Submitted:
07 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
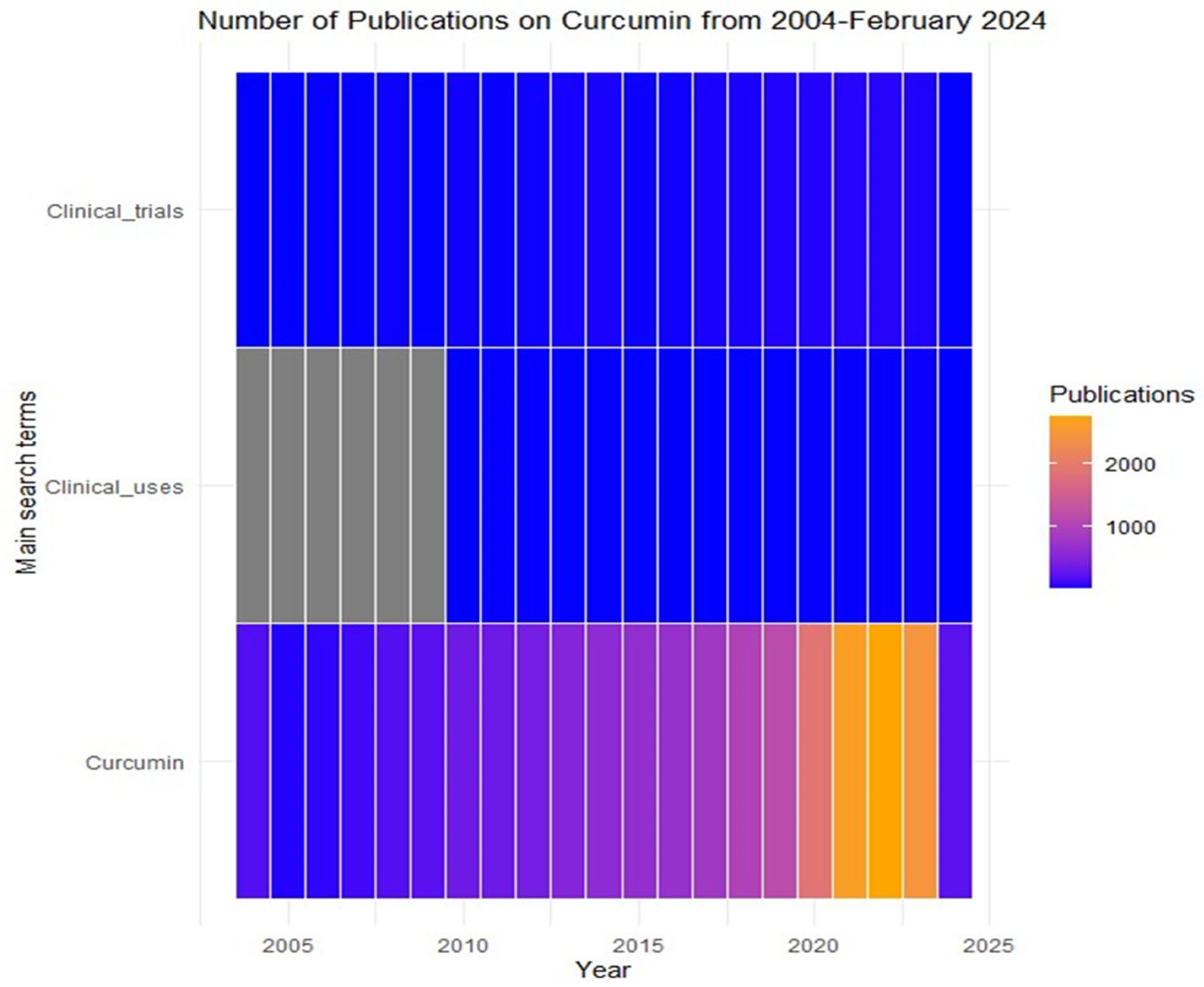
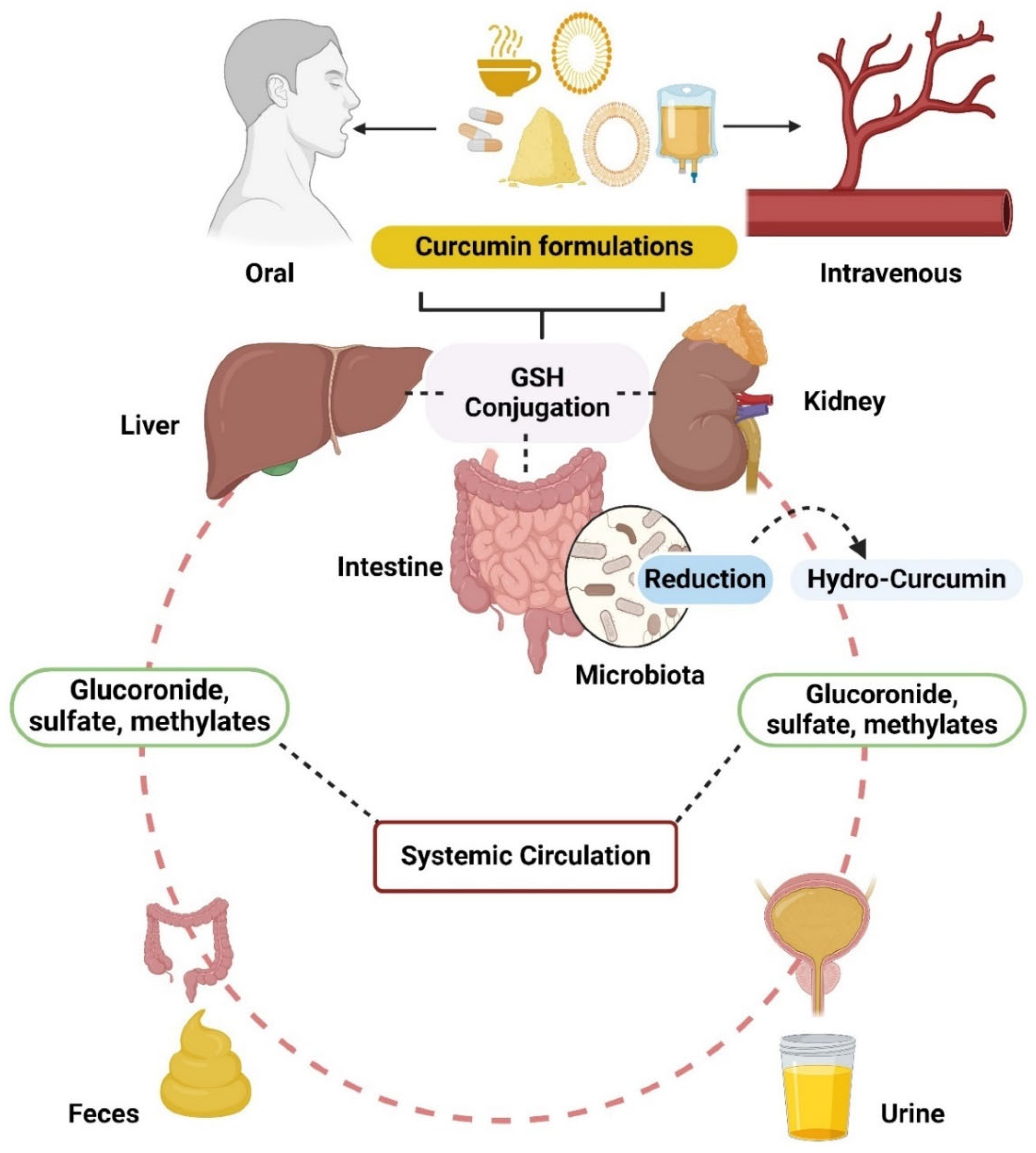
Pharmacokinetic Profile of Curcumin
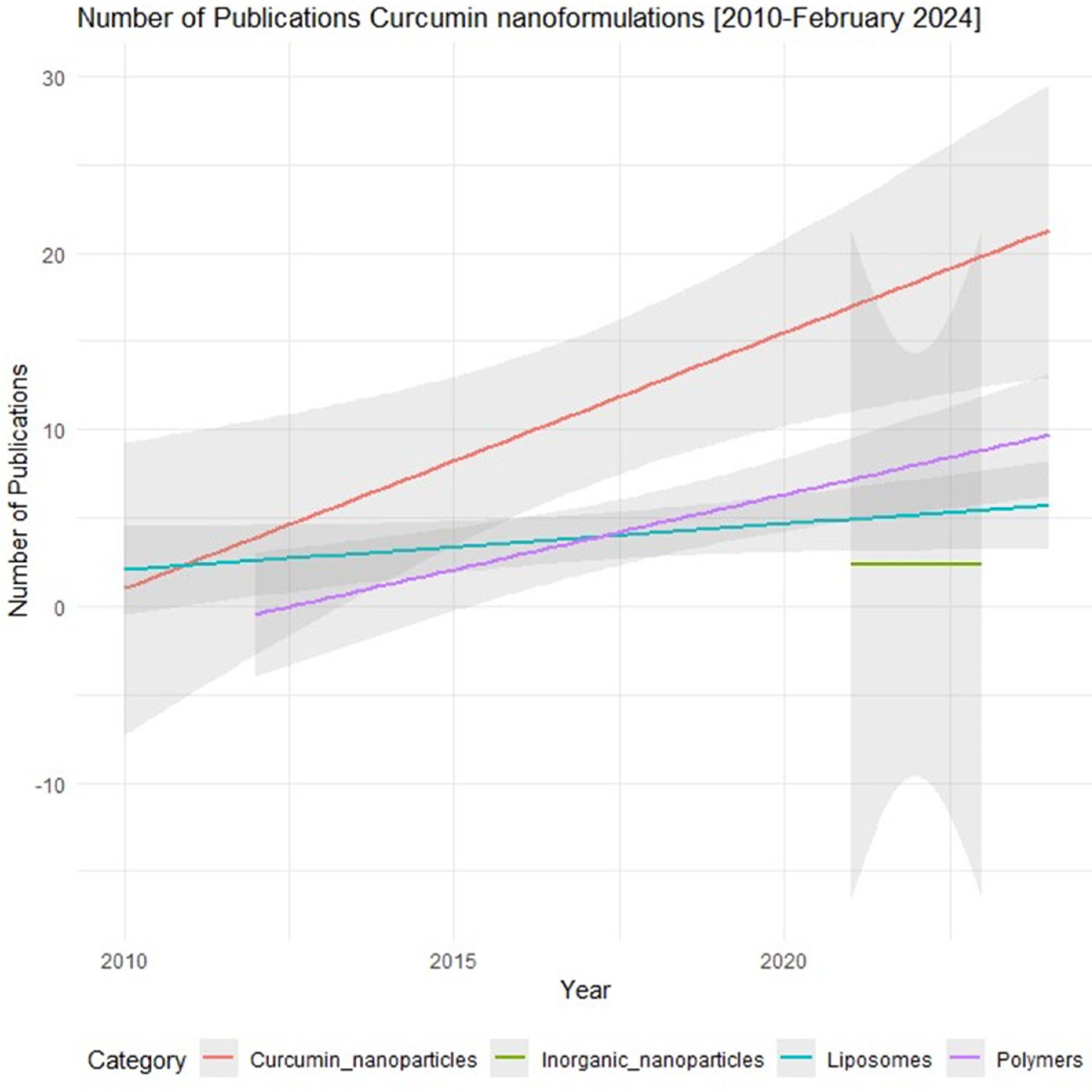
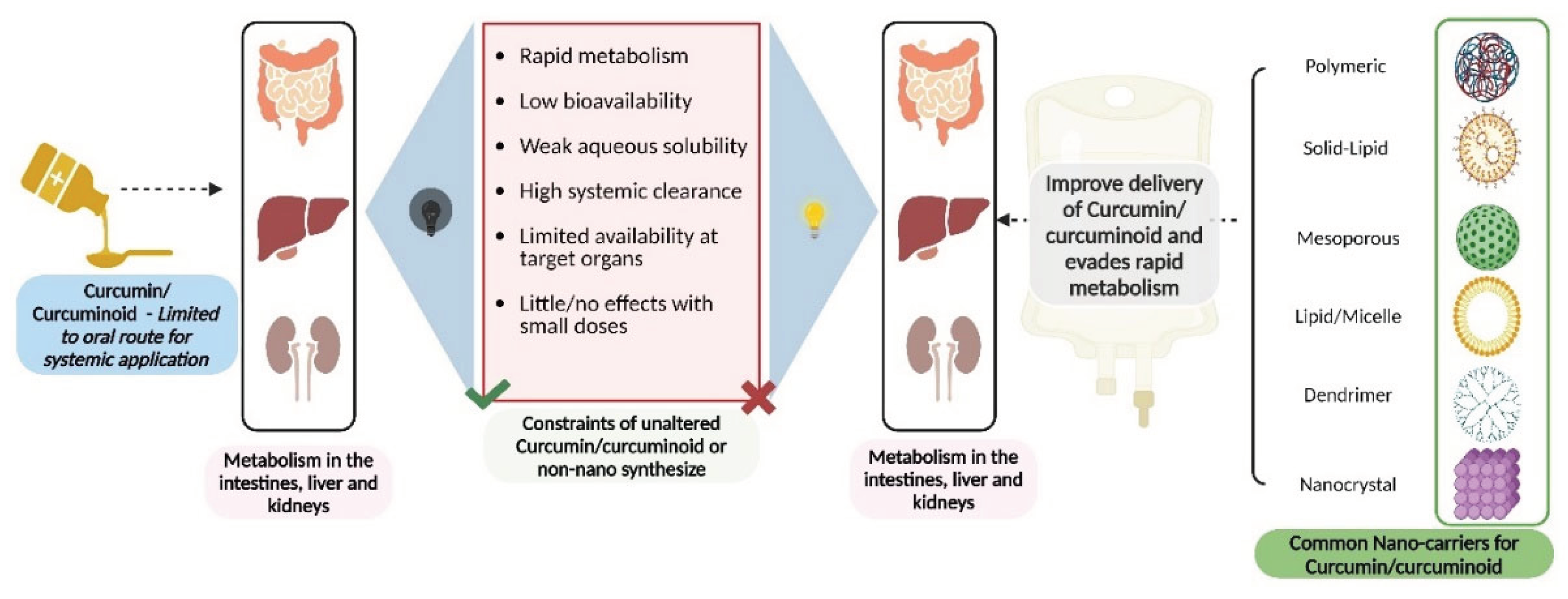
Current Curcumin Nanoformulations and Their Pharmacological Profile
Other Novel Formulations of Curcumin
Combinations of Curcumin with Other Therapeutic Agents
| Form of Curcumin | Combinatory Agent (s) | Clinical Use | Outcome | Type of Study | Reference |
|---|---|---|---|---|---|
| Nano-Curcumin | Docetaxel | Glioma | Easy passage through the BBB and reduce the toxic effects of high dose docetaxel | Preclinical | [24] |
| Curcumin | Omeprazole, amoxicillin, and metronidazole | Gastritis– associated Helicobacter pylori infection. | Eradication of Helicobacter pylori infection | Clinical | [87] |
| Curcumin | Long-chain omega-3 polyunsaturated fatty acids | Type II Diabetes | Reduces blood lipids, increase insulin sensitivity but has no effect on blood glucose | Clinical | [93,94] |
| Curcumin | Chlorogenic acid and coconut yogurt | Inflammation | Anti-inflammatory effects | Clinical | [91] |
| Curcumin | Fennel essential oil | Irritable bowel syndrome | Safe and effective | Clinical | [88] |
| Curcumin | Docetaxel | Metastatic castration-resistant prostate cancer | No therapeutic benefit | Clinical | [97] |
| Curcumin | Aloe Vera gel | Oral Submucous Fibrosis | Anti-inflammatory | Clinical | [85] |
| Curcumin | Piperine | COVID-19, ischemic stroke | Increase bioavailability and Energy booster | Preclinical and Clinical | [77,78,79] |
| Curcumin | Alendronate | Osteoporosis | Anti-inflammatory | Clinical | [99] |
| Curcumin | Dexamethasone and hyaluronidase | Oral Submucous Fibrosis | Anti-inflammatory | Clinical | [86] |
| Curcumin and curcuminoids, entrapped in a patented delivery system (LipiSperse®) | Ferrous sulphate | Inflammation | Anti-inflammatory effects | Clinical | [90,92] |
| Curcumin and curcuminoid | Blue light, Sodium dodecyl sulphate | Oral disinfectant | Reduction of salivary microorganisms | Clinical | [82,83,84] |
| Curcumin | Resveratrol | Malnutrition | Increase in Bone and Muscle mass, reduction in fat | Clinical | [101] |
| Curcumin and desmethoxycurcumin and bisdemethoxycurcumin | folinic acid-5-fluorouracil-oxaliplatin chemotherapy | Metastatic Colorectal Cancer | Improves the quality of life of Metastatic Colorectal Cancer patients | Clinical | [95,96] |
