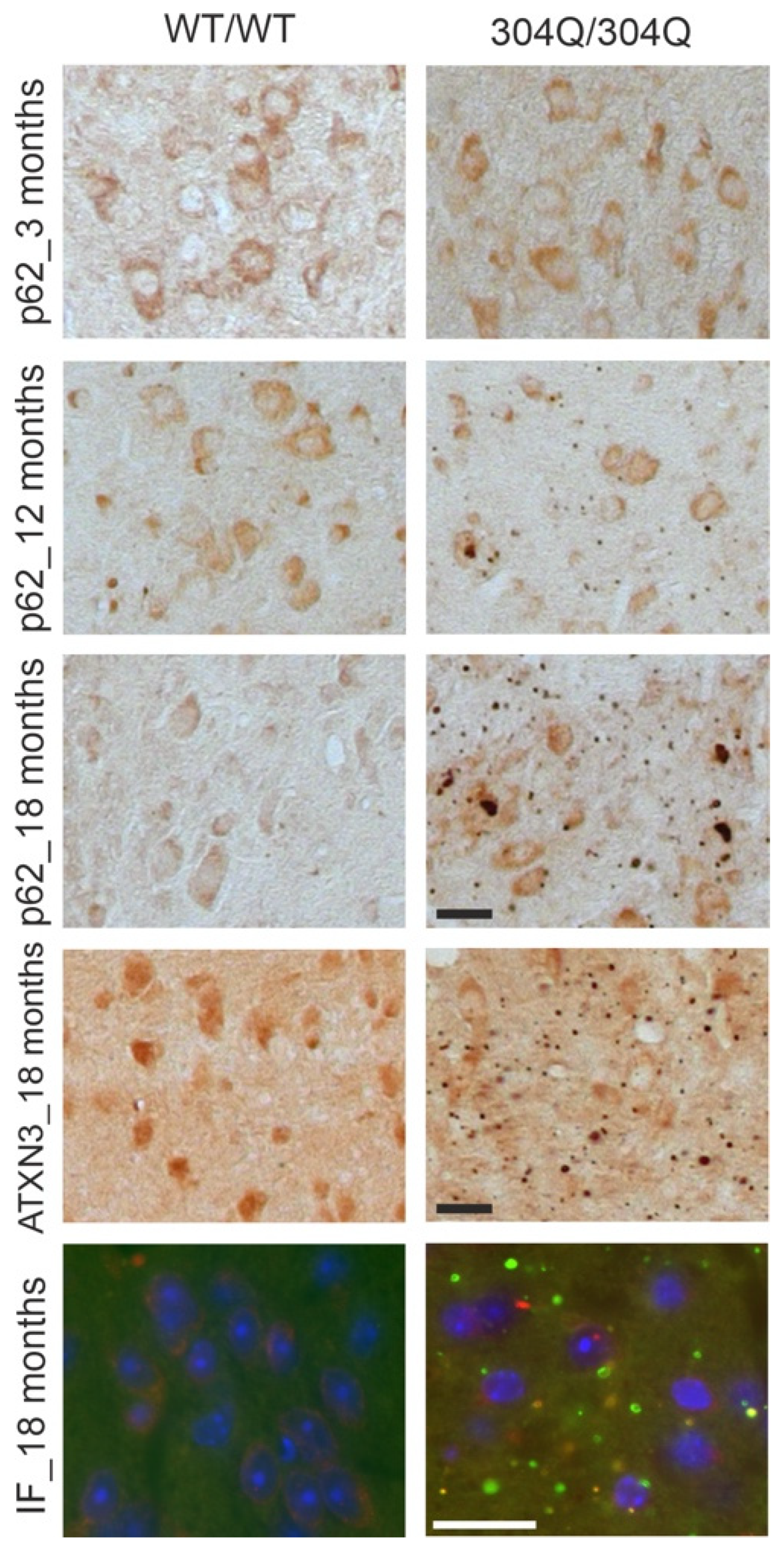
6]. In our study, the strongest co-aggregation of p62 proteins with ATXN3 was observed in homozygous SCA3 mice, which was not present in WT animals. Interestingly, homozygous SCA3 mice showed strong aggregation with aggregates positive for both ATXN3 and p62 or only for one of the proteins, indicating a possible co-localization for some extend of ATXN3 and p62. The interaction and co-aggregation of p62 with disease protein ATXN3 was previously shown in other studies, indicating a role of p62 in aggregate formation in SCA3 disease [
30,
31]. Aggregation of ATXN3 and p62 was not only shown for the 304Q-KI mouse model but also in HEK-293T cells transfected with ATXN3 plasmids and treated with cycloheximide, a drug which inhibits the eukaryotic cytosolic translation. HEK-293T cells transfected with ATXN3 plasmids with expanded polyQ tracts (77Q, 148Q) demonstrated aggregates positive for both ATXN3 and p62, validating the ATXN3 and p62 co-localization.
