Submitted:
08 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
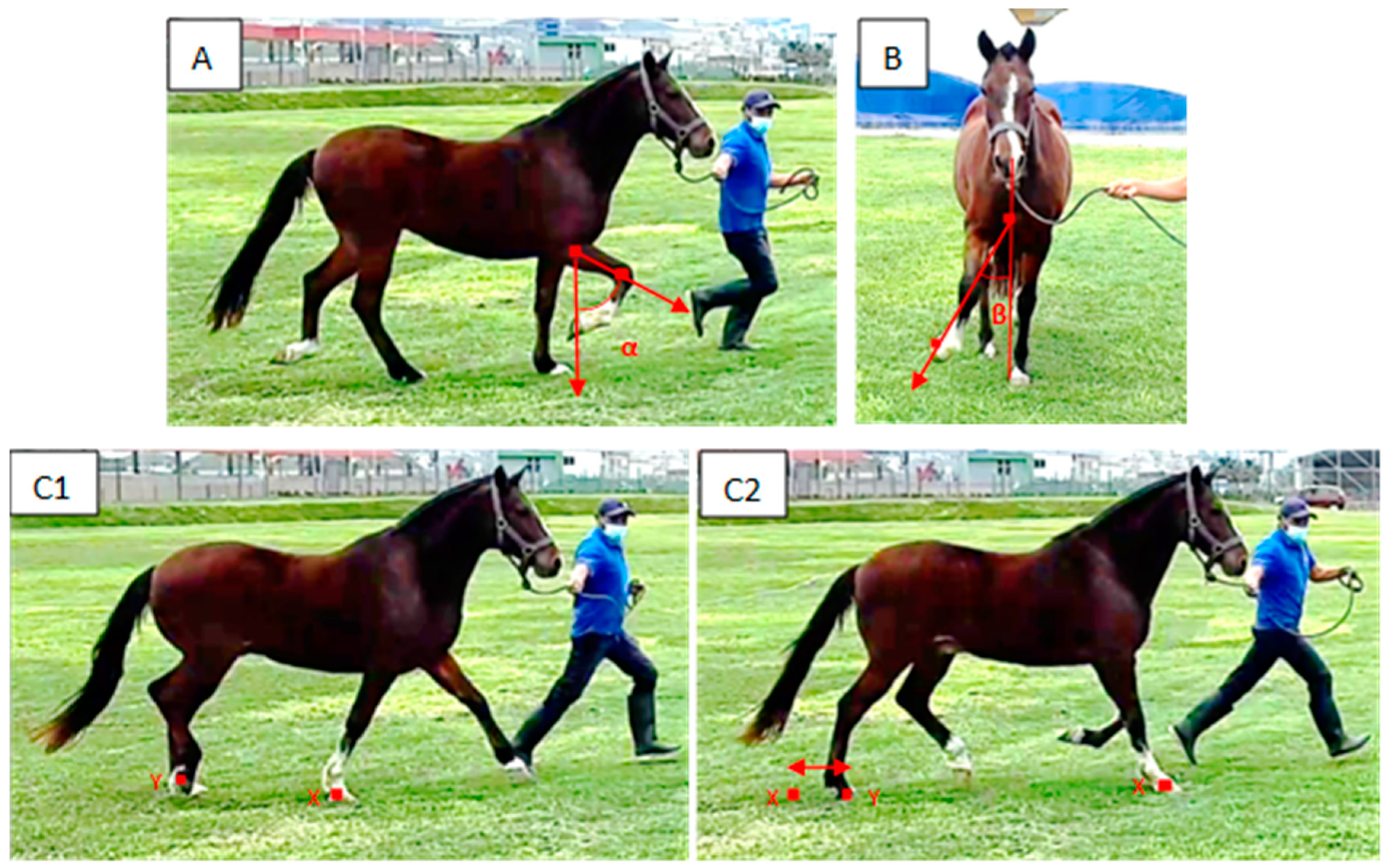
2. Materials and Methods
2.1. Animals
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzales, R.; Li, R.; Kemper, G.; Del Carpio, C.; Ruiz, E. Un algoritmo para estimar la variación de los ángulos articulares de las extremidades del caballo peruano de paso. In Proceedings of the IEEE XXV Conferencia Internacional sobre Electrónica, Ingeniería Eléctrica y Computación (INTERCON), Lima, Perú, 8–10 August 2018. [Google Scholar]
- Asociación Norteamericana de Caballos Peruanos. Available online: https://www.napha.net/about-the-peruvian-horse/ (accessed on 29 December 2022).
