Submitted:
06 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods and Materials
2.1. Participants and Study Design
| Figure 1. | CONSORT flow diagram. |
2.2. Procedure
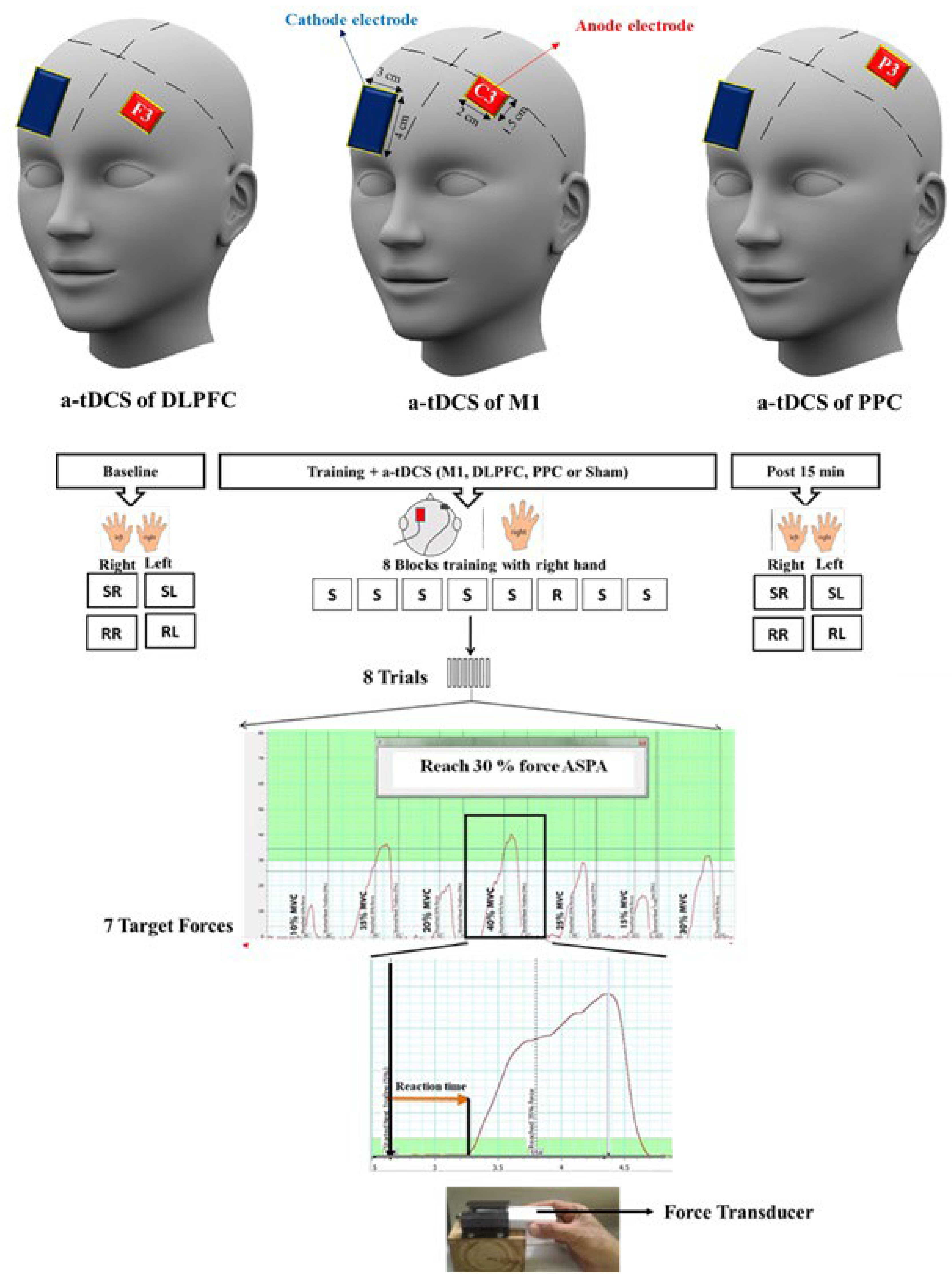
2.3. Transcranial Direct Current Stimulation (tDCS)
2.4. Data Analysis
3. Results
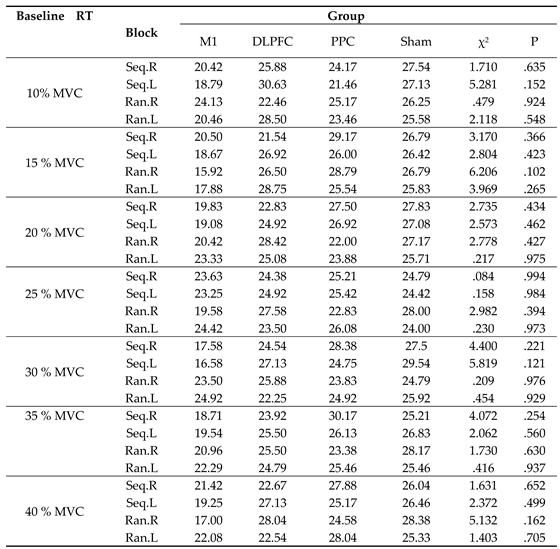 |
3.1. Ratio RT for Sequence Blocks in Both Right and Left Hands
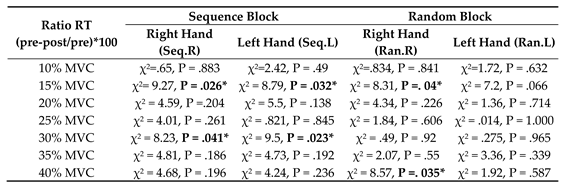 |
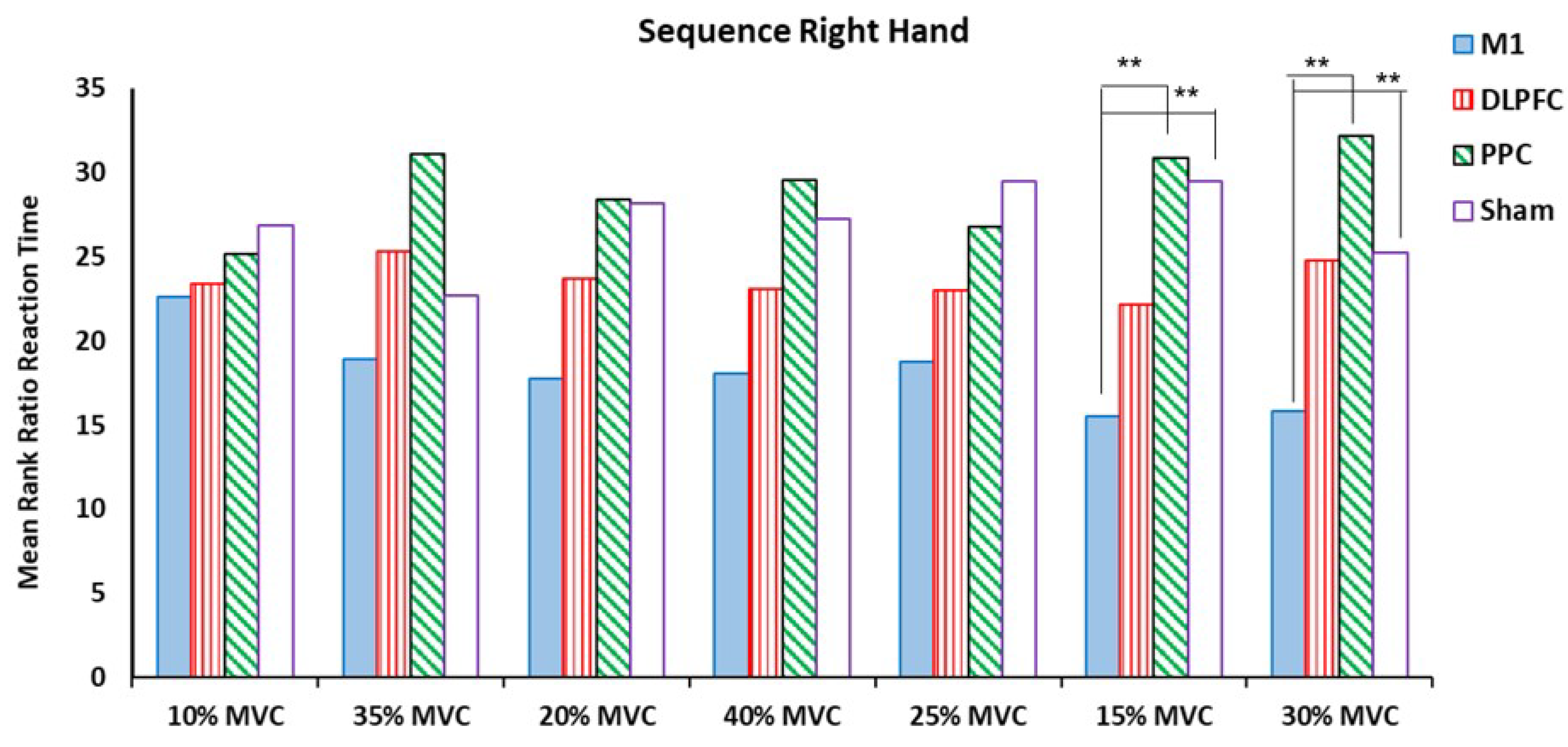
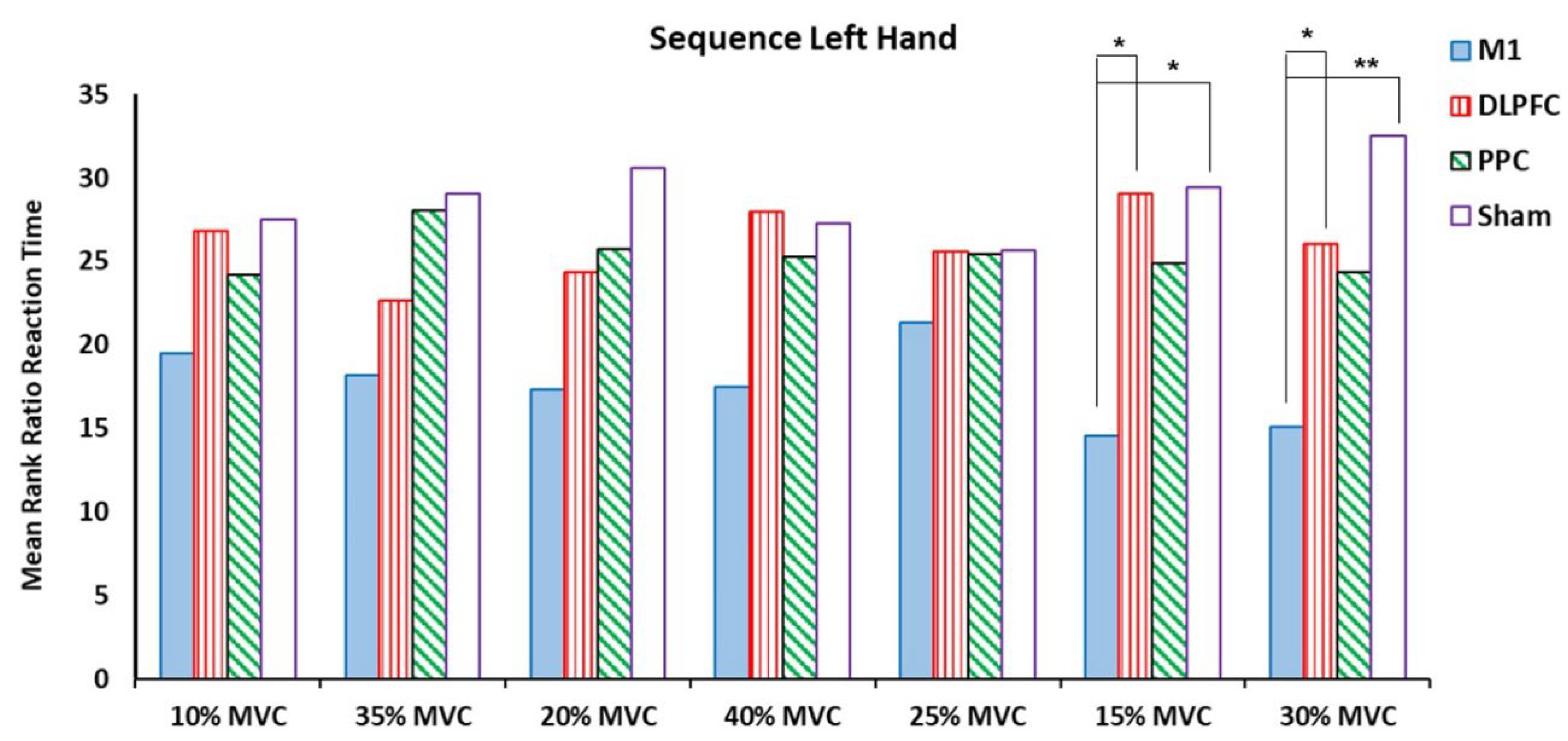
3.2. Ratio RT for Random Blocks in Both Right and Left Hands
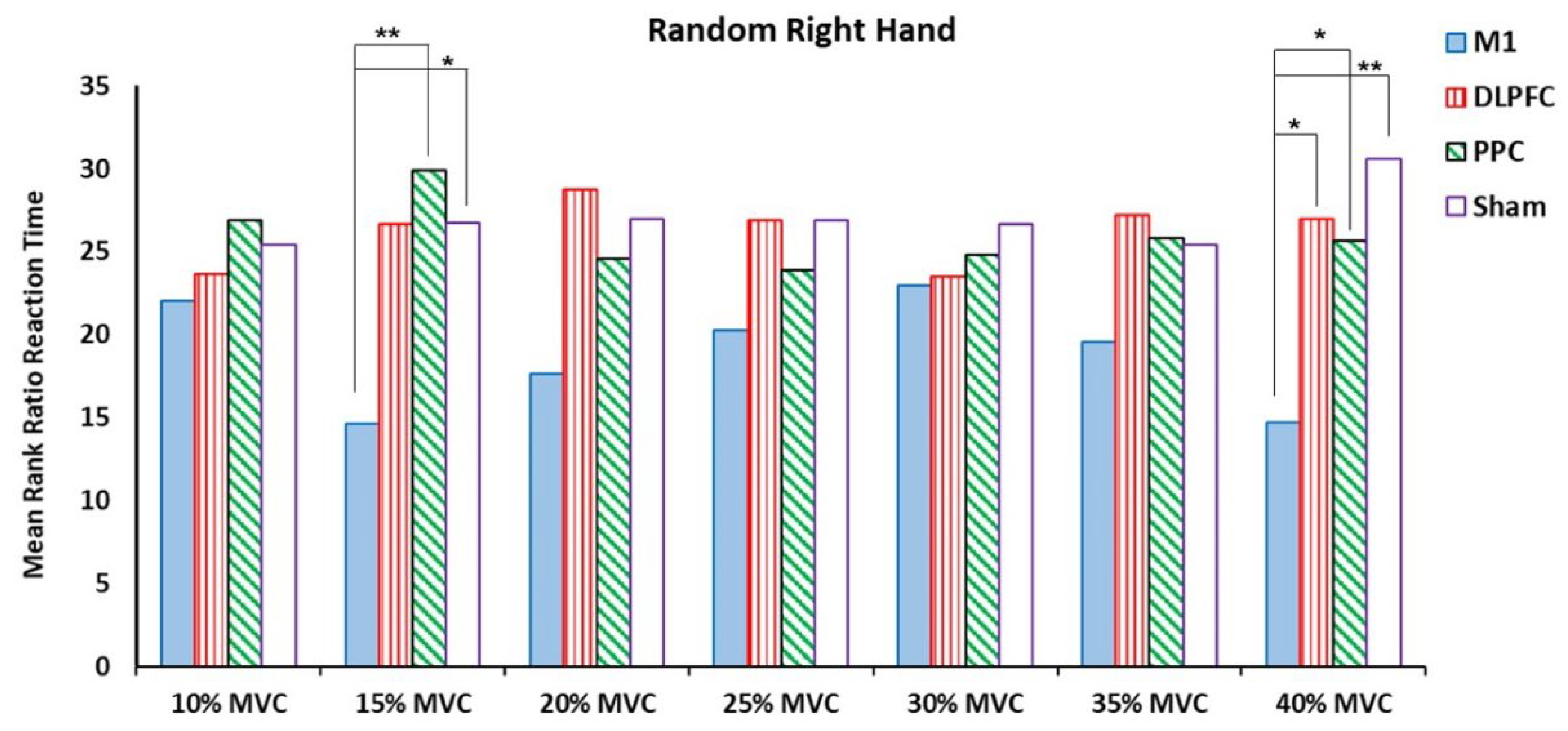
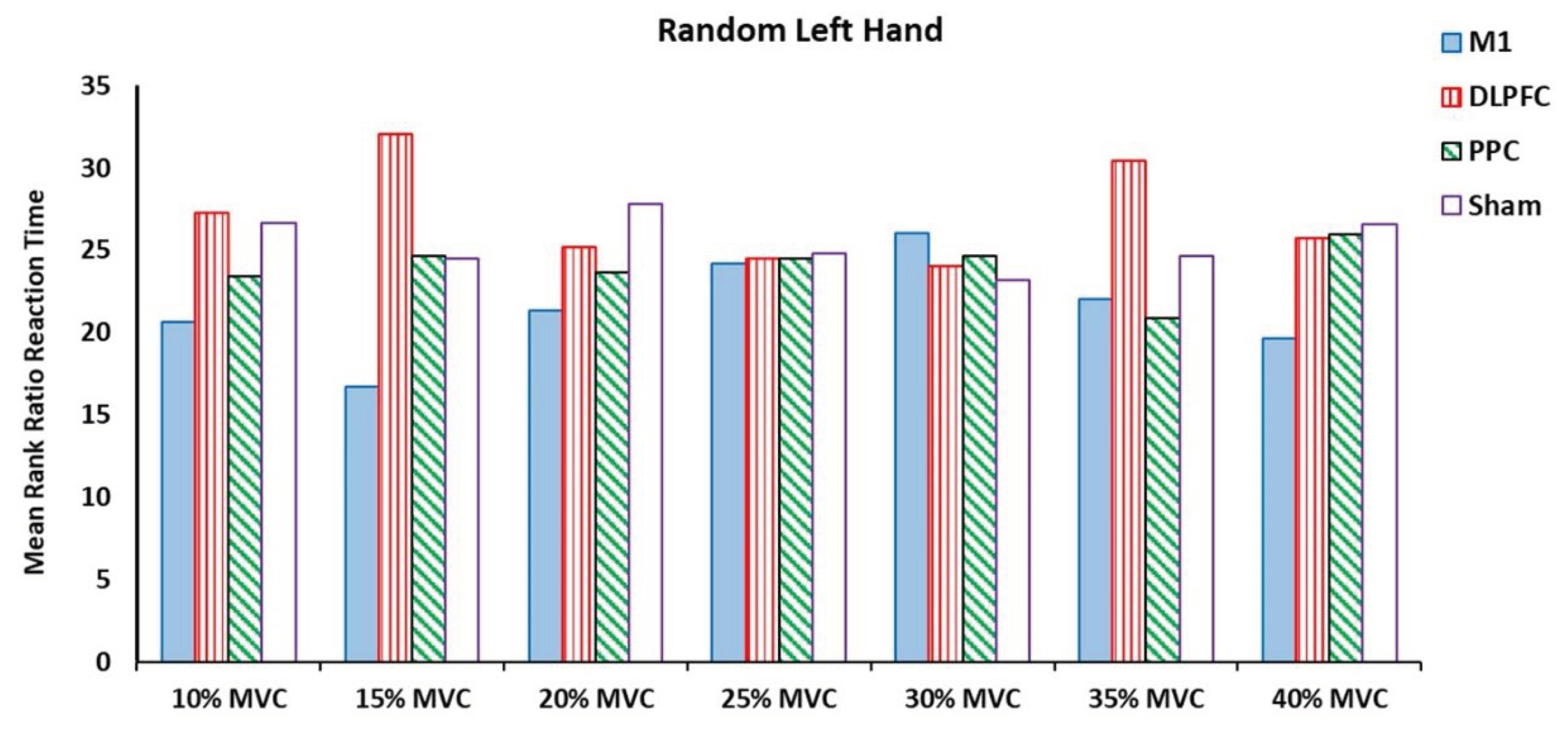
4. Discussion
Limitations
5. Conclusion
Acknowledgments
Conflicts of Interest statement
References
- Cohen, N.J. and L.R. Squire, Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science, 1980, 210, 207–210. [CrossRef]
- Pecenka, N., A. Engel, and P.E. Keller, Neural correlates of auditory temporal predictions during sensorimotor synchronization. Frontiers in human neuroscience, 2013, 7, 380. [CrossRef]
- Karabanov, A., et al., The dorsal auditory pathway is involved in performance of both visual and auditory rhythms. Neuroimage, 2009, 44, 480–488. [CrossRef]