Autophagy is a well-regulated physiological process which is involved in several important cellular processes including development, metabolism, immunity, neuronal health, and tumorigenesis [
18]. Transcriptional regulation of autophagy is linked to the central modulator of autophagy, TOR by controlling the nuclear localization of nutrient-regulated transcriptions factors [
32,
33]. An increase in mRNA levels of certain ATG genes and p62/SQSTM1 regulated by different transcription factors is demonstrated to induce or suppress autophagy [
34]. In this study, RNA sequencing results of SCA3 human and mouse cerebella of aged animals revealed selective dysregulation of autophagic genes. Importantly, the most striking effects were observed in aged controls and aged SCA3 mouse cerebella, underlining the already described mRNA dysregulation of autophagic genes in neurodegenerative diseases and aging [
35,
36]. In 12-month-old SCA3 KI mice only ATG12, an ubiquitin-like protein which get conjugated to ATG5 (ATG5-ATG12 complex important for extension and closure of autophagosome [
37]) and the selective autophagy receptor p62/SQSTM1 were significantly dysregulated compared to controls. For p62/SQSTM1 it is known that several of its protein domains including Phox and Bem1 (PB1) and ubiquitin-associated domain (UBA) are involved in protein-protein interaction and may play a critical role in formation of autophagosomes and cytoplasmic accumulations [
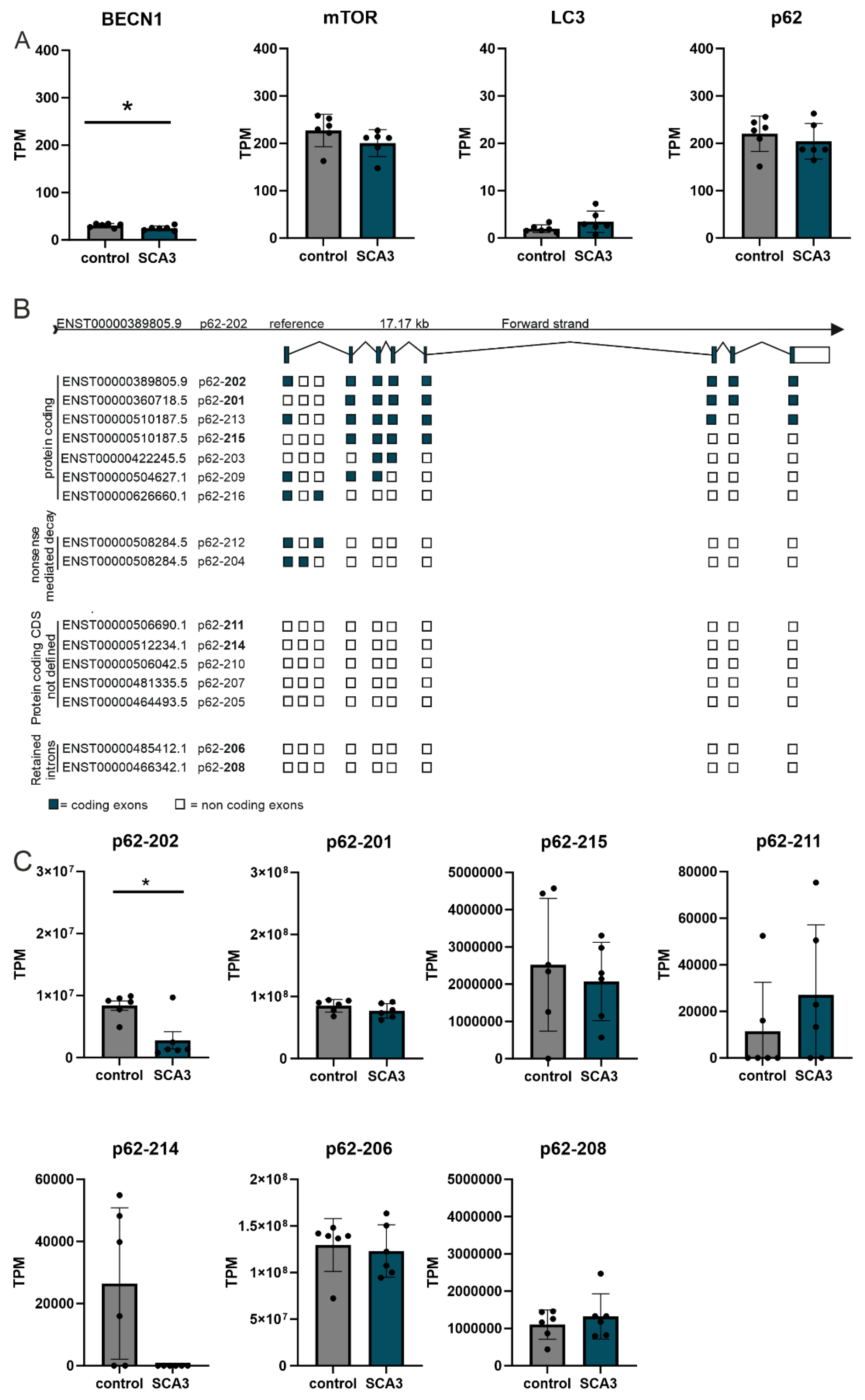
23,
24]. Currently, up to 16 p62 isoforms are annotated (Ensembl, last visit 11.01.2024). The two best characterized isoforms, reference p62-202 (ENST00000389805) and p62-201 (ENST00000360718), are differing at the 5´-UTR. Isoform p62-201 (ENST00000360718) employs an alternative start codon corresponding to amino acid position 85, resulting in a truncated mRNA and protein lacking large parts of the PB1 domain [
23]. Isoform expression analyses in SCA3 human
post-mortem RNA sequencing datasets revealed a significant down regulation of the p62 reference isoform p62-202 in SCA3 but not of the truncated isoform p62-201. Earlier studies demonstrated that the PB1 domain of p62 is important for the binding of several interacting partners and essential for the polymerization scaffold of p62 and aggresome formation [
23,
24]. Interestingly, studies revealed differences in the behavior of both p62 isoforms and demonstrated that the truncated isoform p62-201 forms larger aggregates compared to reference isoform p62-202 [
23]. The finding that in human SCA3
post-mortem datasets the reference isoform p62-202 is lower expressed may explain the very large p62-positive aggregates in the SCA3 KI mouse.