| Curcuminoids and Turmeric oil | Diclofenac | Knee osteoarthritis | Tolerable and analgesic | Clinical | [102] |
| Curcuminoids extract | Hydrolysed collagen, and green tea extract | Osteoarthritis | Modulates key catabolic, inflammatory, and angiogenesis factors associated with osteoarthritis progression. | Clinical | [98] |
| Nanocurcumin | Omega-3 fatty acids | Episodic migraine | Reduce serum VCAM level | Clinical | [103] |
| Turmeric Phytosome® (equivalent to 10 mg of curcumin) | Boswellia serrata (BSE) gum resin | Oxidative stress and inflammation | Anti-inflammatory and antioxidant effects | Clinical | [89] |
| Turmeric volatile oil | Boswellic acid extract from Indian frankincense root | Osteoarthritis | Analgesic | Clinical | [100] |
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Tomeh, M.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. International Journal of Molecular Sciences 2019, 20, 1033. [Google Scholar] [CrossRef]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive Review on Physiological Effects of Curcumin. Drug Research 2020, 70, 441–447. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in Cancer Chemoprevention: Molecular Targets, Pharmacokinetics, Bioavailability, and Clinical Trials. Archiv der Pharmazie 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Rodríguez, C.; Parween, P. Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway. International Journal of Molecular Sciences 2019, 20, 4606. [Google Scholar] [CrossRef]
- Mehmet, H.; Ucisik, S.K. Bernhard Schuster & Uwe B Sleytr Characterization of CurcuEmulsomes: nanoformulation for enhanced solubility anddelivery of curcumin. Journal of Nanobiotechnology 2013. [Google Scholar] [CrossRef]
- Giordano and Tommonaro, Curcumin and Cancer. Nutrients 2019, 11, 2376. [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-Y.; Ngai, S.C.; Goh, B.-H.; Lee, L.-H.; Htar, T.-T.; Chuah, L.-H. Is Curcumin the Answer to Future Chemotherapy Cocktail? Molecules 2021, 26, 4329. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Pi, C.; Yang, H.; Zheng, X.; Zhao, L.; Wei, Y. Review of Curcumin Physicochemical Targeting Delivery System. International Journal of Nanomedicine 2020, 15, 9799–9821. [Google Scholar] [CrossRef]
- Putu Yudhistira Budhi, S.; Nyoman, K.; Arief, N.; Subagus, W. Curcumin in combination: Review of synergistic effects and mechanisms in the treatment of inflammation. Journal of Applied Pharmaceutical Science 2020. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M.; Machado-Orellana, M.G. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules 2022, 27, 5055. [Google Scholar] [CrossRef] [PubMed]
