Submitted:
07 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
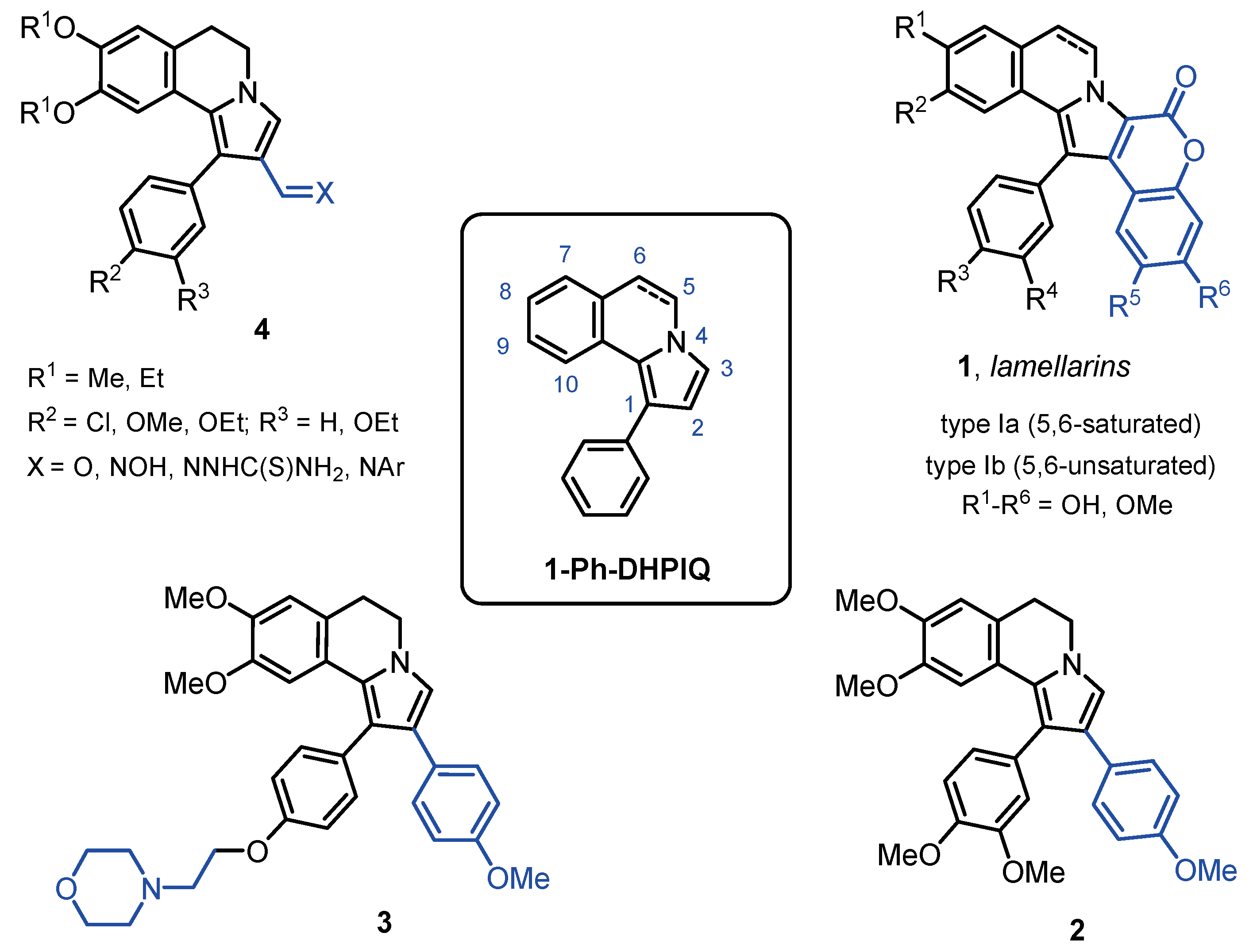
1. Introduction
2. Results and Discussion
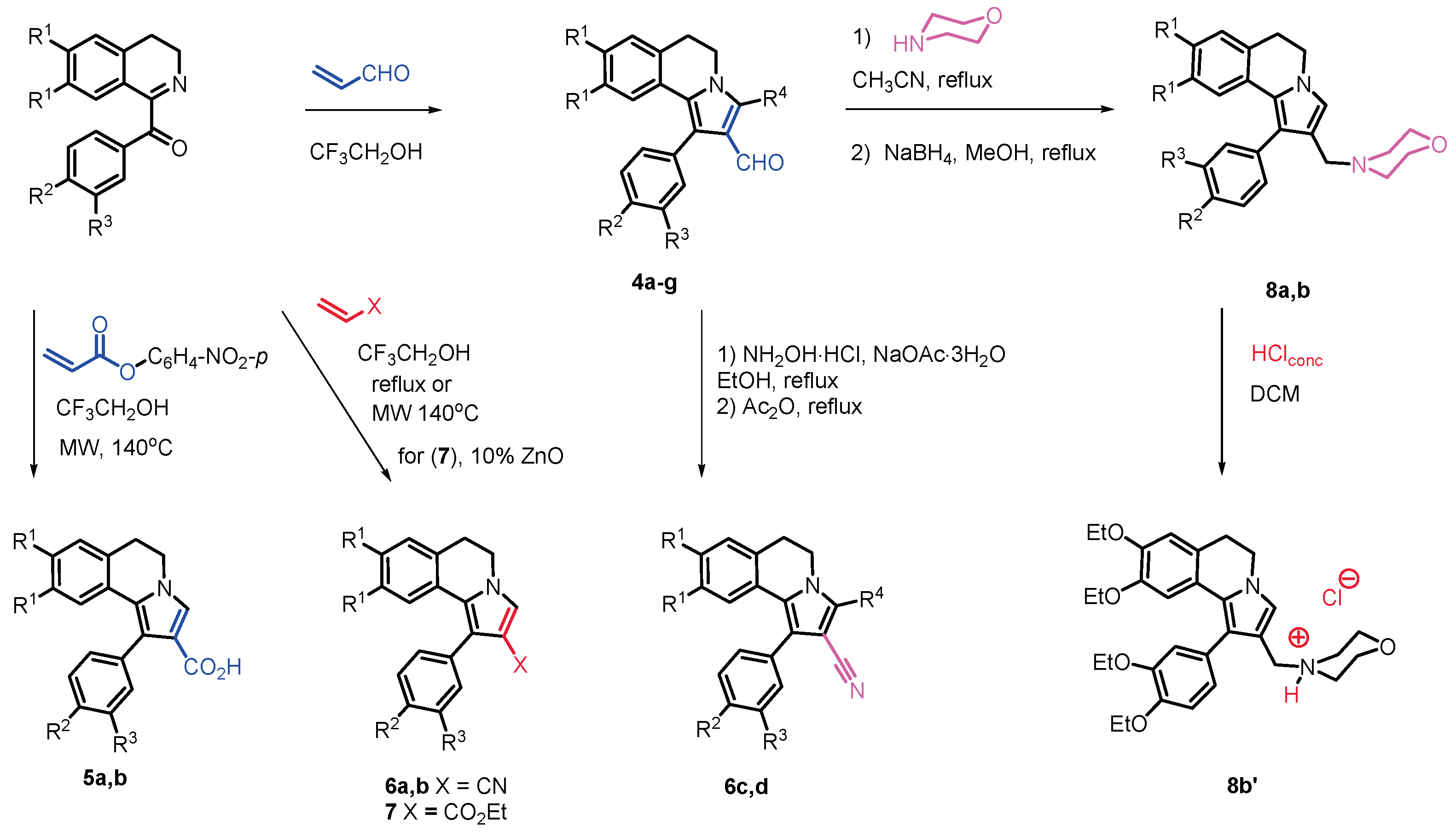