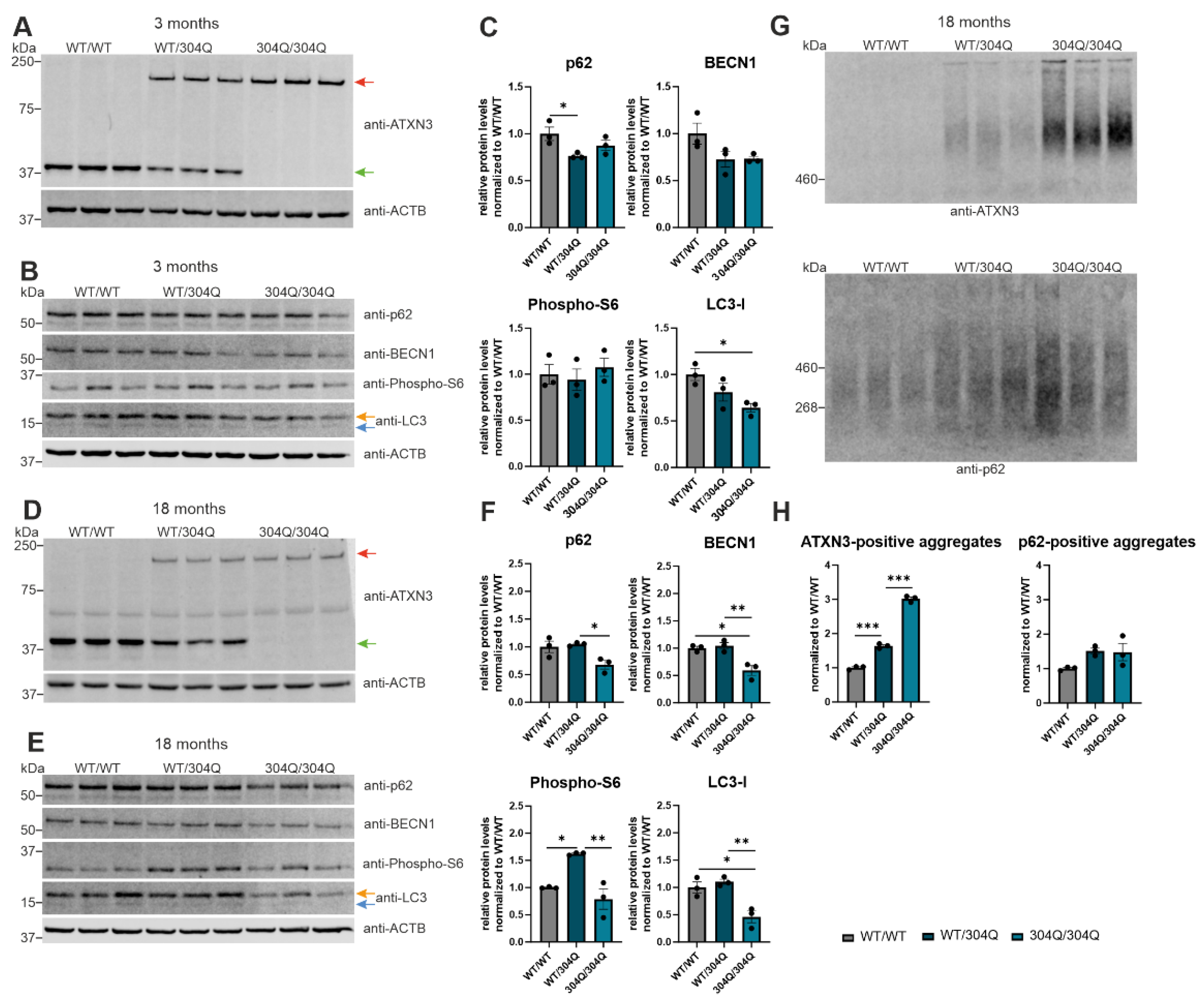
In addition to protein aggregation, autophagy impairment plays a major role in the development of SCA3. For instance, Watchon et al. demonstrated decreased levels of LC3-II, a marker for autophagosomes and autolysosomes, in cells expressing ATXN3 with expanded polyQ tract [
10,
27]. Our current study confirms autophagy impairment with increasing age of 304Q-KI mice. The previously discussed RNA sequencing data of mouse cerebellar RNA demonstrated strong dysregulation of autophagic genes (BECN1, p62, mTOR, LC3) comparing young and old SCA3 KI mice. Additionally, the protein expression of LC3-I which is responsible for the autophagosome formation in autophagy showed decreased levels in homozygous 304Q-KI mice. In 18-months old animals, BECN1 protein expression was significantly reduced with increasing length of ATXN3 polyQ tract. This BECN1 reduction is possibly caused by an aberrant interaction with the polyQ-expanded ATXN3 protein which has been reported in a previous study [
38].
