1. Introduction
Endometriosis is defined as endometrium-like tissue outside the uterus. Historically, the effects of ovarian hormones on both tissues were believed to be similar. Estrogens stimulate the growth of the endometrium and induce progesterone receptors. Progesterone stops endometrial growth and induces secretory changes and decidualization, which are changes preparing the endometrium for the implantation of an embryo. In women, primates, some mice and bats, an abrupt decrease in plasma estrogens and/or progesterone concentrations results in the shedding of the endometrium and withdrawal bleeding [
1].
The human menstrual cycle is a precisely timed cascade of events. A small increase in FSH induced by progesterone withdrawal stimulates a cohort of follicles that had initiated growth, resulting in a race of the fittest with estrogen secretion and a primordial follicle, inducing an LH peak, ovulation some 36 hours later, followed by a corpus luteum and progesterone secretion, lasting for 12 days unless rescued by a pregnancy. This cascade explains that most menstrual cycles are regular, lasting for 28 days and that ovulation occurs 14 days after a progesterone decrease. An exponential increase in plasma estrogen concentrations occurs before ovulation and a bell-shaped progesterone concentration after ovulation. The endometrium growth reflects these plasma changes of estrogens during the follicular phase. During the luteal phase, the secretory changes are strictly timed and used for endometrial biopsy dating [
2,
3].
The concentrations and the standard deviations of estrogens and progesterone in plasma during the menstrual cycle are well-known. However, the clinical implications of the individual variability are poorly understood, as is the variable dose response of concentrations in individual women. Even less documented is the endocrinology of the basal layer of the endometrium with a strong progesterone resistance [
4], the relationship with the junctional zone (JZ) [
5], and whether some cancer driver mutations might signal the susceptibility for endometriosis development.
The estrogen and progesterone effects on metabolic activity and histology have been assumed to be similar to those observed in the eutopic endometrium. However, menstrual bleeding in endometriosis lesions has been rarely observed, which is logical considering the estrogen and progesterone concentrations in peritoneal fluid [
6]. It remains unclear whether the hormonal response of endometriosis tissue is more similar to the functional or the basal layer of the intrauterine endometrium [
7]. Superficial endometriosis is probably more influenced by peritoneal fluid estrogen and progesterone concentrations, which have a different time course and are quantitatively very different from plasma [
6]. Endometriosis is not one disease. The lesions are clonal [
8,
9] and biochemically different, with variable aromatase activity and progesterone resistance [
10]. Understanding these differences between endometrium and endometriosis is important for managing endometriosis and the associated pain symptoms with hormonal therapy.
Therefore, we here reinterpret the effects of medical therapy for endometriosis considering the estrogen and progesterone concentrations in plasma and peritoneal fluid, the direct effects of medical therapy on the different endometriosis lesions, and the individual variability of the dose-response relationship.
2. Basics of Steroid Hormones, Such as Estrogens and Progesterone
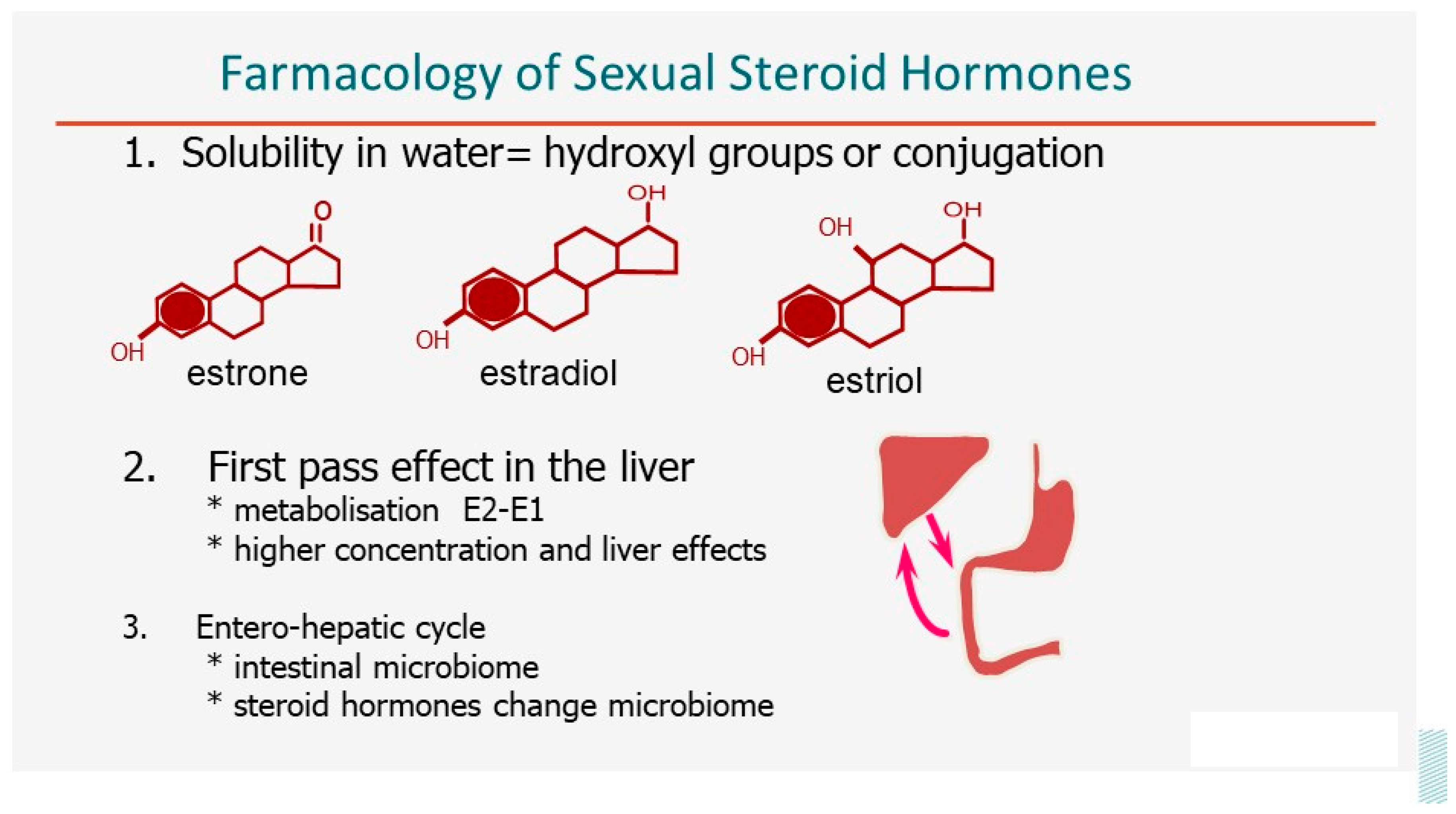
Steroid hormones are poorly soluble in water [
11], especially those with no hydroxyl groups, such as progesterone (