- Peruvian Horse Association of Canada. Available online: https://phac.ca/breed-information/ (accessed on 29 December 2022).
- Nicodemus, M.C.; Clayton, H.M. Variables temporales de cuatro tiempos, pasos de caballos de marcha. Appl Anim Behav Sci 2003, 80, 133–142. [Google Scholar] [CrossRef]
- Asociacion nacional de criadores y propietarios de caballos peruanos de paso. Available online: https://www.ancpcpp.org.pe/glosario-preliminar-por-practicas-del-caballo-peruano-de-paso (accessed on 26 December 2022).
- Crolle, R.C. See, analyze and use our Peruvian Paso Horse. LVXII Concurso nacional del Caballo Peruano de Paso. Lima; Asociación nacional de criadores y propietarios de caballos peruanos de paso. Lima, Peru, 2017.
- La Rosa, A. The phenotype of my horse. LVXII Concurso nacional del Caballo Peruano de Paso. Lima; Asociación nacional de criadores y propietarios de caballos peruanos de paso. Lima, Peru, 2017.
- Nicodemus, M.C.; Clayton, H.M.; Lanovaz, J.L. Comparison of a joint coordinate system versus multi-planar analysis for equine carpal and fetlock kinematics. Comparative Exercise Physiology 2008, 5, 43–55. [Google Scholar] [CrossRef]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; Van der Zwaag, B.J.; Voskamp, J.; Back, W.; Van Weeren, R.; Havinga, P. Equimoves: A wireless networked inertial measurement system for objective examination of horse gait. Sensores 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Santos, R.; Molina, A.; Galisteo, A.; Valera, M. Genetic analysis of kinematic traits at the trot in Lusitano horse subpopulations with different types of training. Animal 2014, 8, 192–199. [Google Scholar] [CrossRef]
- Cano, M.R.; Miró, F.; Vivo, J.; Galisteo, A.M. Comparative Biokinematic Study of Young and Adult Andalusian Horses at the Trot. J Vet Med A 1999, 46, 91–102. [Google Scholar] [CrossRef]
- De Souza, M.V.; Galisteo, A.M.; Novales, M.; Miró, F. Influence of camped under associated with upright pastern in front conformation in the forelimb movement of horses. J Equine Vet Sci 2004, 24, 341–346. [Google Scholar] [CrossRef]
- Miró, F.; Vivo, J.; Cano, R.; Diz, A.; Galisteo, A.M. Walk and trot in the horse at driving: Kinematic adaptation of its natural gaits. Anim Res 2006, 55, 603–613. [Google Scholar] [CrossRef]
- Novoa-Bravo, M.; Fegraeus, K.J.; Rhodin, M.; Strand, E.; García, L.F.; Lindgren, G. Selection on the Colombian Paso horse's gaits has produced kinematic differences partly explained by the DMRT3 gene. PLoS ONE 2018, 13, e202584. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; Brama, P.; McGrath, D. Research trends in equine movement analysis, future opportunities and potential barriers in the digital age: A scoping review from 1978 to 2018. Equine Veterinary Journal 2019, 51, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Santosuosso, E.; Leguillette, R.; Vinardell, T.; Filho, S.; Massie, S.; McCrae, P.; Johnson, S.; Rolian, C.; David, F. Kinematic Analysis During Straight Line Free Swimming in Horses: Part 1 - Forelimbs. Front. Vet. Sci. 2021. 8, 752375. [CrossRef]
- Kristjansson, T.; Bjornsdottir, S.; Albertsdóttir, E.; Sigurdsson, A.; Pourcelot, P.; Crevier-Denoix, N.; Arnason, T. Association of conformation and riding ability in Icelandic horses. Livest Sci 2016, 189, 91–101. [Google Scholar] [CrossRef]
- Viswakumar, A.; Rajagopalan, V.; Ray, T.; Gottipati, P.; Parimi, C. Development of a Robust, Simple, and Affordable Human Gait Analysis System Using Bottom-Up Pose Estimation With a Smartphone Camera. Frente Physiol 2022, 12, 784865. [Google Scholar] [CrossRef]