Since autophagy is impaired during SCA3, activation of autophagy and reduction of aggregate formation was shown to ameliorate SCA3 disease progression [
11,
39,
40]. In the current study, autophagy was activated with the inhibitor of mTOR, rapamycin. Rapamycin treatment of ATXN3 transfected HEK-293T cells showed a significant reduction of soluble p62. These results suggest a successful autophagic activation, since p62 itself is an autophagy substrate [
9]. Additionally, this was confirmed by the increased LC3-II and reduced phospho-S6 expression due to rapamycin treatment. LC3-II activation indicates formation of autophagosomes whereas inhibiting mTOR also leads to blocking protein S6 phosphorylation [
26,
27]. Since no significant effects were seen regarding BECN1 expression, autophagy in SCA3 is regulated in a mTOR-dependent manner. Unfortunately, no reduction of protein aggregation was achieved in ATXN3 HEK-293T cells using rapamycin treatment. Previous studies of Ravikumar et al. showed that autophagy induction due to rapamycin treatment reduced aggregate formation and cell death in a Huntington’s Disease (HD) cell model [
41]. However, rapamycin treatment did only reduce protein aggregation 9 h after transfection of the cells [
41]. Rapamycin treatment at later time point showed no significant reduction in aggregation or cell death in the HD cell model [
41]. Since in the current study treatment with rapamycin was performed 72 h after transfection of HEK-293T cells, an earlier time point should be tested for reducing protein aggregates.
Due to the detected autophagic impairment in 304Q-KI mice as well as ATXN3 HEK-293T cells transfected with Atxn3-15Q, Atxn3-77Q or Atxn3-148Q, degradation of the autophagic proteins was examined using CHX. ATXN3 as well as p62 expression was significantly lower in ATXN3 148Q HEK-239T cells treated with CHX compared to ATXN3 15Q or 77Q HEK-293T cells. This could be caused by a faster degradation of ATXN3 and p62 in cells transfected with longer ATXN3 polyQ tracts. However, DD-AGE analysis demonstrated an increased aggregation of ATXN3 in HEK-293T cells transfected with longer ATXN3 polyQ tracts (77Q, 148Q). Therefore, CHX treatment leads to an increased aggregation of ATXN3 and possibly of p62. CHX treatment of ATXN3 HEK-293T cells transfected with 15Q, 77Q or 148Q increased phospho-S6 levels compared to untreated ATXN3 HEK-293T cells of all genotypes. Since increased phospho-S6 indicates an activation of mTOR, CHX treatment led to an inhibition of autophagy in ATXN3 HEK-293T cells transfected with 15Q, 77Q or 148Q, which is consistent with former studies [
42,
43]. A study of Dang et al. where the impact of CHX on autophagic pathways was investigated, demonstrated that CHX-mediated inhibition of protein synthesis leads to a problem in autophagosome-lysosome fusion [
43]. Therefore, treating cells with CHX triggers an autophagic impairment, which explains the increased aggregation of the disease protein ATXN3 and the possible aggregation of p62.
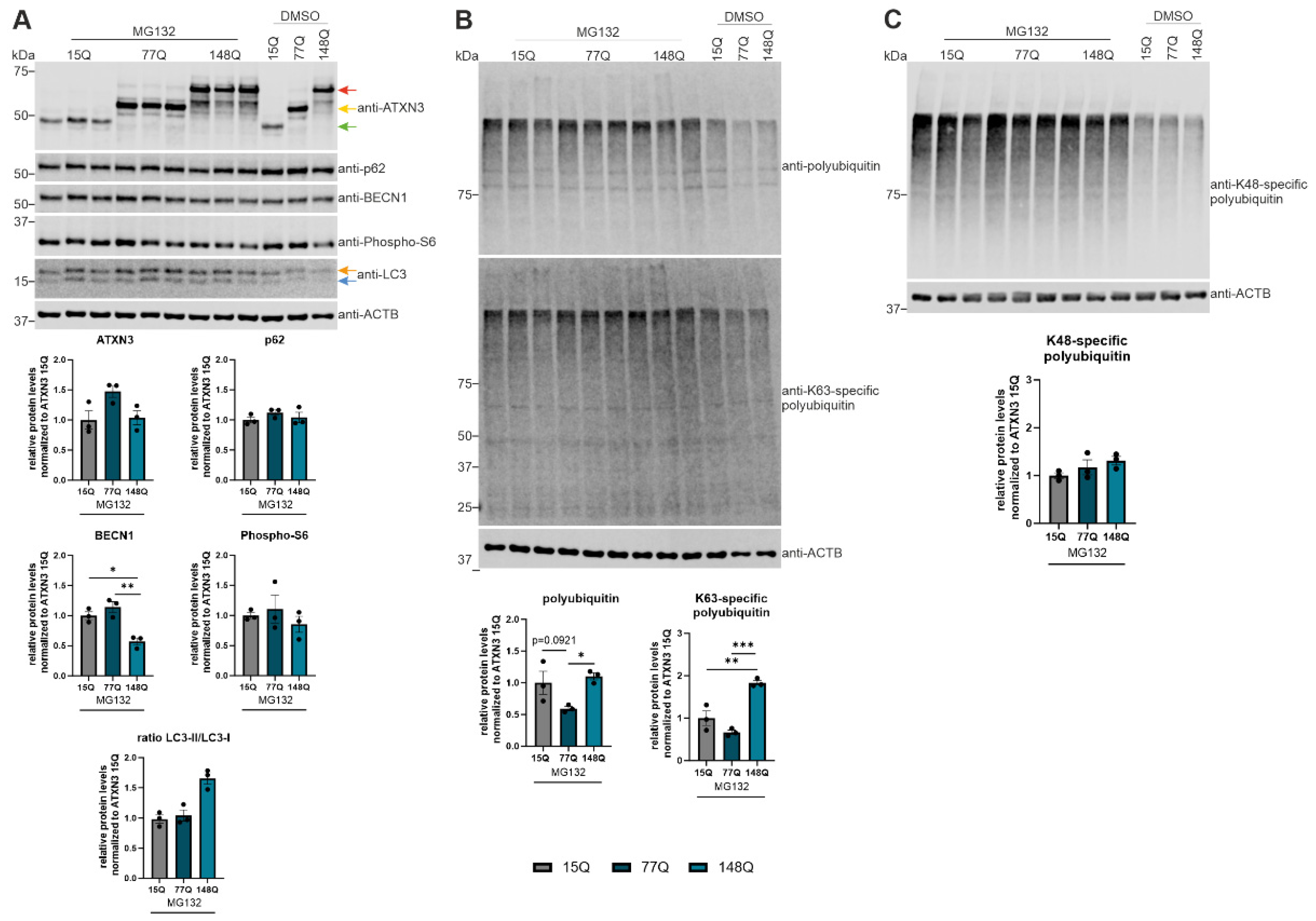
For further investigation of the autophagy pathway in ATXN3 HEK-293T cells with different repeat length (15Q, 77Q, 148Q), MG132 was used to block the proteasomal degradation. Expression of p62 was reduced in all genotypes according to MG132 treatment compared to control cells (DMSO treatment), which indicates an activation of autophagy. Since phospho-S6 showed no significant decrease in MG132-treated ATXN3 HEK-293T cells (15Q, 77Q, 148Q) and BECN1 expression increased due to treatment with MG132 in ATXN3 HEK-293T cells transfected with 15Q or 77Q, blocking the proteasome activates BECN1-dependent autophagy. These observations are confirmed by two studies where inhibiting the proteasomal degradation led to an autophagy activation via BECN1 [