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxicity Screening
2.2.2. P-gp and MRP1 Inhibitory Potency
2.2.3. Binding Affinity to Human Serum Albumin (HSA)
2.3. Structure Activity Relationships
2.3.1. Solubility-Related Parameters
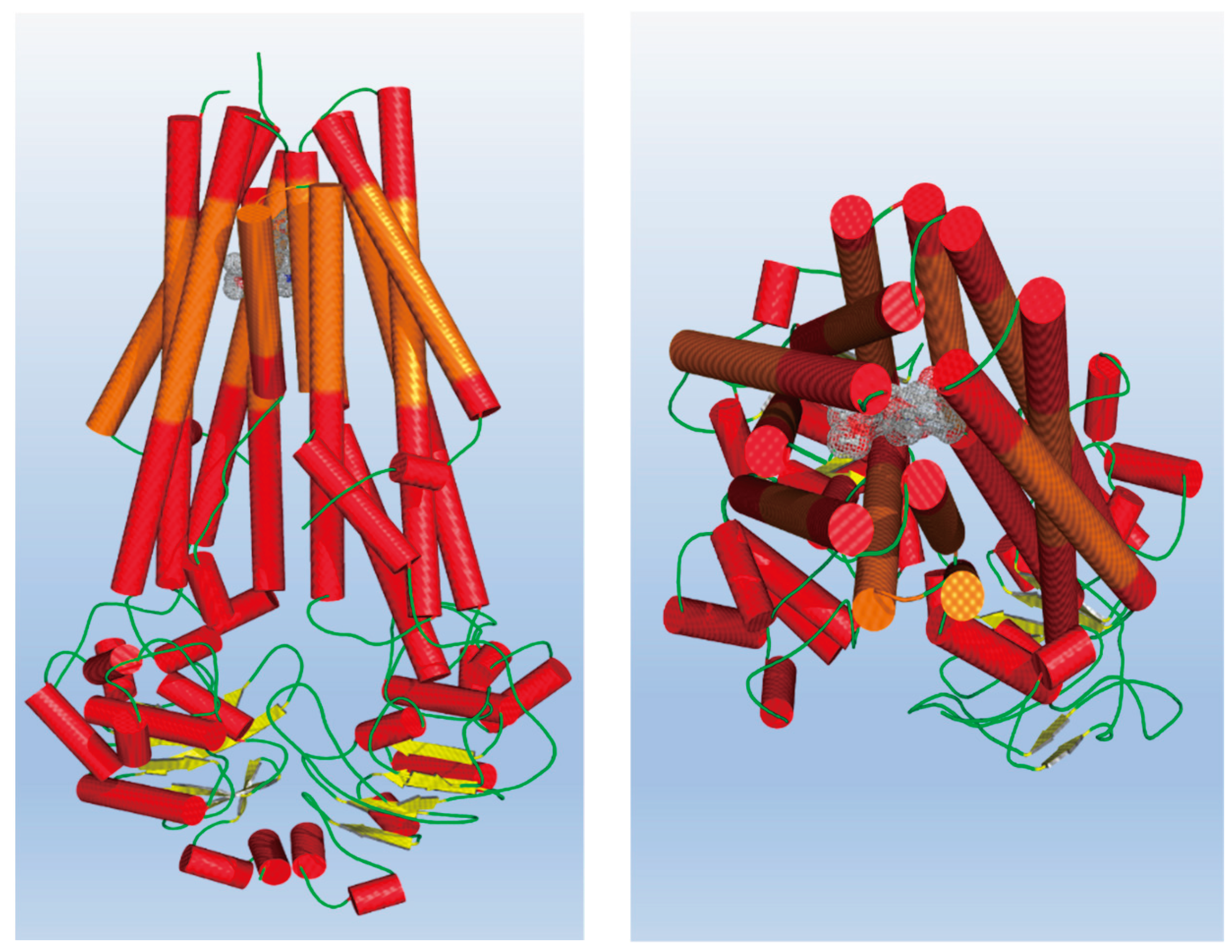
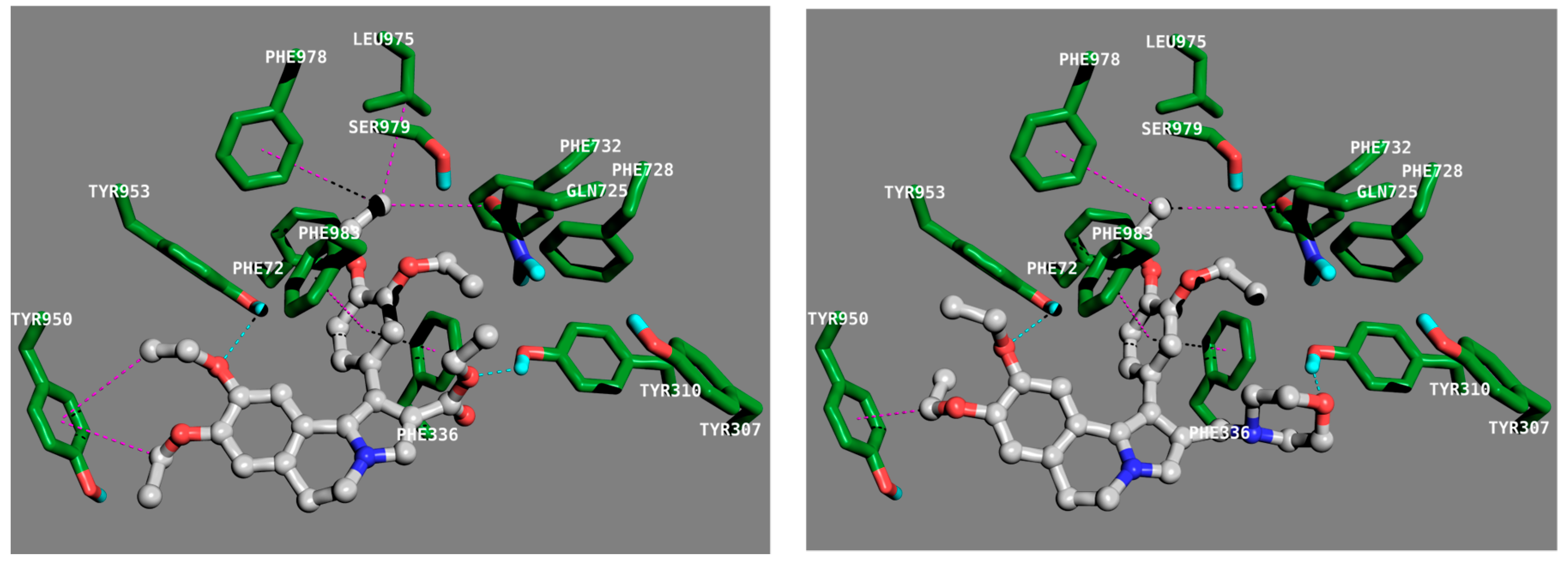
2.3.2. Molecular Docking Calculation
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of (1-(4-Methoxyphenyl)-8,9-dimethoxy-5,6-dihydropyrrolo[2,1-a]isoquinoline-2-carbaldehyde (4b)