- Coull, J. and A. Nobre, Dissociating explicit timing from temporal expectation with fMRI. Current opinion in neurobiology. 2008, 18, 137–144. [CrossRef]
- Coull, J.T., J. Cotti, and F. Vidal, Differential roles for parietal and frontal cortices in fixed versus evolving temporal expectations: Dissociating prior from posterior temporal probabilities with fMRI. Neuroimage. 2016, 141, 40–51. [CrossRef]
- Rivera-Urbina, G.N., et al., Parietal transcranial direct current stimulation modulates primary motor cortex excitability. Eur J Neurosci. 2015, 41, 845–855. [CrossRef]
- Brazovskaya, F., A. Malikova, and R. Pavlygina, After-effects of anodal polarization in the cat cerebral cortex. Neurophysiology. 1972, 4, 194–199. [CrossRef]
- Hodgson, R., et al., Training-induced and electrically induced potentiation in the neocortex. Neurobiology of learning and memory. 2005, 83, 22–32. [CrossRef]
- Monfils, M.H. and G.C. Teskey, Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004, 125, 329–336. [CrossRef]
- Rioult-Pedotti, M.S., D. Friedman, and J.P. Donoghue, Learning-induced LTP in neocortex. Science. 2000, 290, 533–536. [CrossRef]
- Muellbacher, W., et al., Early consolidation in human primary motor cortex. Nature. 2002, 415, 640–644. [CrossRef]