44,
45]. However, BECN1 activation after MG132 was not found in ATXN3 148Q cells, suggesting no BECN1-dependent autophagic activation in HEK-293T cells transfected with very long ATXN3 polyQ tracts. This supports the assumption of strong autophagic impairment according to longer polyQ tracts in the ATXN3 protein.
An inhibition of the proteasomal degradation with MG132 led to increased levels of K48-specific and K63-specific polyubiquitination in ATXN3 HEK-293T cells of all genotypes compared to DMSO controls. Therefore, we detected an elevated autophagic degradation (K63) and proteasomal degradation (K48) in ATXN3 15Q, 77Q or 148Q HEK-293T cells. The different ATXN3 polyQ lengths showed no impact on K48-polyubiquitination and thereby on proteasomal degradation. However, ATXN3 148Q HEK-293T cells treated with MG132 showed significantly higher amounts of K63-polyubiquitin chains compared to ATXN3 15Q and 77Q HEK-293T cells. Since the deubiquitinase protein ATXN3 preferentially cleaves K63-linked polyubiquitin chains, increased levels of K63 chains in ATXN3 148Q HEK-392T cells indicate impaired binding of mutant ATXN3 protein to polyubiquitin chains [
46].
The present study demonstrates a role of the receptor for selective autophagy, p62, in aggregate formation seen in the neurodegenerative disease SCA3. Investigation of the SCA3 304Q-KI mouse model as well as cell culture experiments with overexpression of ATXN3 plasmids with different polyQ lengths (15Q, 77Q, 148Q) confirmed a co-localized aggregation of p62 and ATXN3 with increasing polyQ length of ATXN3. The overserved elevated aggregation of the autophagic protein p62 or the disease protein ATXN3 is probably caused by the increase of autophagy impairment triggered by disease progression of SCA3 304Q-KI mice or by elevated polyQ tract length in ATXN3 plasmids.
Author Contributions
Conceptualization, J.H-S, O.R and A.J.Z.; methodology, A.J.Z., C.B., J.M.S, J.J.W; software, A.J.Z, C.B., J.M.S; validation, A.J.Z., J.H-S. and O.R.; formal analysis, J.H-S.; investigation, A.J.Z.; resources, J.H-S.; data curation, A.J.Z.; writing—original draft preparation, A.J.Z.; writing—review and editing, O.R., J.J.W, J.H-S; visualization, A.J.Z.; supervision, O.R., J-H-S; project administration, J.H-S.; funding acquisition, J.H-S. All authors have read and agreed to the published version of the manuscript.
Figure 2.
Activation of autophagy with disease progression in SCA3 mice. (A-E) Protein levels of ATXN3, p62, BECN1, phospho-S6 and LC3 were measured in 3- and 18-months old 304Q-KI mice. Beta-Actin (ACTB) is shown as loading control. All expression levels were normalized to WT/WT levels. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents endogenous ATXN3, while red arrow represents expanded ATXN3. Orange arrow symbolizes LC3-I while blue arrow label LC3-II. (G) Aggregation of ATXN3 and p62 were analyzed in 18 months old 304Q-KI mice using DD-AGE. All aggregation values were normalized to WT/WT. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001 by one-way ANOVA. Values are shown as mean +/− SEM. N = 3 for each experiment, mixed sex of 304Q-KI mice.
Figure 2.