- Charmant, J. Kinovea. Versión 0.9.5. Available online: https://www.kinovea.org (accessed on 22 January 2021).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative genetics, 4th ed.; Longman Group: Essex, UK, 1996; pp. 160–204. [Google Scholar]
- Johnson, D.L.; Thompson, R. Restricted maximum verosimilitud estimation of variance components for univariate animal models using sparse matrix techniques and average information. J Dairy Sci. 1995, 78, 449–456. [Google Scholar] [CrossRef]
- Meyer, K. WOMBAT: A tool for mixed model analysis in quantitative genetics by restricted maximum likelihood (REML). J Zhejiang Univ Sci B 2007, 8, 815–821. [Google Scholar] [CrossRef]
- Ablondi, M.; Summer, A.; Vasini, M.; Simoni, M.; Sabbioni, A. Genetic parameters estimation in an Italian horse native breed to support the conversion from agricultural uses to riding purposes. J Anim Breed Gen 2020, 137, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Stock, K.F.; Distl, O. Genetic analyses of new movement traits using detailed evaluations of warmblood foals and mares. J Anim Breed Gen 2012, 129, 390–401. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.; Silva, F.F.E.; Carvalho, R.S.B.; Ventura, R.V.; De Oliveira, H.N.; Abreu Silva, B.D.C.; Fonseca, M.G.; dos Santos, B.A.; Pereira, G.L.; Eler, J.P.; et al. Model comparisons for genetic evaluation of gait type in Mangalarga Marchador horses. Livest Sci 2020, 239, 104168. [Google Scholar] [CrossRef]
- De Oliveira, F.; Perez, B.D.C.; Ventura, R.V.; Silva, F.F.E.; Peixoto, M.G.C.D.; Vizoná, R.G.; Mattos, E.C.; Ferraz, J.B.S.; Eler, J.P.; Curi, R.A.; et al. Genetic analysis of morphological and functional traits in Campolina horses using Bayesian multi-trait model. Livest Sci 2018, 216, 119–129. [Google Scholar] [CrossRef]
- Dugué, M.; Dumont Saint Priest, B.; Crichan, H.; Danvy, S.; Ricard, A. Genomic Correlations Between the Gaits of Young Horses Measured by Accelerometry and Functional Longevity in Jumping Competition. Genet frontal 2021, 12, 619947. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Valera, M.; Galisteo, A.M.; Vivo, J.; Gómez, M.D.; Rodero, A.; Agüera, E. Genetic parameters of biokinematic variables at walk in the Spanish Purebred (Andalusian) horse using experimental treadmill records. Livest Sci 2008, 116, 137–145. [Google Scholar] [CrossRef]
- Novotná, A.; Svitáková, A.; Schmidová, J.; Pribyl, J.; Vostrá-Vydrová, H. Variance components, inherititability estimates, and breeding values for performance test traits in Old Kladruber horses. Czech J Anim Sci 2016, 61, 369–376. [Google Scholar] [CrossRef]
- Medeiros, B.R.; Garbade, P.; Seixas, L.; Peripolli, V.; McManus, C. Brazilian Sport Horse: Genetic parameters for approval of Brasileiro de Hipismo stallions. Trop Anim Health Prod 2020, 52, 1669–1680. [Google Scholar] [CrossRef]
- Rustin, M.; Janssens, S.; Buys, N.; Gengler, N. Multi-trait animal model estimation of genetic parameters for linear type and gait traits in the Belgian warmblood horse. J Anim Breed Gen 2009, 126, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Sabeva, I. Phenotypic and genetic parameters of the complex assessment of BV in two-year-old tested horses from the east Bulgarian breed. Bulg J Agric Sci 2019, 25, 1266–1270. [Google Scholar]