- Matthewman, C.; Krishnakumar, I.M.; Swick, A.G. Review: bioavailability and efficacy of ‘free’ curcuminoids from curcumagalactomannoside (CGM) curcumin formulation. Nutrition Research Reviews 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Jarvis, M.; Krishnan, V.; Mitragotri, S. Nanocrystals: A perspective on translational research and clinical studies. Bioengineering & Translational Medicine 2018, 4, 5–16. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: history, challenges, and latest developments. Journal of Biological Engineering 2022, 16. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Jangde, R.K. Current updated review on preparation of polymeric nanoparticles for drug delivery and biomedical applications. Next Nanotechnology 2023, 2, 100013. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. International Journal of Molecular Sciences 2023, 24, 2643. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Seko, I.; Tonbul, H.; Tavukçuoğlu, E.; Şahin, A.; Akbas, S.; Yanık, H.; Öztürk, S.C.; Esendagli, G.; Khan, M.; Capan, Y. Development of curcumin and docetaxel co-loaded actively targeted PLGA nanoparticles to overcome blood brain barrier. Journal of Drug Delivery Science and Technology 2021, 66, 102867. [Google Scholar] [CrossRef]
- R. H. Müller a, M.R.b., S.A. Wissing b. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Pareek, A.; Kothari, R.; Pareek, A.; Ratan, Y.; Kashania, P.; Jain, V.; Jeandet, P.; Kumar, P.; Khan, A.A.; Alanazi, A.M.; et al. Development of a new inhaled swellable microsphere system for the dual delivery of naringenin-loaded solid lipid nanoparticles and doxofylline for the treatment of asthma. European Journal of Pharmaceutical Sciences 2024, 193, 106642. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Scientia Pharmaceutica 2019, 87, 17. [Google Scholar] [CrossRef]
- Preeti; et al. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 1–25. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-inflammatory effects of oral supplementation with curcumin: a systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews 2021, 79, 1043–1066. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discovery Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, C.Y.O.; Preuss, H.G.; Ray, S.D.; Bucci, L.R.; Ji, J.; Ruff, K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: a comparative study. BMC Complementary and Alternative Medicine 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Kothaplly, S.; Alukapally, S.; Nagula, N.; Maddela, R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Advances in Therapy 2022, 39, 2128–2138. [Google Scholar] [CrossRef]