Activation of autophagy with disease progression in SCA3 mice. (A-E) Protein levels of ATXN3, p62, BECN1, phospho-S6 and LC3 were measured in 3- and 18-months old 304Q-KI mice. Beta-Actin (ACTB) is shown as loading control. All expression levels were normalized to WT/WT levels. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents endogenous ATXN3, while red arrow represents expanded ATXN3. Orange arrow symbolizes LC3-I while blue arrow label LC3-II. (G) Aggregation of ATXN3 and p62 were analyzed in 18 months old 304Q-KI mice using DD-AGE. All aggregation values were normalized to WT/WT. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001 by one-way ANOVA. Values are shown as mean +/− SEM. N = 3 for each experiment, mixed sex of 304Q-KI mice.
Figure 3.
Co-localization of ATXN3- and p62-positive accumulations in the pons of SCA3 mice. First three rows show IHC staining of 3- and 18-months old WT and 304Q/304Q SCA3 mice pons sections. Staining was performed with ATXN3 or p62- specific antibodies. Last row represents IF staining of 18 months old SCA3 and WT/WT mice pons sections labeled with antibodies against ATXN3 and p62. Green = ATXN3, red = p62, orange = ATXN3 and p62-double-positive aggregates. Scale bar indicates 20 µm.
Figure 3.
Co-localization of ATXN3- and p62-positive accumulations in the pons of SCA3 mice. First three rows show IHC staining of 3- and 18-months old WT and 304Q/304Q SCA3 mice pons sections. Staining was performed with ATXN3 or p62- specific antibodies. Last row represents IF staining of 18 months old SCA3 and WT/WT mice pons sections labeled with antibodies against ATXN3 and p62. Green = ATXN3, red = p62, orange = ATXN3 and p62-double-positive aggregates. Scale bar indicates 20 µm.
Figure 4.
Rapamycin activated autophagy in ATXN3 expressing HEK-293T cells did not reduce ATXN3 aggregation. (A, B) Protein levels of ATXN3, BECN1, p62, phospho-S6 and LC3 were measured in HEK-293T cells transfected with ATXN3 (15Q, 77Q, 148Q) and treated with rapamycin and compared to untreated controls (DMSO). Beta-Actin (ACTB) is shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow label LC3-II. (C) Aggregation of ATXN3 was investigated in rapamycin treated ATXN3 transfected HEK-293T cells compared to untreated cells (DMSO) using filter trap analysis. Amount of ATXN3-positive aggregates was normalized to the respective ATXN3 15Q expression values. Statistical analysis was performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05 by one-way ANOVA. Values are shown as mean +/− SEM.
Figure 4.
Rapamycin activated autophagy in ATXN3 expressing HEK-293T cells did not reduce ATXN3 aggregation. (A, B) Protein levels of ATXN3, BECN1, p62, phospho-S6 and LC3 were measured in HEK-293T cells transfected with ATXN3 (15Q, 77Q, 148Q) and treated with rapamycin and compared to untreated controls (DMSO). Beta-Actin (ACTB) is shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow label LC3-II. (C) Aggregation of ATXN3 was investigated in rapamycin treated ATXN3 transfected HEK-293T cells compared to untreated cells (DMSO) using filter trap analysis. Amount of ATXN3-positive aggregates was normalized to the respective ATXN3 15Q expression values. Statistical analysis was performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05 by one-way ANOVA. Values are shown as mean +/− SEM.
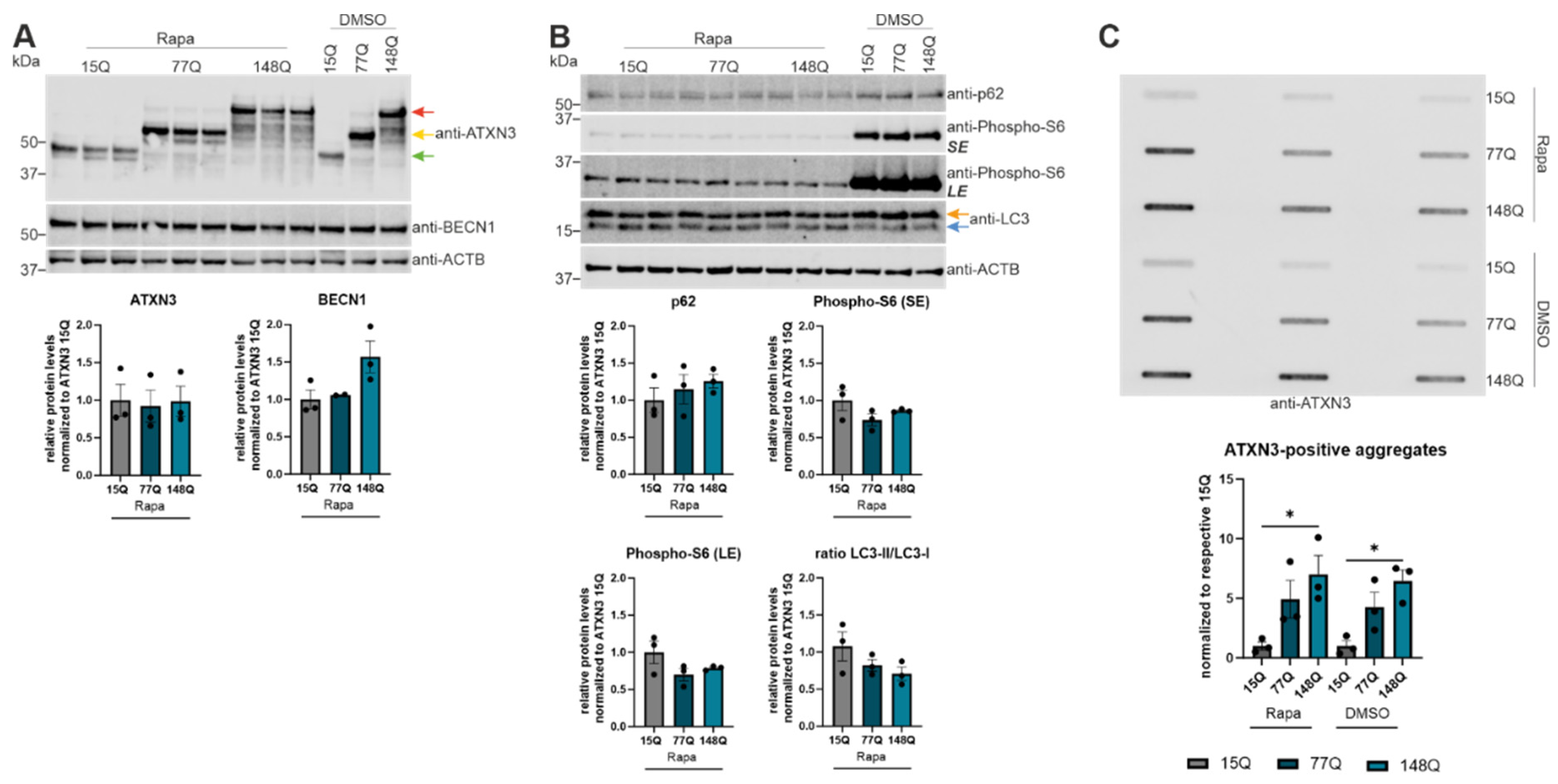
Figure 5.