Figure 1). Therefore, the free or soluble concentrations are small and transport in plasma requires binding to proteins such as sex hormone binding globulin (SHBG) and transcortin. Since lipophilic, steroid hormones easily cross the lipid bilayer of the cellular membrane, bind to a specific receptor -if present- and affect DNA transcription after translocation to the nucleus. The poor solubility in water is important for the oral intake and resorption. Oestriol can be taken orally, and 17β-estradiol, with two hydroxyl groups, can be taken orally after micronization to increase the surface area. However, after micronization, the plasma concentrations vary with the degree of micronization and not only with the dose. After oral intake, all steroid hormones have a first-pass effect in the liver, with active metabolization and specific liver effects because of the higher concentrations. Estradiol is almost quantitatively, around 98%, transformed into estrone, which is the main hormone in plasma after the administration of 17β-estradiol. This metabolization cannot occur in ethinylestradiol since the 17β-hydroxyl group is protected from oxidation by the ethinyl group. The role of the intestinal microbiome and the entero-hepatic cycle is important, although still poorly understood. The microbiome permits the readsorption of the conjugated steroid hormones secreted in the bile by hydrolyzing the conjugate. This prolongs the effective half-life sufficiently for one intake a day to become sufficient. The intake of estrogens and progesterone also affects the intestinal microbiome, but the direct clinical consequences remain unclear.
The physiology and the effects of estrogens and progesterone and the dose-response effects have been known since the 1980s. A specific cellular receptor is required for steroid hormones to have an effect. Therefore, progesterone has no effect on the endometrium without prior estrogen stimulation since estrogens are needed to induce the progesterone receptor [
12]. The effect of steroid hormones increases with the binding to the receptor. According to Michaelis Menten's law, the affinity constant is the product of the free receptor (R) and free hormone (H) concentrations divided by the concentration of the hormone-receptor complex (HR) (K
d = [H][R]/[HR]). The clinical importance is that the effect of a hormone (or the binding) increases sigmoidal with the logarithm of the concentration [
13]. Therefore, estrogens and progesterone do not have an overdose for the endometrium since increasing the dose of hormones will not increase the effect once saturation is obtained. This also explains that giving a weak estrogen in addition to a strong estrogen can decrease the effect by decreasing the binding of the strong estrogen by competitive inhibition. Unfortunately, the effect is difficult to predict in the individual cell [
14] because of the complexity of the subsequent DNA transcription and translation due to many interfering factors, such as epigenetic changes. This individual variability might explain the wide range of normal concentrations of estrogens (varying preovulatory from 140 to 400 pg/ml) and progesterone (varying in the mid-luteal phase from 8 to 20 ng/ml) in plasma. A nice example of this individual variability is the sigmoidal relationship between estrogen concentrations in plasma and menopausal symptoms: below 50pgr/ml estradiol, most women will have menopausal symptoms, but 180pg/ml is needed before most women are symptom-free. This variability in effect, together with the variable resorption and metabolization of estrogen and progesterone [
15], explains the need to individualize hormone replacement therapy. Another example of this variability is that the intake of 50 µgr ethinyloestradiol results in a huge range of plasma concentrations from 100 to 1000 pg/ml. Not surprisingly, 100pg/ml is the limit to have a contraceptive effect, while 1000 pg/ml can result in nausea. We will not discuss the brain effects of the different estrogens and progesterone and its metabolites, such as the 5-α reduced pregnanes [
16], or the different cellular effects between fast resorbable steroids with a peak concentration after 30 minutes and slow resorption over 5 hours such as micronized 17β-estradiol.