3.1.2. Synthesis of 1-Aryl-5,6-dihydropyrrolo[2,1-a]isoquinoline-2-carboxylic acids 5a-b
3.1.3. Synthesis of Carbonitriles 6c,d.
3.1.4. Synthesis of Ethyl 1-(3,4-Diethoxyphenyl)-8,9-diethoxy-5,6-dihydro pyrrolo[2,1-a]isoquinoline-2-carboxylate (7)
3.1.5. Synthesis of 2-(Morpholin-4-yl-methyl)-5,6-dihydropyrrolo[2,1-a]isoquinolines 8a,b.
3.1.6. Synthesis of 4-{[1-(3,4-Diethoxyphenyl)-8,9-diethoxy-5,6-dihydropyrrolo[2,1-a]isoquinolin-2-yl]methyl}morpholin-4-ium chloride (8b’)
3.2. Biological Evaluation
3.2.1. Cell Cultures
3.2.2. Cytotoxicity Assay
3.2.3. Inhibition Assays of P-Glycoprotein (P-gp) and Multidrug-Resistance-Associated Protein-1 (MRP1)
3.2.4. Affinity to Human Serum Albumin (HSA) by Surface Plasmon Resonance (SPR)
3.3. Solubility and Lipophilicity
3.3.1. Determination of Kinetic Solubility in PBS
3.3.2. Determination of Lipophilicity by RP-HPLC
3.4. Molecular Doocking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, T.; Ishibashi, F.; Iwao, M. Synthesis and biological activity of lamellarin alkaloids: an overview. Heterocycles 2011, 83, 491–529. [Google Scholar] [CrossRef]
- Matveeva, M.D.; Purgatorio, R.; Voskressensky, L.G.; Altomare, C.D. Pyrrolo[2,1- a ]isoquinoline scaffold in drug discovery: Advances in synthesis and medicinal chemistry. Future Med. Chem. 2019, 11, 2735–2755. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, S.; Shahosseini, S.; Zarghi, A. Design, synthesis and cytotoxicity evaluation of new 2-aryl-5, 6-dihydropyrrolo[2,1-a]isoquinoline derivatives as topoisomerase inhibitors. Iran. J. Pharm. Res. 2014, 13, 71–77. [Google Scholar]
- Kakhki, S.; Shahosseini, S.; Zarghi, A. Design and synthesis of pyrrolo [2,1-a] isoquinoline-based derivatives as new cytotoxic agents. Iran J. Pharm. Res. 2016, 15, 743–751. [Google Scholar] [PubMed]
- Nevskaya, A.A.; Miftyakhova, A.R.; Anikina, L.V.; Borisova, T.N.; Varlamov, A.V.; Voskressensky, L.G. Synthesis and Cytotoxicity of Novel 1-Arylindolizines and 1-Arylpyrrolo[2,1-a]isoquinolines. Tetrahedron Lett. 2021, 87, 153552. [Google Scholar] [CrossRef]
- Nevskaya, A.A.; Anikina, L.V.; Purgatorio, R.; Catto, M.; Nicolotti, O.; de Candia, M.; Pisani, L.; Borisova, T.N.; Miftyakhova, A.R.; Varlamov, A.V.; et al. Homobivalent Lamellarin-Like Schiff Bases: In Vitro Evaluation of Their Cancer Cell Cytotoxicity and Multitargeting Anti-Alzheimer’s Disease Potential. Molecules 2021, 26, 359. [Google Scholar] [CrossRef]
- Nevskaya, A.A.; Matveeva, M.D.; Borisova, T.N.; Niso, M.; Colabufo, N.A.; Boccarelli, A.; Purgatorio, R.; de Candia, M.; Cellamare, S.; Voskressensky, L.G.; et al. A New Class of 1-Aryl-5,6-dihydropyrrolo[2,1-a]isoquinoline derivatives as reversers of P-glycoprotein-mediated multidrug resistance in tumor cells. ChemMedChem 2018, 13, 1588–1596. [Google Scholar] [CrossRef]
- Dufour, E.; Storer, A.C.; Menard, R. Peptide aldehydes and nitriles as transition state analog inhibitors of cysteine proteases. Biochemistry 1995, 34, 9136–9143. [Google Scholar] [CrossRef]