- Nitsche, M.A. and W. Paulus, Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000, 527 Pt 3, 633–639. [CrossRef]
- Nitsche, M.A., et al., Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [CrossRef]
- Priori, A., et al., Polarization of the human motor cortex through the scalp. Neuroreport. 1998, 9, 2257–2260. [CrossRef]
- Nitsche, M.A. and W. Paulus, Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001, 57, 1899–1901. [CrossRef]
- Nitsche, M.A., et al., Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003, 56, 255–276. [CrossRef]
- Liebetanz, D., et al., Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002, 125 Pt 10, 2238–2247. [CrossRef]
- Tecchio, F., et al., Anodal transcranial direct current stimulation enhances procedural consolidation. J Neurophysiol. 2010, 104, 1134–1140. [CrossRef]
- Nitsche, M.A., et al., Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience. 2003, 15, 619–619. [CrossRef]
- Cuypers, K., et al., Is Motor Learning Mediated by tDCS Intensity? PLoS ONE 2013, 8, e67344. [CrossRef]
- Stagg, C.J., et al., Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011, 49, 800–804. [CrossRef]
- Kantak, S.S., C.K. Mummidisetty, and J.W. Stinear, Primary motor and premotor cortex in implicit sequence learning - Evidence for competition between implicit and explicit human motor memory systems. European Journal of Neuroscience. 2012, 36, 2710–2715. [CrossRef]
- Schambra, H.M., et al., Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol. 2011, 106, 652–661. [CrossRef]
- Saucedo Marquez, C.M., et al., Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci. 2013, 7, 333. [CrossRef]
- Reis, J., et al., Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009, 106, 1590–1595. [CrossRef]
- Parlow, S.E. and M. Kinsbourne, Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989, 11, 98–113. [CrossRef]
- Japikse, K.C., et al., Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp Brain Res. 2003, 148, 38–49. [CrossRef]
- Perez, M., et al., Neural substrates of intermanual transfer of a newly acquired motor skill. Current Biology. 2007, 17, 1896–1902. [CrossRef]
- Calford, M.B. and R. Tweedale, Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990, 249, 805–807. [CrossRef]
- Gordon, A.M., H. Forssberg, and N. Iwasaki, Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia. 1994, 32, 555–568. [CrossRef]