3. The Concentrations of Estrogens and Progesterone in Plasma, Peritoneal Cavity, the Ovary and the Uterus.
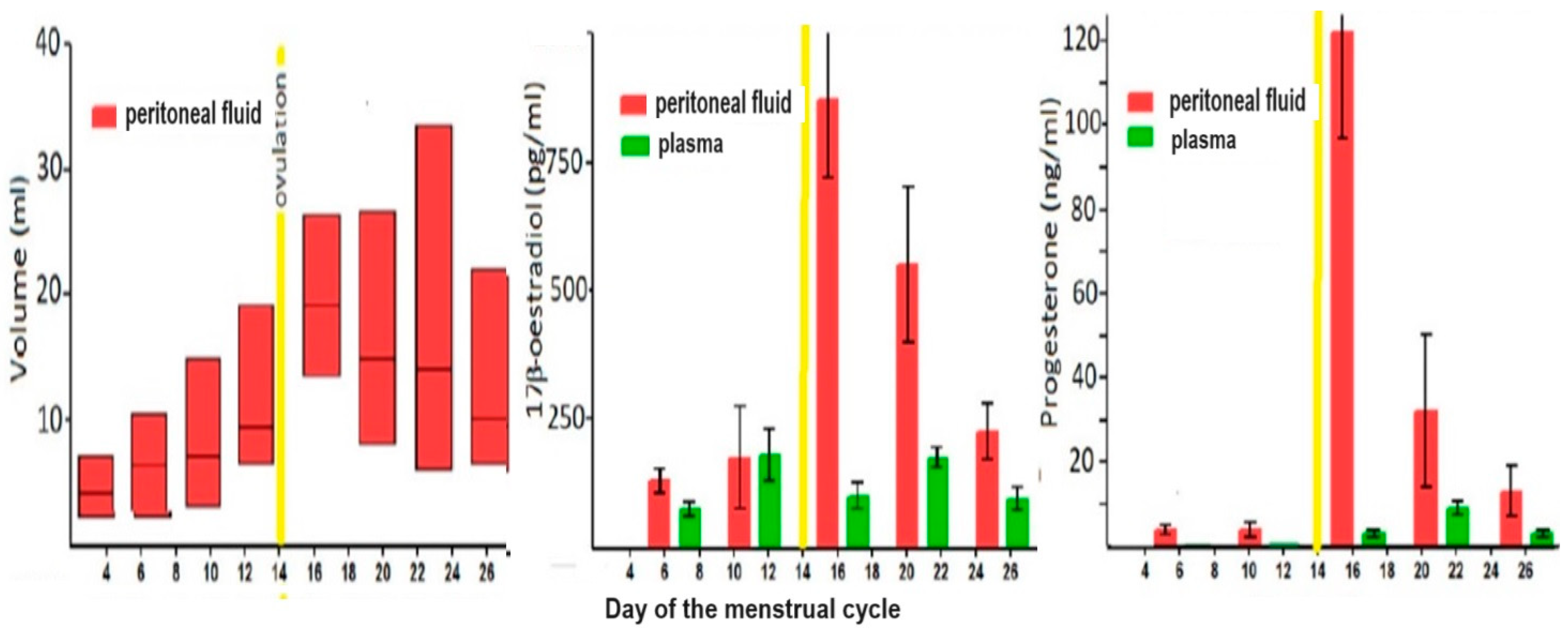
The cyclic variations in plasma concentrations of estrogens and progesterone during the menstrual cycle are considered well-known (Figure 2). However, the wide range of individual variability received little attention, although it is expected that individual women will always have high-normal or low-normal concentrations, as known for other hormones. Also, the logarithmic distribution of plasma concentration [
6,
17] is rarely taken into account, resulting in many publications in mean values minus 2 or 3 SDs being below 0, which is nonsense by definition.
The peritoneal fluid volume increases up to 200 ml before ovulation, and, being an ovarian exudate [
18], during the follicular phase, the estrogen concentrations are twice as high as in plasma and progesterone concentrations are almost as high as during the luteal phase in plasma. Considering the concentrations of albumin, SHBG and corticosteroid-binding globulin in peritoneal fluid, being only 70% of those of plasma [
6], the free concentrations of estrogens and progesterone are much higher than in plasma. After ovulation, the peritoneal fluid concentrations of both steroid hormones increase 10-fold by direct secretion into the peritoneal cavity [
6] (Figure 2).
The intraovarian concentrations are poorly documented but must be much higher than in plasma. In animals, the ovarian vein concentrations of estrogens and progesterone are 5 to 10 times higher than in plasma [
19], which is not surprising since secreted into the ovarian vein. However, more important is the countercurrent exchange between the ovarian artery and vein [
20,
21], resulting in higher concentrations in the oviducts and ovaries, which is important for oocyte pick-up. Although poorly investigated, the uterine concentrations irrigated by the ovarian artery are also much higher than in plasma. A similar mechanism can be expected in women since the ovarian artery and vein run together over several cm.