- Mustafa Usta, H.M.W. , Jacques Vervoort, Marelle G. Boersma, and P.J.v.B. Ivonne M. C. M. Rietjens, and Nicole H. P. Cnubben. Human Glutathione S-Transferase-Mediated Glutathione Conjugation of Curcumin and Efflux of These Conjugates in Caco-2 Cells. Chem. Res. Toxicol. 2007, 1895–1902. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine 2006, 6. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Nurmila, S.; Katanasaka, Y.; Ito, M.; Ogawa, T.; Ozawa-Umeta, H.; et al. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, two-way crossover study. Journal of Functional Foods 2021, 81, 104443. [Google Scholar] [CrossRef]
- Komiyama, M.; Ozaki, Y.; Wada, H.; Yamakage, H.; Satoh-Asahara, N.; Kishimoto, A.; Katsuura, Y.; Imaizumi, A.; Hashimoto, T.; Sunagawa, Y.; Morimoto, T. Study protocol to determine the effects of highly absorbable oral curcumin on the indicators of cognitive functioning: a double- blind randomised controlled trial. BMJ open 2022, 12, e057936. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Katanasaka, Y.; Hirano, S.; Namiki, M.; Watanabe, Y.; Suzuki, H.; Doi, O.; Suzuki, K.; Yamauchi, M.; et al. Drinkable preparation of Theracurmin exhibits high absorption efficiency--a single-dose, double-blind, 4-way crossover study. Biol Pharm Bull 2013, 36, 1708–1714. [Google Scholar] [CrossRef]
- Tanabe, Y.; Maeda, S.; Akazawa, N.; Zempo-Miyaki, A.; Choi, Y.; Ra, S.G.; Imaizumi, A.; Otsuka, Y.; Nosaka, K. Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur J Appl Physiol 2015, 115, 1949–1957. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Murata, S.; Yabumoto, H.; Maeda, T.; Akamatsu, S. The Efficacy and Safety of Highly-Bioavailable Curcumin for Treating Knee Osteoarthritis: A 6-Month Open-Labeled Prospective Study. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders 2020, 13, 1179544120948471. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemotherapy and Pharmacology 2018, 82, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Ouyang, A.; I. M, K.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z.; Reiner, Z.; Soleimani, A.; Aghadavod, E.; Bahmani, F. The Effects of Nano-curcumin on Metabolic Status in Patients With Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran J Kidney Dis 2020, 14, 290–299. [Google Scholar] [PubMed]