- Singh, J.; Petter, R.C.; Baillie, T.A.; Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011, 10, 307. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 80, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, M.; Borisova, T.; Titov, A.; Anikina, L.; Dyachenko, S.; Astakhov, G.; Varlamov, A.; Voskressensky, L. Domino Reactions of 1-Aroyl-3,4-Dihydroisoquinolines with α,β-Unsaturated Aldehydes. Synthesis 2017, 49, 5251–5257. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Shigaev, R.R.; Borisova, T.N.; Ershova, A.A.; Titov, A.A.; Varlamov, A.V.; Voskressensky, L.G.; Matveeva, M.D. Facile Synthesis of Pyrrolo[2,1-a]Isoquinolines by Domino Reaction of 1-Aroyl-3,4-Dihydroisoquinolines with Conjugated Ketones, Nitroalkenes and Nitriles. Mol. Divers. 2021, 25, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Pagliarulo, V.; Berardi, F.; Contino, M.; Inglese, C.; Niso, M.; Ancona, P.; Albo, G.; Pagliarulo, A.; Perrone, R. Bicalutamide Failure in Prostate Cancer Treatment: Involvement of Multi Drug Resistance Proteins. Eur. J. Pharmacol. 2008, 601, 38–42. [Google Scholar] [CrossRef]
- Colabufo, N.A.; Berardi, F.; Cantore, M.; Perrone, M.G.; Contino, M.; Inglese, C.; Niso, M.; Perrone, R.; Azzariti, A.; Simone, G.M.; et al. Small P-Gp Modulating Molecules: SAR Studies on Tetrahydroisoquinoline Derivatives. Bioorg. Med. Chem. 2008, 16, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Trompier, D.; Chang, X.-B.; Barattin, R.; d’Hardemare, A.D.M.; Di Pietro, A.; Baubichon-Cortay, H. Verapamil and Its Derivative Trigger Apoptosis through Glutathione Extrusion by Multidrug Resistance Protein MRP1. Cancer Res. 2004, 64, 4950–4956. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; De Candia, M.; Catto, M.; Rullo, M.; Pisani, L.; Denora, N.; Carrieri, A.; Nevskaya, A.A.; Voskressensky, L.G.; Altomare, C.D. Evaluation of Water-Soluble Mannich Base Prodrugs of 2,3,4,5-Tetrahydroazepino[4,3-b]indol-1(6H)-one as Multitarget-Directed Agents for Alzheimer’s Disease. ChemMedChem 2021, 16, 589–598. [Google Scholar] [CrossRef]
- Fabini, E.; Danielson, U.H. Monitoring Drug–Serum Protein Interactions for Early ADME Prediction through Surface Plasmon Resonance Technology. J. Pharm. Biomed. Anal. 2017, 144, 188–194. [Google Scholar] [CrossRef]
- Frostell-Karlsson, Å.; Remaeus, A.; Roos, H.; Andersson, K.; Borg, P.; Hämäläinen, M.; Karlsson, R. Biosensor Analysis of the Interaction between Immobilized Human Serum Albumin and Drug Compounds for Prediction of Human Serum Albumin Binding Levels. J. Med. Chem. 2000, 43, 1986–1992. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10th Edition, 2019.
- Purgatorio, R.; De Candia, M.; De Palma, A.; De Santis, F.; Pisani, L.; Campagna, F.; Cellamare, S.; Altomare, C.; Catto, M. Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules 2018, 23, 1544. [Google Scholar] [CrossRef]
- Purgatorio, R.; Kulikova, L.N.; Pisani, L.; Catto, M.; De Candia, M.; Carrieri, A.; Cellamare, S.; De Palma, A.; Beloglazkin, A.A.; Reza Raesi, G.; et al. Scouting around 1,2,3,4-Tetrahydrochromeno[3,2- c ]Pyridin-10-ones for Single- and Multitarget Ligands Directed towards Relevant Alzheimer’s Targets. ChemMedChem 2020, 15, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Nosol, K.; Romane, K.; Irobalieva, R.N.; Alam, A.; Kowal, J.; Fujita, N.; Locher, K.P. Cryo-EM Structures Reveal Distinct Mechanisms of Inhibition of the Human Multidrug Transporter ABCB1. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 26245–26253. [Google Scholar] [CrossRef]
- Schrödinger Release 2023-1, Maestro.
- Schrödinger, LLC: New York, NY, USA, 2023.
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Molecular Modeling Software | OpenEye Scientific. Available online: https://www.eyesopen.com (accessed on 20 September 2023).