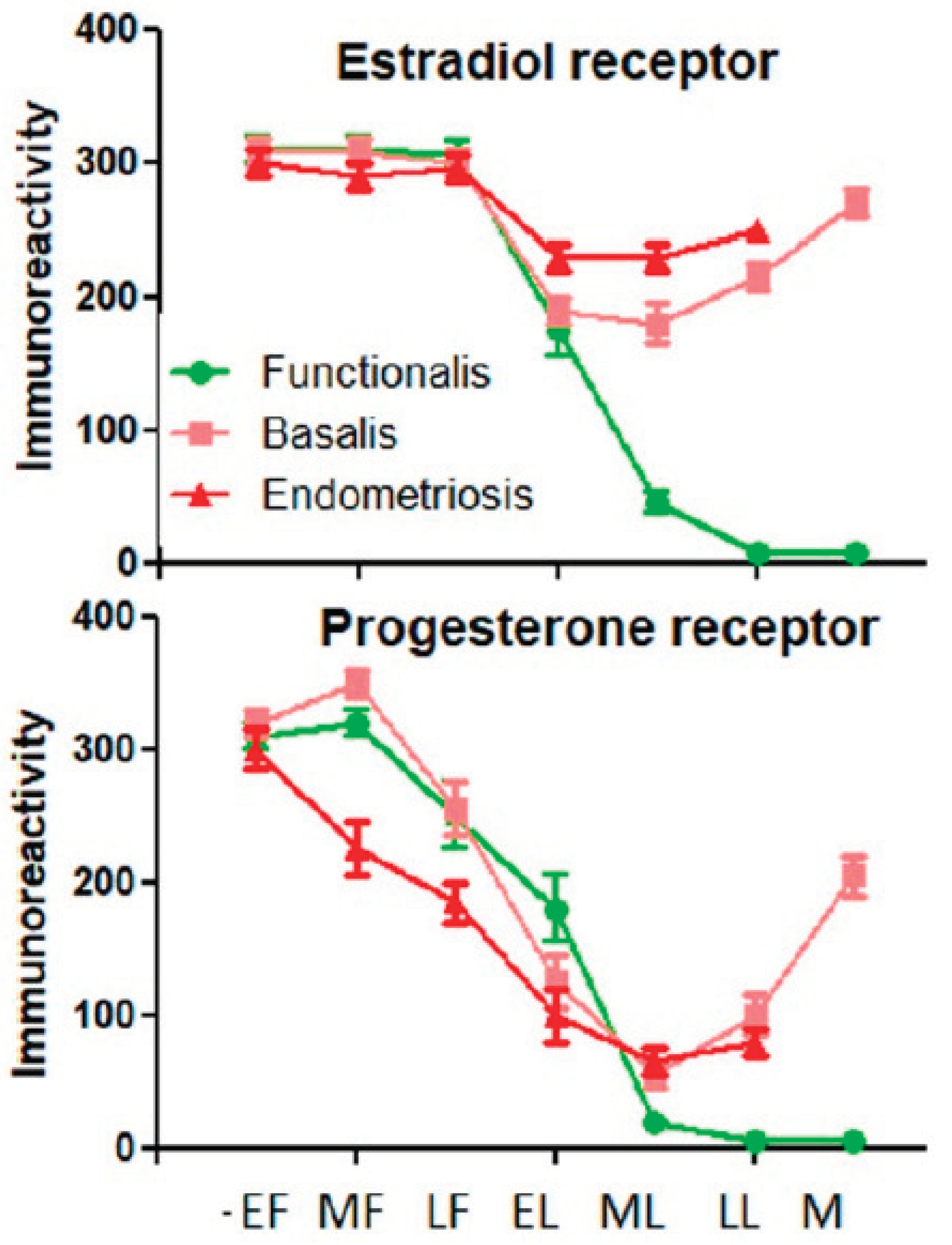
Poorly understood is the hormonal regulation of the basal endometrium. These rhizome-like clonal glands contain estrogen and progesterone receptors but with progesterone resistance (Figure 3).
3. Medical Therapy for Endometriosis.
Medical therapy for endometriosis was initiated in the 1970s [
22] based on the observations that endometriosis-associated pain decreased during pregnancy and during the intake of oral contraceptives, both estro-progestin combination and progestagen-only pills [
23]. Later, more androgenic progestins such as Danazol and gestrinone were used [
24]. Typical lesions were demonstrated to shrink during the use of these medications, but it was soon realized that endometriosis foci were only temporarily inactivated and that their metabolic activity resumed very early after drug discontinuation [
25].
3.1. Endometriosis and Medical Therapy
The peritoneum is similar to the skin and not vascularized, and the peritoneal cavity is a specific micro-environment outside the body with specific hormone concentrations, a specific immunology, and a specific microbiome. It seems logical that superficial layers of the pelvis, including superficial pelvic endometriosis, are mainly influenced by peritoneal fluid steroid hormone concentrations [
4], which are much higher than in plasma and with follicular progesterone concentrations as high as in the luteal phase in plasma. This explains that progesterone resistance must be postulated for subtle lesions to be proliferative. It also explains that typical pelvic endometriosis lesions are rarely proliferative and generally out of phase with the endometrium [
4]. Also, cystic ovarian endometriosis is likely influenced by the local intra-ovarian concentrations of estrogens and progesterone, which are higher because of local production or counter-current exchange from the ovarian vein to the ovarian artery [
26]. This explains that the endometriosis lining of endometriomas is rarely proliferative. However, large cystic ovarian endometriosis opened and drained during two-step surgery often displays proliferative endometriosis during the second intervention three months later (unpublished observation PK). This concept is also consistent with the observation in deep endometriosis, that the superficial parts of deep endometriosis were rather inactive like superficial lesions, while from a depth of 5 mm onwards, the endometriosis was more proliferative and in phase with the endometrium [
27] (Figure 5). Because of these observations, we suggested that the superficial parts were influenced by peritoneal fluid concentrations and the deeper parts by plasma [
28]. For this reason, and because of the biphasic distribution of depth with a nadir around 5 mm [
29], a depth greater than 5mm under the peritoneum was suggested as a definition for deep endometriosis [
30]. However, it should be understood that the exact depth at which the dominance of peritoneal fluid concentrations changes for plasma concentrations is progressive and might be variable by location.
Endometriosis lesions are clonal and biochemically heterogeneous, with some lesions having a variable aromatase activity and other lesions having variable progesterone resistance [
31,
32] and fewer progesterone receptors and less response to progestagenic medical therapy [
33]. Unfortunately, the type of endometriosis that was investigated was not specified. This would be logical for deeper parts of deep endometriosis under plasma hormonal control. Not all endometriosis lesions require estrogens for growth when aromatase activity is sufficient. This might explain why some deep endometriosis lesions can develop and grow after menopause in the absence of exogenous estrogens [
34].