- Kheiridoost, H.; Shakouri, S.K.; Shojaei-Zarghani, S.; Dolatkhah, N.; Farshbaf-Khalili, A. Efficacy of nanomicelle curcumin, Nigella sativa oil, and their combination on bone turnover markers and their safety in postmenopausal women with primary osteoporosis and osteopenia: A triple-blind randomized controlled trial. Food Science & Nutrition 2021, 10, 515–524. [Google Scholar] [CrossRef]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: a randomized clinical trial. BMC Complement Med Ther 2021, 21, 232. [Google Scholar] [CrossRef]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-Inflammatory Effects of Novel Standardized Solid Lipid Curcumin Formulations. J Med Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Gupte, P.A.; Giramkar, S.A.; Harke, S.M.; Kulkarni, S.K.; Deshmukh, A.P.; Hingorani, L.L.; Mahajan, M.P.; Bhalerao, S.S. Evaluation of the efficacy and safety of Capsule Longvida(®) Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: a pilot clinical study. J Inflamm Res 2019, 12, 145–152. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hassaniazad, M.; Eftekhar, E.; Inchehsablagh, B.R.; Kamali, H.; Tousi, A.; Jaafari, M.R.; Rafat, M.; Fathalipour, M.; Nikoofal-Sahlabadi, S.; Gouklani, H.; et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytotherapy Research 2021, 35, 6417–6427. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Landolf, K.M.; Barlow, B.; Yeung, S.Y.A.; Heavner, J.J.; Claassen, C.W.; Heavner, M.S. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy 2020, 40, 416–437. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Abdolmohammadi-vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. International Immunopharmacology 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Conterato, G.M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: implications for COVID-19. The Journal of Nutritional Biochemistry 2021, 97, 108787. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.; Freire, D.T.; Pereira, D.T.; Amaral-Machado, L.; Alencar, É.N.; de Barros, A.L.B.; Egito, E.S.T. Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomedicine & Pharmacotherapy 2021, 139, 111578. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mehrabi, Z.; Zare, M.; Ghadir, S.; Masoumi, S.J.; de Souza, L.N. Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial. International Journal of Clinical Practice 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicology Reports 2021, 8, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Saji, S.; Asha, S.; Svenia, P.J.; Ratheesh, M.; Sheethal, S.; Sandya, S.; Krishnakumar, I.M. Curcumin-galactomannoside complex inhibits pathogenesis in Ox-LDL-challenged human peripheral blood mononuclear cells. Inflammopharmacology 2018, 26, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- T Krishnareddy, N.; Thomas, J.V.; Nair, S.S.; N. Mulakal, J.; Maliakel, B.P.; Krishnakumar, I.M. A Novel Curcumin-Galactomannoside Complex Delivery System Improves Hepatic Function Markers in Chronic Alcoholics: A Double-Blinded, randomized, Placebo-Controlled Study. BioMed Research International 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- M, R.; Jose, S.; Im, K.; S, S.; Saji, S.; S, S. Curcumin-Galactomannoside Complex inhibits the Proliferation of Human Cervical Cancer Cells: Possible Role in Cell Cycle Arrest and Apoptosis. Asian Pacific Journal of Cancer Prevention 2021, 22, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gastrointestin Liver Dis 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European Journal of Nutrition 2019, 59, 477–483. [Google Scholar] [CrossRef]
- Morimoto, T.; Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. Journal of Diabetes Research 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dost, F.S.; Kaya, D.; Ontan, M.S.; Erken, N.; Bulut, E.A.; Aydin, A.E.; Isik, A.T. Theracurmin Supplementation May be a Therapeutic Option for Older Patients with Alzheimer's Disease: A 6-Month Retrospective Follow-Up Study. Curr Alzheimer Res 2021, 18, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Delost, M.D.; Qureshi, M.H.; Smith, D.T.; Njardarson, J.T. A Survey of the Structures of US FDA Approved Combination Drugs. J Med Chem 2019, 62, 4265–4311. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H. Repurposing approved drugs on the pathway to novel therapies. Med Res Rev 2020, 40, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Sahebkar, A.; Soleimani, D.; Mahdavi, A.; Rafiee, S.; Majeed, M.; Khorvash, F.; Iraj, B.; Elyasi, M.; Rouhani, M.H.; et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials 2022, 23. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: a randomized controlled trial. Nutrition Journal 2023, 22. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Cai, Q.; Ren, Q.; Wei, L. Red Light Combined with Blue Light Irradiation Regulates Proliferation and Apoptosis in Skin Keratinocytes in Combination with Low Concentrations of Curcumin. PLOS ONE 2015, 10, e0138754. [Google Scholar] [CrossRef]