- Forli, S.; Olson, A.J. A Force Field with Discrete Displaceable Waters and Desolvation Entropy for Hydrated Ligand Docking. J. Med. Chem. 2012, 55, 623–638. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- El Khoury, L.; Santos-Martins, D.; Sasmal, S.; Eberhardt, J.; Bianco, G.; Ambrosio, F.A.; Solis-Vasquez, L.; Koch, A.; Forli, S.; Mobley, D.L. Comparison of Affinity Ranking Using AutoDock-GPU and MM-GBSA Scores for BACE-1 Inhibitors in the D3R Grand Challenge 4. J. Comput.-Aided Mol. Des. 2019, 33, 1011–1020. [Google Scholar] [CrossRef]
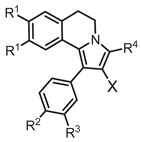
| N | X | R1 | R2 | R3 | R4 | RD | HCT116 | HeLa | A549 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4ab | CHO | OMe | Cl | H | H | 17.6 (3.2) | 22.0 (4.0) | 33.0 (4.5) | 38.6 (3.2) | ||
| 4b | CHO | OMe | OMe | H | H | 95.2 (7.1) | > 100 | > 100 | > 100 | ||
| 4cc | CHO | OEt | OEt | OEt | H | 21.3 (1.2) | 11.8 (0.2) | 44.5 (2.0) | 19.7 (0.3) | ||
| 5a | CO2H | OMe | Cl | H | H | > 100 | > 100 | > 100 | > 100 | ||
| 5b | CO2H | OEt | OEt | OEt | H | n.a. | n.a. | n.a. | n.a. | ||
| 6a | CN | OMe | Cl | H | H | > 100 | > 100 | > 100 | > 100 | ||
| 6b | CN | OEt | OEt | OEt | H | > 100 | > 100 | > 100 | > 100 | ||
| 6c | CN | OEt | OEt | OEt | Me | > 100 | > 100 | > 100 | > 100 | ||
| 6d | CN | OEt | OEt | OEt | Ph | > 100 | n.a. | n.a. | n.a. | ||
| 7 | CO2Et | OEt | OEt | OEt | H | n.a. | n.a. | n.a. | n.a. | ||
| 8a | MMd | OMe | OMe | H | H | 37.7 (1.6) | 56.4 (1.7) | 65.4 (4.5) | 66.0 (1.6) | ||
| 8b’ | MM·HCl | OEt | OEt | OEt | H | 18.6 (2.7) | 15.7 (0.7) | 17.5 (1.4) | 20.8 (3.6) | ||
| Camptothecin | 16.0 (0.2) | 12.3 (0.5) | 0.33 (0.07) | 3.32 (0.02) | |||||||
| Doxorubicin | 0.29 (0.02) | 0.14 (0.01) | 0.89 (0.01) | 0.38 (0.02) | |||||||
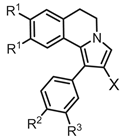
| N | X | R1 | R2 | R3 | P-gp, IC50 (μM)a |
MRP1, IC50 (μM)a |
HSA, KD (μM)b |
S (μM)c | CLog Pd | Log k’we |
|---|---|---|---|---|---|---|---|---|---|---|
| 4a | CHO | OMe | Cl | H | 25.3 (1.9) | 21.9 (1.5) | 1.60 (0.04) | 6.66 (0.09) | 5.62 | 4.32 |
| 4b | CHO | OMe | OMe | H | 4.48 (0.17) | 6.42 (0.27) | 1.95 (0.06) | 19.8 (0.1) | 4.86 | 3.39 |
| 5b | CO2H | OEt | OEt | OEt | 0.35 (0.04) | > 100 | 5.20 (0.20) | 14.6 (0.6) | 4.70 | 6.91 |
| 6a | CN | OMe | Cl | H | 5.89 (0.42) | 16.6 (0.5) | 4.60 (0.10) | 1.42 (0.01) | 5.75 | 4.42 |
| 6b | CN | OEt | OEt | OEt | 0.39 (0.06) | > 100 | 1.80 (0.20) | 1.06 (0.05) | 6.79 | 5.09 |
| 7 | CO2Et | OEt | OEt | OEt | 0.32 (0.08) | 3.23 (0.29) | 12.1 (0.1) | 41.7 (1.1)g | 7.75 | 5.49 |
| 8a | MMf | OMe | OMe | H | 0.36 (0.02) | 1.80 (0.31) | 19.3 (0.5) | 112 (4)g | 4.73 | 3.86 |
| 8b’ | MM·HCl | OEt | OEt | OEt | 0.45 (0.03) | 12.1 (2.1) | 26.9 (0.5) | 41.7 (1.2)g | 6.53 | 4.31 |
| MC18 | 1.20 (0.3) | |||||||||
| Verapamil | 4.53 (0.50) | |||||||||
| Warfarin | 5.30 (0.35) | |||||||||
| N | FEB(a) | ΔE(b) | EFF(c) | TAN(d) | POP(e) |
|---|---|---|---|---|---|
| 7 | – 11.04 | 0.08 | – 0.307 | 0.307 | 265/1000 |
| 8b | – 10.62 | 0.21 | – 0.279 | 0.276 | 121/1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