3.2. Clinical Results of Medical Therapy for Endometriosis
Medical therapy of endometriosis with estro-progestogen combination [
35], progestogen monotherapy [
36,
37,
38], and GN-Rh agonists [
39] or antagonists [
40] suppress ovarian function and decrease estrogen synthesis and abolish progesterone secretion. Without addressing smaller differences between drugs, the overall efficacy of medical therapy on pelvic pain assumed to be caused by endometriosis seems to be around 70%, even in women with deep endometriosis [
41], with 10% to 30% of women experiencing little or no reduction in pain [
42]. For superficial pelvic endometriosis, the efficacy seems to parallel the decrease in estrogen concentrations in the peritoneal fluid, which can be more important for GnRH agonists or antagonists than for combined oral contraceptives. A specific effect of the different progestagenic compounds is not clearly demonstrated and is unlikely, considering the high concentrations in peritoneal fluid in the absence of any therapy. For deep endometriosis, the efficacy in the superficial parts can be considered similar to superficial lesions, whereas, for the deeper parts, a direct progestagenic effect seems logical. Unfortunately, the literature does not permit a clear stratification of the effect by depth of endometriosis. Since deep endometriosis lesions shrink during medical therapy [
43], they will become increasingly under peritoneal fluid control during therapy. Also, the size of cystic ovarian endometriosis decreases [
44].
Unfortunately, a full discussion of the possible placebo effects when blinding is problematic or of the inclusion of women with pain not due to endometriosis is beyond the scope of this manuscript. Also, the erroneous use of standard frequentist statistics in a heterogeneous population [
45] with variable aromatase activity and progesterone resistance suggests that more attention should be given to individual responses. Important to realize is that with 70% responders and 30% poor responders, the efficacy in the responders group is much better than the efficacy calculated for the whole group.
Aromatase inhibitors inhibit aromatase activity and, thus, estrogen production. Since monotherapy stimulates gonadotropin secretion [
46], aromatase inhibitors are used in combination with ovulation inhibitors for endometriosis therapy. It is not clear whether their efficacy results from a further reduction in plasma estrogen concentration or whether they decrease the local estrogen production within endometriosis lesions [
47,
48].
Central sensitization might also explain the lack of efficacy of medical therapy and surgery in some women, as reviewed recently [
49,
50].
4. Hormone Replacement Therapy and Endometriosis
It is unclear whether a history of endometriosis is a contra-indication for hormone replacement therapy (HRT) after menopause, since HRT might stimulate endometriosis lesions. Data are limited to anecdotal and clinical observations. A recent review [
51] concluded that low-quality evidence suggests that HRT does not stimulate endometriosis growth. Although guidelines do not recommend HRT, two-thirds of practitioners continue to prescribe HRT, apparently without endometriosis being stimulated [
52]. In the recent Relugolix Spirit studies, the addition of 1mg estradiol and 0.5mg norethisterone did not affect the results on pelvic pain [
53,
54].
4. Discussion
5.1. Mechanism of Action of Medical Therapy
Medical therapy inhibiting ovarian function is believed to decrease endometriosis stimulation by reducing estrogen concentrations in plasma and even more in peritoneal fluid and in the ovary. Aromatase inhibitors might decrease in addition intralesional estrogen production.
It is unclear whether, for superficial lesions, the progestogenic component of estro-progestins or progestogen monotherapies have an additional or specific effect on endometriosis besides estrogen reduction. Unfortunately, these effects are difficult to separate since they are associated with the strong ovulation inhibition effect of oral nor-testo progestins. Considering the concentrations of estrogens and progestins in peritoneal fluid and, thus, the effect on superficial endometriosis, it seems uncertain whether medical therapy may affect endometriosis besides decreasing estrogens [
4]. However, the deeper parts of endometriosis lesions, estimated around 5mm, are probably controlled by plasma hormone concentrations, and the progestagen content of medical therapy might have a specific effect.
Placebo effects exist and are difficult to quantify without blinding. Placebo effects can be strong, as judged during the TNFα trial. Performed with perfect blinding, some women coming monthly to the emergency for morphine injections were pain-free with a placebo. Similar effects must exist during medical therapy but are difficult to quantify outside rigorous research settings [
55]. Unfortunately, blinding of medical treatment is difficult, if not impossible, since women readily recognize active therapy.
5.2. Clinical Considerations of Medical Therapy
Medical therapy is highly effective in decreasing pelvic pain in about 70% of women with endometriosis. Therefore, considering the limited side effects, medical therapy is indicated as a first line of treatment in women with pelvic pain and suspicion of endometriosis, irrespective of whether the efficacy is a consequence of lower estrogens, a progesterone effect or a placebo effect. However, if pain relief is insufficient after three months of treatment, although it is difficult to define what is insufficient, diagnosis and treatment should be reconsidered. Other causes of pelvic pain might exist, such as venous congestion, adenomyosis, vascular pain such as Nutcrackers syndrome [
56,
57,
58] and many others. Surgery is also an option for patients who do not tolerate the side effects of hormonal medications. If endometriosis is found during laparoscopy, surgical excision should be considered. Medical therapy and surgery, thus, are complementary [
59,
60]. Another consequence of the biochemical and probably genetic or epigenetic variability of endometriosis is that some lesions might progress during medical therapy, similar to postmenopausal endometriosis. Therefore, a yearly follow-up with imaging is indicated even during hormonal suppression to detect eventual endometriosis growth [
61]. Alternatively, considering the individual variability in responses to estrogen concentrations, individualization of therapy might be considered, and individual women could have equal pain relief with less reduction of estrogen concentrations.