- Alkahtani, S.; S. Al-Johani, N.; Alarifi, S.; Afzal, M. Cytotoxicity Mechanisms of Blue-Light-Activated Curcumin in T98G Cell Line: Inducing Apoptosis through ROS-Dependent Downregulation of MMP Pathways. International Journal of Molecular Sciences 2023, 24, 3842. [Google Scholar] [CrossRef]
- Araújo, N.C.; Fontana, C.R.; Gerbi, M.E.; Bagnato, V.S. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed Laser Surg 2012, 30, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: a randomized controlled trial. Photomed Laser Surg 2014, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Panhóca, V.H.; Esteban Florez, F.L.; Corrêa, T.Q.; Paolillo, F.R.; de Souza, C.W.; Bagnato, V.S. Oral Decontamination of Orthodontic Patients Using Photodynamic Therapy Mediated by Blue-Light Irradiation and Curcumin Associated with Sodium Dodecyl Sulfate. Photomed Laser Surg 2016, 34, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nerkar Rajbhoj, A.; Kulkarni, T.M.; Shete, A.; Shete, M.; Gore, R.; Sapkal, R. A Comparative Study to Evaluate Efficacy of Curcumin and Aloe Vera Gel along with Oral Physiotherapy in the Management of Oral Submucous Fibrosis: A Randomized Clinical Trial. Asian Pacific Journal of Cancer Prevention 2021, 22, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Rimal, J.; Maharjan, I.; Shrestha, A. Efficacy of Curcumin in Combination with Intralesional Dexamethasone with Hyaluronidase in the Treatment of Oral Submucous Fibrosis: A Randomized Controlled Trial. Asian Pacific Journal of Cancer Prevention 2022, 23, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Judaki, A.; Rahmani, A.; Feizi, J.; Asadollahi, K.; Hafezi Ahmadi, M.R. Curcumin in Combination with Triple Therapy Regimes Ameliorates Oxidative Stress and Histopathologic Changes in Chronic Gastritis-Associated Helicobacter Pylori Infection. Arquivos de Gastroenterologia 2017, 54, 177–182. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Grimaldi, M.; Fogli, M.V.; Taddia, M.; Guarino, M.; Di Rienzo, T.; Campanale, M.C.; Festi, D.; Caporaso, N.; Kohn, A.; et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. Journal of Gastrointestinal and Liver Diseases 2016, 25, 151–157. [Google Scholar] [CrossRef]
- Chilelli, N.; Ragazzi, E.; Valentini, R.; Cosma, C.; Ferraresso, S.; Lapolla, A.; Sartore, G. Curcumin and Boswellia serrata Modulate the Glyco-Oxidative Status and Lipo-Oxidation in Master Athletes. Nutrients 2016, 8, 745. [Google Scholar] [CrossRef]
- Lorinczova, H.T.; Begum, G.; Renshaw, D.; Zariwala, M.G. Acute Administration of Bioavailable Curcumin Alongside Ferrous Sulphate Supplements Does Not Impair Iron Absorption in Healthy Adults in a Randomised Trial. Nutrients 2021, 13, 2300. [Google Scholar] [CrossRef]