- Sainburg, R.L. and J. Wang, Interlimb transfer of visuomotor rotations: independence of direction and final position information. Experimental brain research. 2002, 145, 437–447. [CrossRef]
- Liang, N., et al., Effects of intermanual transfer induced by repetitive precision grip on input–output properties of untrained contralateral limb muscles. Experimental brain research. 2007, 182, 459–467. [CrossRef]
- Criscimagna-Hemminger, S.E., et al., Learned dynamics of reaching movements generalize from dominant to nondominant arm. Journal of neurophysiology. 2003, 89, 168–176. [CrossRef]
- Almeida, R. and M. Stetter, Modeling the link between functional imaging and neuronal activity: synaptic metabolic demand and spike rates. Neuroimage. 2002, 17, 1065–1079. [CrossRef]
- Tinazzi, M. and G. Zanette, Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neuroscience letters. 1998, 244, 121–124. [CrossRef]
- Bloom, J.S. and G.W. Hynd, The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychology review. 2005, 15, 59–71. [CrossRef]
- Daselaar, S.M., et al., Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiology of aging. 2003, 24, 1013–1019. [CrossRef]
- Bischoff-Grethe, A., et al., Neural substrates of response-based sequence learning using fMRI. Journal of cognitive neuroscience. 2004, 16, 127–138. [CrossRef]