Planning the duration of medical therapy for endometriosis is complex. The side effects and risks of combined oral contraceptives are well documented, with a slight increased incidence of venous thrombosis from 2‰ to 6‰ and possibly a slight increase in cardiovascular incidents. However, considering the non-contraceptive benefits of oral contraception [
62], such as the decreased risk of ovarian and endometrial cancer, we suggest continuing medical therapy with low-dose oral contraceptives if effective. Medical therapy with more severe ovarian suppression, as obtained with LH-RH agonists or antagonists, is associated with a risk of osteoporosis, at least in women who are rapid bone losers. It should, therefore, not be used for more than three months unless combined with adjuvant add-back therapy. Moreover, GnRH agonists and antagonists should not be proposed as first-line therapies but selectively when patients do not respond, do not tolerate, or have contraindications to very low-dose oral contraceptives and progestogens.
Data for the perimenopausal period are not available. Besides a slight reduction in estrogen secretion, irregular cycles are often anovulatory. Therefore, It seems wise to treat anovulation with intermittent progestins to prevent unopposed estrogen stimulation of the endometrium, particularly when metrorrhagia is an issue. A similar attitude seems indicated for medical treatment of endometriosis: it will not harm if not beneficial.
5.3. Hormone Replacement Therapy and Endometriosis
The risks and benefits of hormone replacement therapy after menopause [
63] and the individualization of the type, dose and regimen of estroprogestins are beyond the scope of this manuscript. Also, the oncologic risk or endometriosis lesions after menopause will not be discussed.
However, in the literature, no robust data are available demonstrating a contraindication to HRT use in women with a history of endometriosis. Personally, I (PK) never refrained from giving HRT to the many women with a history of endometriosis, given our clinical focus on endometriosis combined with a menopause clinic since 1981 at KU Leuven. This attitude was based on data from the early 1980s on peritoneal fluid steroid hormone concentrations [
6] and the hypothesis that more than twice the normal dose of estrogens given during HRT would be needed to match peritoneal fluid concentrations. Out of prudence, however, continuous combined therapy was prescribed even in women without a uterus. Not considering HRT a contraindication in women with a history of endometriosis was later indirectly confirmed by our observation that in the large majority of women, endometriosis is a self-limiting disease, not progressive beyond a certain stage [
64] and the hypothesis that the risk of developing endometriosis after 30 years is minimal [
64]. That continuous combined hormone replacement is not contra-indicated in women with a history of endometriosis was recently confirmed in the Spirit trials [
54,
65], in which no difference in pain relief was observed between users of relugolix treatment with and without 1 mg estradiol and 0.5mg norethisterone. Although beyond the scope of this manuscript, a similar concept of higher uterine concentrations because of a countercurrent exchange between the ovarian artery and vein can be developed for adenomyosis.
5. Conclusions
Considering the much higher concentrations of estrogens and progesterone in peritoneal fluid compared to plasma is fundamental for understanding the natural history of endometriosis, the eventual progesterone resistance, and the planning of medical therapy for endometriosis. It is suggested that the efficacy of hormonal treatments for endometriosis-associated pain is likely to result from decreased estrogen concentrations in the peritoneal cavity and reduced peripheral blood levels. If true, progestogens might exert limited or no additive local effect on superficial endometriosis lesions. For the same reason, a history of endometriosis does not seem to constitute a contraindication to hormone replacement therapy.
Author Contributions
The content of this manuscript was extensively discussed during a session at the ENDO-Dubai meeting. PK wrote the draft, and all authors discussed and agreed on the text.
Funding
This research received no external funding.
Institutional Review Board Statement
The IRB board of KULeuven Gasthuisberg confirmed in writing that for experiments not involving human or animal tissues, IRB board approval was not requested.
Informed Consent Statement
Not applicable.
Data Availability Statement
not applicable
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: science and society. Am J Obstet Gynecol 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Am J Obstet Gynecol 1975, 122, 262–263. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Goddeeris, P.G.; Lauweryns, J.M.; De Hertogh, R.C.; Brosens, I.A. Accuracy of endometrial biopsy dating in relation to the midcycle luteinizing hormone peak. Fertil. Steril 1977, 28, 443–445. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal fluid progesterone and progesterone resistance in superficial endometriosis lesions. Human Reproduction 2022, 28, 209–219. [Google Scholar] [CrossRef]
- Gordts, S.; Grimbizis, G.; Tanos, V.; Koninckx, P.; Campo, R. Junctional zone thickening: an endo-myometrial unit disorder. Facts Views Vis Obgyn 2023, 15, 309–316. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Heyns, W.; Verhoeven, G.; Van, B.H.; Lissens, W.D.; De, M.P.; Brosens, I.A. Biochemical characterization of peritoneal fluid in women during the menstrual cycle. J Clin. Endocrinol. Metab 1980, 51, 1239–1244. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: the evolution of a disease and its extant presentation : Pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch Gynecol Obstet 2022. [Google Scholar] [CrossRef]