- Ahmed Nasef, N.; Thota, R.N.; Mutukumira, A.N.; Rutherfurd-Markwick, K.; Dickens, M.; Gopal, P.; Singh, H.; Garg, M.L. Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients 2022, 14, 4619. [Google Scholar] [CrossRef] [PubMed]
- Tiekou Lorinczova, H.; Begum, G.; Temouri, L.; Renshaw, D.; Zariwala, M.G. Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Acharya, S.H.; Abbott, K.A.; Garg, M.L. Curcumin and long-chain Omega-3 polyunsaturated fatty acids for Prevention of type 2 Diabetes (COP-D): study protocol for a randomised controlled trial. Trials 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids in Health and Disease 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. The Journal of Nutrition 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Passildas-Jahanmohan, J.; Eymard, J.C.; Pouget, M.; Kwiatkowski, F.; Van Praagh, I.; Savareux, L.; Atger, M.; Durando, X.; Abrial, C.; Richard, D.; et al. Multicenter randomized phase II study comparing docetaxel plus curcumin versus docetaxel plus placebo in first-line treatment of metastatic castration-resistant prostate cancer. Cancer Medicine 2021, 10, 2332–2340. [Google Scholar] [CrossRef]
- Lammi, M.; Comblain, F.; Dubuc, J.-E.; Lambert, C.; Sanchez, C.; Lesponne, I.; Serisier, S.; Henrotin, Y. Identification of Targets of a New Nutritional Mixture for Osteoarthritis Management Composed by Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract. Plos One 2016, 11, e0156902. [Google Scholar] [CrossRef]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Archives of Endocrinology and Metabolism 2018, 62, 438–445. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complementary and Alternative Medicine 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Murillo Ortiz, B.O.; Fuentes Preciado, A.R.; Ramírez Emiliano, J.; Martínez Garza, S.; Ramos Rodríguez, E.; de Alba Macías, L.A. Recovery Of Bone And Muscle Mass In Patients With Chronic Kidney Disease And Iron Overload On Hemodialysis And Taking Combined Supplementation With Curcumin And Resveratrol. Clinical Interventions in Aging 2019, 14, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Karimi, E.; Sarraf, P.; Tafakhori, A.; Siri, G.; Salehinia, F.; Sedighiyan, M.; Asanjarani, B.; Badeli, M.; Abdollahi, H.; et al. The omega-3 and Nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial. BMC Research Notes 2021, 14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