Increased aggregation of ATXN3 according to cycloheximide (CHX) treatment. (A, B) Protein levels of ATXN3, phospho-S6, p62, BECN1 and LC3 were measured in HEK-293T cells transfected with ATXN3 (15Q, 77Q, 148Q) and treated with cycloheximide or untreated controls (DMSO) by Western Blotting. GAPDH or Beta-Actin (ACTB) are shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. (C) Protein levels of ATXN3, p62, and LC3 were measured in ATXN3 (15Q, 77Q, 148Q) transfected HEK-293T cells treated with CHX for 1 h, 5 h, and 12 h or untreated controls (DMSO) by Western Blotting. GAPDH or Beta-Actin (ACTB) are shown as loading control. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. (D) Aggregation of ATXN3 was analyzed in ATXN3 (15Q, 77Q, 148Q) transfected HEK-293T cells treated with CHX or untreated controls (DMSO) using DD-AGE analysis. Aggregation values were normalized to the respective ATXN3 15Q aggregation. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05, ** p < 0.01 by one-way ANOVA. Values are shown as mean +/− SEM.
Figure 5.
Increased aggregation of ATXN3 according to cycloheximide (CHX) treatment. (A, B) Protein levels of ATXN3, phospho-S6, p62, BECN1 and LC3 were measured in HEK-293T cells transfected with ATXN3 (15Q, 77Q, 148Q) and treated with cycloheximide or untreated controls (DMSO) by Western Blotting. GAPDH or Beta-Actin (ACTB) are shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. (C) Protein levels of ATXN3, p62, and LC3 were measured in ATXN3 (15Q, 77Q, 148Q) transfected HEK-293T cells treated with CHX for 1 h, 5 h, and 12 h or untreated controls (DMSO) by Western Blotting. GAPDH or Beta-Actin (ACTB) are shown as loading control. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. (D) Aggregation of ATXN3 was analyzed in ATXN3 (15Q, 77Q, 148Q) transfected HEK-293T cells treated with CHX or untreated controls (DMSO) using DD-AGE analysis. Aggregation values were normalized to the respective ATXN3 15Q aggregation. Statistical analysis was performed with one-way ANOVA and Tukey´s multiple comparison test. * p < 0.05, ** p < 0.01 by one-way ANOVA. Values are shown as mean +/− SEM.