- Stock, K.F.; Distl, O. Genetic correlations between performance traits and radiographic findings in the limbs of German Warmblood riding horses. J Anim Sci 2007, 85, 31–41. [Google Scholar] [CrossRef]
- Valera, M.; Galisteo, A.M.; Molina, A.; Miró, F.; Gómez, M.D.; Cano, M.R.; Agüera, E. Genetic parameters of biokinematic variables of the trot in Spanish Purebred horses under experimental treadmill conditions. Vet J 2008, 178, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.A.; Carolino, N.; Ralão-Duarte, J.; Gama, L.T. Selection for morphology, gaits and functional traits in Lusitano horses: I. Genetic parameter estimates. Livest Sci 2014, 164, 1–12. [Google Scholar] [CrossRef]
- Bussiman, F.D.O.; Perez, B.D.C.; Ventura, R.V.; Silva, F.F.E.; Peixoto, M.G.C.D.; Vizoná, R.G.; Mattos, E.C.; Ferraz, J.B.S.; Eler, J.P.; Curi, R.A.; et al. Genetic analysis of morphological and functional traits in Campolina horses using Bayesian multi-trait model. Livest Sci. 2018, 216, 119–129. [Google Scholar] [CrossRef]
- Peham, C.; Licka, T.; Schobesberger, H.; Meschan, E. Influence of the rider on the variability of the equine gait. Hum Mov Sci. 2004, 23, 663–671. [Google Scholar] [CrossRef]
- Ripollés-Lobo, M.; Perdomo-González, D.I.; Sánchez-Guerrero, M.J.; Bartolomé, E.; Valera, M. Genetic relationship between free movement and under rider gaits in young Pura Raza Española horses. Livest Sci. 2022, 263, 105031. [Google Scholar] [CrossRef]
- Thorén Hellsten, E.; Viklund, Å.; Koenen, E.P.C.; Ricard, A.; Bruns, E.; Philipsson, J. Review of genetic parameters estimated at stallion and young horse performance tests and their correlations with later results in dressage and show-jumping competition. Livestock Science 2006, 103, 1–12. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Gómez, D.M.; Peña, F.; García, J.; Morales, J.L.; Molina, A.; Valera, M. Relationship between conformation traits and gait characteristics in Pura Raza Español horses. Arch Anim Breed. 2013, 56, 137–148. [Google Scholar] [CrossRef]
- Solé, M.; Santos, R.; Gómez, M.D.; Galisteo, A.M.; Valera, M. Evaluation of conformation against traits associated with dressage ability in unridden Iberian horses at the trot. Res Vet Sci. 2013, 95, 660–666. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turkish Journal of Emergency Medicine 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Lewczuk, D. Phenotypic correlations between jump and gaits characteristics measured by inertial measurement units in horse jumping training - preliminary results. Livest Sci. 2022, 266, 105112. [Google Scholar] [CrossRef]
- Nazari-Ghadikolaei, A.; Fikse, F.; Gelinder Viklund, Å.; Eriksson, S. Factor analysis of evaluated and linearly scored traits in Swedish Warmblood horses. Journal of Animal Breeding and Genetics 2023, 140, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guerrero, M.J.; Cervantes, I.; Molina, A.; Gutiérrez, J.P.; Valera, M. Designing an early selection morphological linear traits index for dressage in the Pura Raza Español horse. Animal 2017, 11, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda Caviedes, M.F.; Forbes, B.S.; Pfau, T. Repeatability of gait analysis measurements in Thoroughbreds in training. Equine Vet J. 2018, 50, 513–518. [Google Scholar] [CrossRef]