- Hashemirad, F., et al., Single-session anodal tDCS with small-size stimulating electrodes over frontoparietal superficial sites does not affect motor sequence learning. Frontiers in human neuroscience. 2017, 11, 153. [CrossRef]
- Koechlin, E., et al., The role of the anterior prefrontal cortex in human cognition. Nature. 1999, 399, 148–151. [CrossRef]
- Poreisz, C., et al., Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain research bulletin. 2007, 72, 208–214. [CrossRef]
- Waters-Metenier, S., et al., Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. Journal of Neuroscience. 2014, 34, 1037–1050. [CrossRef]
- Horvath, J.C., O. Carter, and J.D. Forte, No significant effect of transcranial direct current stimulation (tDCS) found on simple motor reaction time comparing 15 different simulation protocols. Neuropsychologi. 2016, 91, 544–552. [CrossRef]
- Nitsche, M.A., et al., Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of neurophysiology. 2007, 97, 3109–3117. [CrossRef]
- Boros, K., et al., Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. European Journal of Neuroscience. 2008, 27, 1292–1300. [CrossRef]
- Elbert, T., et al., The influence of low-level transcortical DC-currents on response speed in humans. International Journal of Neuroscience. 1981, 14, 101–114. [CrossRef]
- Marshall, L., et al., Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC neuroscience. 2005, 6, 1–7. [CrossRef]
- Friehs, M.A. and C. Frings, Pimping inhibition: Anodal tDCS enhances stop-signal reaction time. Journal of Experimental Psychology: Human Perception and Performance. 2018, 44, 1933.
- Krause, V., et al., 1 Hz rTMS of the left posterior parietal cortex (PPC) modifies sensorimotor timing. Neuropsychologia. 2012, 50, 3729–3735. [CrossRef]
- Heinen, K., et al., Cathodal transcranial direct current stimulation over posterior parietal cortex enhances distinct aspects of visual working memory. Neuropsychologia. 2016, 87, 35–42. [CrossRef]
- Keitel, A., et al., Anodal transcranial direct current stimulation (tDCS) over the right primary motor cortex (M1) impairs implicit motor sequence learning of the ipsilateral hand. Frontiers in Human Neuroscience. 2018, 12, 289. [CrossRef]
- Doyon, J. and H. Benali, Reorganization and plasticity in the adult brain during learning of motor skills. Current opinion in neurobiology. 2005, 15, 161–167. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