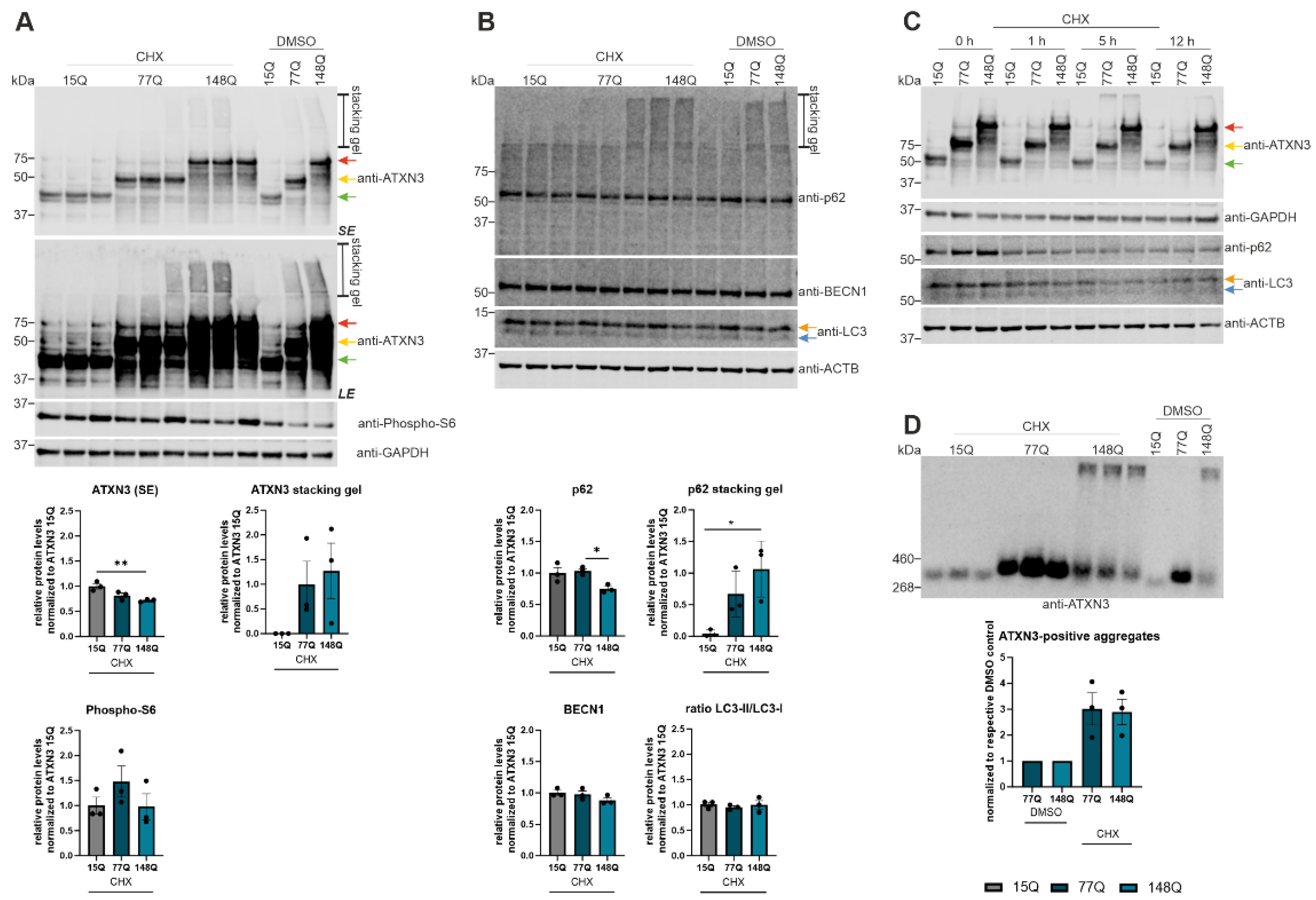
Figure 6.
BECN1-dependent autophagic activation caused by proteasomal inhibition. (A, B, C) Protein levels of ATXN3, p62, BECN1, phospho-S6, LC3, total polyubiquitin, K63- and K48-specific polyubiquitin were measured in ATXN3 (15Q, 77Q, 148Q) HEK-293T cells treated with MG132 or untreated controls (DMSO). Beta-Actin (ACTB) is shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. * p < 0.05, ** p < 0.01, *** p < 0.001 by one-way ANOVA. Values are shown as mean +/− SEM.
Figure 6.
BECN1-dependent autophagic activation caused by proteasomal inhibition. (A, B, C) Protein levels of ATXN3, p62, BECN1, phospho-S6, LC3, total polyubiquitin, K63- and K48-specific polyubiquitin were measured in ATXN3 (15Q, 77Q, 148Q) HEK-293T cells treated with MG132 or untreated controls (DMSO). Beta-Actin (ACTB) is shown as loading control. Protein levels were normalized to the respective ATXN3 15Q protein expression. Statistical analyses were performed from three independent experiments with one-way ANOVA and Tukey´s multiple comparison test. Green arrow represents 15Q ATXN3, yellow arrow 77Q ATXN3 and red arrow 148Q ATXN3. Orange arrow symbolizes LC3-I while blue arrow labels LC3-II. * p < 0.05, ** p < 0.01, *** p < 0.001 by one-way ANOVA. Values are shown as mean +/− SEM.
Table 1.
Antibodies used for Western Blot.
Table 1.
Antibodies used for Western Blot.
| Target Protein |
Product number |
Species |
Manufacturer |
Dilution |
| ACTB |
A2228 |
mouse |
Merck |
1:5000 |
| ATXN3 |
clone 13H9L9
702788 |
rabbit |
Thermo Fisher Scientific |
1:1000 |
| BECN1 |
3738S |
rabbit |
Cell Signaling Technology, Danvers, MA, USA |
1:1000 |
| GAPDH |
clone GA1R
ab125247 |
mouse |
Abcam, Cambridge, UK |
1:2000 |
| K63-polyubiquitin |
5621S |
rabbit |
Cell Signaling Technology |
1:500 |
| K48-polyubiquitin |
8081S |
rabbit |
Cell Signaling Technology |
1:1000 |
| LC3B |
2775S |
rabbit |
Cell Signaling Technology |
1:500 |
| SQSTM1/p62 |
5114S |
rabbit |
Cell Signaling Technology |
1:1000 |
| phospho-S6 ribosomal protein (Ser235/236) |
2211S |
rabbit |
Cell Signaling Technology |
1:1000 |
| Ubiquitin |
clone P4D1
sc-8017 |
mouse |
Santa Cruz Biotechnology,
Inc., Texas, USA |
1:1000 |
Table 2.
Primary antibodies used for Immunohistochemistry staining.
Table 2.
Primary antibodies used for Immunohistochemistry staining.
| Target Protein |
Product number |
Species |
Manufacturer |
Dilution |
| ATXN3 |
clone 1H9
MAB5360 |
mouse |
Merck |
1:1000 |
| p62 |
5114S |
rabbit |
Cell Signaling Technology |
1:500 |