- Gerber Olsson, E.; Arnason, T.; Nasholm, A.; Philipsson, J. Genetic parameters for traits at performance test of stallions and correlations with traits at progeny tests in Swedish warmblood horses. Livestock Production Science 2000, 65, 81–89. [Google Scholar] [CrossRef]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of repeatability of a wireless, inertial sensor–based lameness evaluation system for horses. Am J Vet Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef]
- Dohm, M.R. Repeatability estimates do not always set an upper limit to heritability. Funct Ecol. 2002, 16, 273–280. [Google Scholar]
- Larrea Izurieta, C.O.L.; Carpio, M.G.; Landi, V.; Hurtado, E.A.; Andrade, J.I.M.; Loor, L.E.V.; Lozada, E.; Cartuche, L. Evaluation of inbreeding and genetic variability of the Peruvian Paso Horse registered in Ecuador. Revista de Investigaciones Veterinarias del Peru 2022, 33, e21672. [Google Scholar] [CrossRef]
- Montenegro, V.; Vilela, J.L.; Wurzinger, M. Assessment of generation interval and inbreeding in Peruvian Paso Horse. In Proceedings of the XI World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 15 February 2018. [Google Scholar]
- Santosuosso, E.; Leguillette, R.; Vinardell, T.; Filho, S.; Massie, S.; McCrae, P.; Johnson, S.; Rolian, C.; David, F. Kinematic Analysis During Straight Line Free Swimming in Horses: Part 2 - Hindlimbs. Front Vet Sci 2022, 8, 761500. [Google Scholar] [CrossRef] [PubMed]
| Overreach | Term | Acuteness | ||
|---|---|---|---|---|
| Animals | Stallions | 28 | 28 | 28 |
| Mares | 106 | 109 | 106 | |
| Median | 25.9 | 25.3 | 72 | |
| Mean | 25.338 | 25.111 | 71.512 | |
| Records | 500 | 481 | 500 | |
| Std. Error of Mean | 0.909 | 0.288 | 0.308 | |
| 95% CI Mean Upper | 29.119 | 25.675 | 72.117 | |
| 95% CI Mean Lower | 25.556 | 24.546 | 70.907 | |
| Std. Deviation | 20.33 | 6.315 | 6.902 | |
| Coefficient of variation | 0.744 | 0.251 | 0.097 | |
| Skewness | 0.219 | 0.177 | -0.298 | |
| Kurtosis | -0.445 | 0.038 | 0.087 | |
| Minimum | -18.080 | 6.800 | 49.000 | |
| Maximum | 86.180 | 44.200 | 88.600 | |
| p-value Anderson Darling Test | 0.881 | 0.814 | 0.897 | |
| CI. Confidence interval | ||||
| Overreach | Term | Acuteness | ||
|---|---|---|---|---|
| Number of animals in pedigree file | 1615 | 1641 | 1615 | |
| Number of animals with records | 134 | 137 | 134 | |
| Animals with | 3 records | 90 | 100 | 86 |
| 4 records | 2 | 12 | 8 | |
| 5 records | 42 | 25 | 40 | |
| Number of animals with | unknown sire | 79 | 81 | 79 |
| unknown dam | 211 | 211 | 211 | |
| both parents unknown | 56 | 57 | 56 | |
| Number of animals without offspring | 123 | 126 | 123 | |
| Number of animals with offspring | 1234 | 1257 | 1234 | |
| Number of animals with offspring and records | 11 | 11 | 11 | |
| Number of sires | 458 | 467 | 458 | |
| Number of sires with progeny in data | 51 | 52 | 51 | |
| Number sires with records and progeny in data | 3 | 3 | 3 | |
| Number of dams | 774 | 788 | 774 | |
| Number of dams with progeny in data | 102 | 104 | 102 | |
| Number of dams with records and progeny in data | 8 | 8 | 8 | |
| Average inbreeding coefficient (%) | 5.42 | 5.43 | 5.44 | |
| amongst inbreed animals (%) | 8.51 | 8.41 | 8.45 | |
| Average inbreeding coefficient amongst animals phenotyped (%) | 8.31 | 8.29 | 8.30 | |
| Overreach | Term | Acuteness | |
|---|---|---|---|
| Overreach |
h2 = 0.411 (0.199) R = 0.856 (0.020) |
-0.697 (0.374) | 0.493 (0.360) |
| Term | -0.213 (0.079) |
h2 = 0.476 (0.197) R = 0.783 (0.029) |
-0.301 (0.432) |
| Acuteness | 0.189 (0.081) | 0.183 (0.081) |
h2 = 0.405 (0.224) R = 0.854 (0.019) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