- Praetorius, T.H.; Leonova, A.; Lac, V.; Senz, J.; Tessier-Cloutier, B.; Nazeran, T.M.; Köbel, M.; Grube, M.; Kraemer, B.; Yong, P.J.; et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil Steril 2022. [Google Scholar] [CrossRef]
- Suda, K.; Cruz Diaz, L.A.; Yoshihara, K.; Nakaoka, H.; Yachida, N.; Motoyama, T.; Inoue, I.; Enomoto, T. Clonal lineage from normal endometrium to ovarian clear cell carcinoma through ovarian endometriosis. Cancer Science 2020, 111, 3000–3009. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front Endocrinol (Lausanne) 2021, 12, 745548. [Google Scholar] [CrossRef]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J Steroid Biochem Mol Biol 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Gorski, J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proceedings of the National Academy of Sciences 1996, 93, 15180–15184. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.C.; Ong, K.M.; Dougherty, E.J.; Simons, S.S., Jr. Inferring mechanisms from dose-response curves. Methods Enzymol 2011, 487, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Estrogen and related compounds: biphasic dose responses. Critical reviews in toxicology 2001, 31, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef]
- Giatti, S.; Diviccaro, S.; Falvo, E.; Garcia-Segura, L.M.; Melcangi, R.C. Physiopathological role of the enzymatic complex 5α-reductase and 3α/β-hydroxysteroid oxidoreductase in the generation of progesterone and testosterone neuroactive metabolites. Front. Neuroendocrinol. 2020, 57, 100836. [Google Scholar] [CrossRef]
- Kletzky, O.A.; Nakamura, R.M.; Thorneycroft, I.H.; Mishell, D.R., Jr. Log normal distribution of gonadotropins and ovarian steroid values in the normal menstrual cycle. Am J Obstet Gynecol 1975, 121, 688–694. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Renaer, M.; Brosens, I.A. Origin of peritoneal fluid in women: an ovarian exudation product. Br. J. Obstet. Gynaecol 1980, 87, 177–183. [Google Scholar] [CrossRef]
- Hunter, R.H.; Cook, B.; Poyser, N.L. Regulation of oviduct function in pigs by local transfer of ovarian steroids and prostaglandins: a mechanism to influence sperm transport. Eur J Obstet Gynecol Reprod Biol 1983, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zervomanolakis, I.; Ott, H.W.; Müller, J.; Seeber, B.E.; Friess, S.C.; Mattle, V.; Virgolini, I.; Heute, D.; Wildt, L. Uterine mechanisms of ipsilateral directed spermatozoa transport: Evidence for a contribution of the utero-ovarian countercurrent system. Eur J Obstet Gynecol Reprod Biol 2009, 144 Suppl 1, S45–49. [Google Scholar] [CrossRef]
- Einer-Jensen, N.; Hunter, R. Counter-current transfer in reproductive biology. Reproduction 2005, 129, 9–18. [Google Scholar] [CrossRef]
- Kistner, R.W. Management of endometriosis in the infertile patient. Fertil. Steril 1975, 26, 1151–1166. [Google Scholar] [CrossRef]
- Moghissi, K.S. Pseudopregnancy induced by estrogen-progestogen or progestogens alone in the treatment of endometriosis. Prog. Clin. Biol. Res 1990, 323, 221–232. [Google Scholar] [PubMed]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane. Database. Syst. Rev 2007, CD000068. [Google Scholar]
- Evers, J.L.H. The second-look laparoscopy for evaluation of the result of medical treatment of endometriosis should not be performed during ovarian suppression. Fertil. Steril 1987, 47, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Muyldermans, M.; Moerman, P.; Meuleman, C.; Deprest, J.; Cornillie, F. CA 125 concentrations in ovarian 'chocolate' cyst fluid can differentiate an endometriotic cyst from a cystic corpus luteum. Hum. Reprod 1992, 7, 1314–1317. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Oosterlynck, D.; D'Hooghe, T.; Meuleman, C. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann N Y Acad Sci 1994, 734, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, F.J.; Oosterlynck, D.; Lauweryns, J.M.; Koninckx, P.R. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil. Steril 1990, 53, 978–983. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril 2019, 111, 327–339. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Martin, D.C. Deep endometriosis: a consequence of infiltration or retraction or possibly adenomyosis externa? Fertil. Steril 1992, 58, 924–928. [Google Scholar] [CrossRef]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum Reprod Update 2019. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J Clin Endocrinol Metab 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Asencio, F.; Ribeiro, H.A.; Ribeiro, P.A.; Malzoni, M.; Adamyan, L.; Ussia, A.; Gomel, V.; Martin, D.C.; Koninckx, P.R. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: a systematic review and seven case reports. Gynecological Surgery 2019, 16, 3. [Google Scholar] [CrossRef]
- Brown, J.; Crawford, T.J.; Datta, S.; Prentice, A. Oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Dragoman, M.V.; Gaffield, M.E. The safety of subcutaneously administered depot medroxyprogesterone acetate (104 mg/0.65 mL): A systematic review. Contraception 2016, 94, 202–215. [Google Scholar] [CrossRef]
- Andres Mde, P.; Lopes, L.A.; Baracat, E.C.; Podgaec, S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet 2015, 292, 523–529. [Google Scholar] [CrossRef]
- Brown, J.; Kives, S.; Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2012. [Google Scholar] [CrossRef]
- Veth, V.B.; van de Kar, M.M.; Duffy, J.M.; van Wely, M.; Mijatovic, V.; Maas, J.W. Gonadotropin-releasing hormone analogues for endometriosis. Cochrane Database Syst Rev 2023, 6, Cd014788. [Google Scholar] [CrossRef]
- Xin, L.; Ma, Y.; Ye, M.; Chen, L.; Liu, F.; Hou, Q. Efficacy and safety of oral gonadotropin-releasing hormone antagonists in moderate-to-severe endometriosis-associated pain: a systematic review and network meta-analysis. Arch Gynecol Obstet 2023, 308, 1047–1056. [Google Scholar] [CrossRef]
- Vercellini, P.; Sergenti, G.; Buggio, L.; Frattaruolo, M.P.; Dridi, D.; Berlanda, N. Advances in the medical management of bowel endometriosis. Best Practice & Research Clinical Obstetrics & Gynaecology 2021, 71, 78–99. [Google Scholar] [CrossRef]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertility and Sterility 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Bianchi, S.; Zanconato, G.; Tozzi, L.; Raffaelli, R. Gonadotropin-releasing hormone agonist treatment for endometriosis of the rectovaginal septum. Am J Obstet Gynecol 2000, 183, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.; Nguyen, D.B.; Smith, J.P.; Mansour, F.W.; Krishnamurthy, S.; Zakhari, A. Medical Management of Ovarian Endometriomas: A Systematic Review and Meta-analysis. Obstet Gynecol 2024, 143, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Heterogeneity of endometriosis lesions requires individualization of diagnosis and treatment and a different approach to research and evidence based medicine. Facts Views Vis Obgyn 2020, 11, 263. [Google Scholar] [PubMed]
- Cantineau, A.E.; Rutten, A.G.; Cohlen, B.J. Agents for ovarian stimulation for intrauterine insemination (IUI) in ovulatory women with infertility. Cochrane Database of Systematic Reviews 2021, 2021. [Google Scholar] [CrossRef]
- Peitsidis, P.; Tsikouras, P.; Laganà, A.S.; Laios, A.; Gkegkes, I.D.; Iavazzo, C. A Systematic Review of Systematic Reviews on the Use of Aromatase Inhibitors for the Treatment of Endometriosis: The Evidence to Date. Drug Des Devel Ther 2023, 17, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Perrone, U.; Evangelisti, G.; Laganà, A.S.; Bogliolo, S.; Ceccaroni, M.; Izzotti, A.; Gustavino, C.; Ferrero, S.; Barra, F. A review of phase II and III drugs for the treatment and management of endometriosis. Expert opinion on emerging drugs 2023, 28, 333–351. [Google Scholar] [CrossRef]
- Cetera, G.E.; Merli, C.E.M.; Barbara, G.; Caia, C.; Vercellini, P. Questionnaires for the Assessment of Central Sensitization in Endometriosis: What Is the Available Evidence? A Systematic Review with a Narrative Synthesis. Reproductive Sciences 2023. [Google Scholar] [CrossRef]
- Ping, Z.; Wen, Z.; Jinhua, L.; Jinghe, L. <p>Research on central sensitization of endometriosis-associated pain: a systematic review of the literature</p>. J. Pain Res. 2019, Volume 12, 1447–1456. [Google Scholar] [CrossRef]
- Zanello, M.; Borghese, G.; Manzara, F.; Degli Esposti, E.; Moro, E.; Raimondo, D.; Abdullahi, L.O.; Arena, A.; Terzano, P.; Meriggiola, M.C.; Seracchioli, R. Hormonal Replacement Therapy in Menopausal Women with History of Endometriosis: A Review of Literature. Medicina 2019, 55, 477. [Google Scholar] [CrossRef]
- Amer, S.; Bazmi, S. HRT in Women Undergoing Pelvic Clearance for Endometriosis—A Case Report and a National Survey. Journal of Clinical Medicine 2023, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Dynowski, K.; Wilk, K.; Li, Y.; Mathur, V.; et al. A Plain Language Summary to learn about relugolix combination therapy for the treatment of pain associated with endometriosis. Pain Management 2023, 13, 631–640. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Brown, E.; Dynowski, K.; Wilk, K.; Li, Y.; et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomized, double-blind, studies (SPIRIT 1 and 2). The Lancet 2022, 399, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum. Reprod 2008, 23, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Dewald, C.L.A.; Becker, L.S.; Meyer, B.C. Interventional Therapy of Pelvic Venous Disorders (PeVD). Rofo 2024. [Google Scholar] [CrossRef]
- Ozsvath, K.; Raffetto, J.D.; Lindner, E.; Murphy, E.H. Venous compression syndromes in females: A descriptive review. Semin. Vasc. Surg. 2023, 36, 550–559. [Google Scholar] [CrossRef]
- Maharaj, D.; Mohammed, S.R.; Caesar, K.; Dindyal, S. Nutcracker syndrome: a case-based review. Ann R Coll Surg Engl 2023. [Google Scholar] [CrossRef]
- Mijatovic, V.; Vercellini, P. Towards comprehensive management of symptomatic endometriosis: beyond the dichotomy of medical versus surgical treatment. Hum Reprod 2024, 39, 464–477. [Google Scholar] [CrossRef]
- Vercellini, P.; Vigano, P.; Buggio, L.; Somigliana, E. "We Can Work It Out:" The Hundred Years' War between Experts of Surgical and Medical Treatment for Symptomatic Deep Endometriosis. J Minim Invasive Gynecol 2018, 25, 356–359. [Google Scholar] [CrossRef]
- Wattiez, A.; Schindler, L.; Ussia, A.; Campo, R.; Keckstein, J.; Grimbizis, G.; Exacoustos, C.; Kondo, W.; Nezhat, C.; Canis, M.; et al. A proof of concept that experience-based management of endometriosis can complement evidence-based guidelines. Facts, Views and Vision in ObGyn 2023, 15, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.C.; Bentz, E.-K.; Ott, J.; Tempfer, C.B. Non-contraceptive benefits of oral contraceptives. Expert Opin. Pharmacother. 2008, 9, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Voedisch, A.J. Counseling on hormone replacement therapy: the real risks and benefits. Curr Opin Obstet Gynecol 2023, 35, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Wattiez, A.; Adamyan, L.; Martin, D.C.; Gordts, S. The severity and frequency distribution of endometriosis subtypes at different ages: a model to understand the natural history of endometriosis based on single centre/single surgeon data. Facts Views Vis Obgyn 2021, 13, 211–221. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Brown, E.; Dynowski, K.; Wilk, K.; Li, Y.; et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomized, double-blind, studies (SPIRIT 1 and 2). Lancet 2022, 399, 2267–2279. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).