1. Introduction
The COVID-19 pandemic raised people
’s concerns about infectious diseases [
1,
2,
3,
4,
5]. Although the impact of the pandemic has subsided in recent years, antimicrobial and antiviral measures remain an important issue. This study focuses on the antiviral properties involving materials and, in particular, how metallic materials affect viral infections. Infectious diseases occur between organisms and are caused by microorganisms such as bacteria and viruses[
6]. Viruses cannot multiply independently and complete their multiplication cycle by invading host cells. Highly pathogenic viruses multiply by hijacking cellular functions and destroying cells, causing the host to develop various symptoms[
7]. In recent years, it has been noted that materials play an essential role in the viral infection pathway[
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Since inorganic materials are not living organisms by nature, there is no mechanism to sustain infectivity. However, viruses adhere to the surface of products and materials in human-to-human transmission and remain active for a certain period. Since bacteria are living organisms, they can continue to grow on material surfaces as long as they have nutrients and energy. Viruses, on the other hand, are not living organisms and maintain their infectivity (activity) by maintaining their structure on the material surface. The type of material to which the virus adheres significantly impacts the maintenance of viral activity.
Viruses are classified into two structures: those with envelopes and those without. Viruses with envelopes have RNA covered by a protein capsid and a lipid envelope. The envelope plays a vital role in viral infection and facilitates entry into the host cell. On the other hand, viruses without an envelope consist only of capsid and are more stable than those with an envelope.
In this study, we conducted antiviral tests mainly on influenza A virus (H3N2), which has an envelope. Feline calicivirus (F-9) ATCC VR-782, which does not have an envelope, was used to compare. Then, we report mainly the results of antiviral tests of the virus with an envelope and discuss the mechanism of maintaining the activity of the virus on the surface of the material.
2. Materials and Methods
2.1. Specimens, Viruses, and Cells
The virus used was influenza A virus (H3N2) ATCC VR-1680. Feline calicivirus (F-9 ATCC VR-782) was used for comparison. Six materials were used: pure iron (99.9%), commercial rolled steel (JIS SS400), pure titanium (99.9%), pure nickel (99.9%), two types of commercial stainless steel (JIS SUS304, SUS316L), and a control (PE film, Organo) with no correlation with the materials. Three sheets from each sample were used in the experiment. The film used to cover the samples was PE film cut into 4×4 strips. MDCK (NBL-2) was used for the influenza virus, and CRFK (ATCC CCL-94) was used for the feline calicivirus. Preliminary experiments were conducted to determine the concentration of the virus solution used in this study. The test was performed under N number = 6.
2.2. Cell passaging Culture and 6-Well Plate Preparation
In conducting this experiment, performing periodic passaging of MDCK (NBL-2 ) in the case of influenza virus and CRFK cells in the case of feline calicivirus was necessary. The procedure for periodic passaging was as follows:
After confirming 90~100% confluency of cells cultured in 75 cm2 flasks under a microscope, trypsin EDTA (TE) frozen in a 15-mL centrifuge tube was thawed in a beaker filled with water. The thawed TE was inverted and mixed, then subjected to 1000 GRP for 5 min in a centrifuge, and the TE was collected. 75 cm2 flasks of cultured cells were washed twice with 10 mL of PBS. After 5 min, the 75 cm2 flask was removed and the cells attached to the bottom of the flask were peeled off by tilting the flask. After 5 minutes, the 75-cm2 flask was removed and tilted to remove cells adhering to the bottom of the flask. 10 mL of 10% FBS-EMEM was added to the removed 75-cm2 flask, and floating cells were collected in a 15-mL centrifuge tube by repeated pipetting a few times. For passaging, 18 mL of 10% FBS-EMEM was added to a new 75 cm2 flask, and the suspended cells in the 15-mL centrifuge tube were added to the previously prepared flask after repeated pipetting a few times. To prepare a 6-well plate, 36 mL of 10% FBS-EMEM was added to a 50-mL centrifuge tube. To this, 4 mL of 10% FBS-EMEM containing the collected suspended cells was added, making a total of 40 mL of 10% FBS-EMEM solution containing suspended cells. Then, 3 mL was added to each well of a 6-well plate to make a 6-well plate. Both the flasks and 6-well plates were tested within 2~4 days while confirming confluency by microscopic observation.
2.3. Film Covering Method
The PE film was cut out 4 x 4 cm and stored in a petri dish filled with alcohol. The water bath was set at 36°C in advance. Ten freeze-dried viruses (100 μm) were placed in the water bath for 2 minutes to thaw. The test material was placed on a petri dish, vortexed with the virus solution prepared earlier, and thoroughly agitated. Then, 0.4 ml of the virus solution was inoculated and covered with PE film. The samples and PE film were briefly sterilized with alcohol at 25°C, 90%RH or higher for 24, 6, and 3 hours to allow the virus to interact with the samples. After incubation, 10 ml of washing solution (SCDLP medium) was added and pipetted several times in a petri dish. The virus solution was then collected in a centrifuge tube [
18,
19,
20].
2.4. Plaque Assay
The plaque method is a method to evaluate the antiviral activity of cells cultured in a monolayer on the bottom of a well by seeding the cells with virus solution, infecting them, and then staining them. The plaque method was selected for this study because it is the simplest method, and the results are visible from a third-party perspective, although there are other methods, such as the TCID
50 method[
21]. Usually, when cells at the bottom of the well are infected with a virus, the cells are destroyed and peeled off from the bottom. The hole formed by the detached cells is called a plaque. The antiviral activity is evaluated by measuring the plaque formed[
22].
The virus solution obtained by the film covering method is diluted to a concentration determined in a preliminary experiment. If the concentration of the virus solution is too high, plaques may form all over the cells, making it impossible to measure the number of plaques. Next, 0.1 ml of the diluted virus solution was seeded onto the cells. After seeding, the cells were incubated at 35°C for 1 hour with a CO2 concentration of 5% and tilted every 15 minutes to spread the virus solution over the entire surface of the well plate. After the tilting, each well was fixed with agar medium containing a certain amount of medium solution and incubated in a CO2 incubator under the same conditions for 2 days. After the incubation, the cells were fixed with glutaraldehyde for 1 hour, the agar medium was removed, and the cells were stained with methylene blue for 30 minutes. This staining identified uninfected cells and plaque. In this experiment, however, plaques were not formed in all results. However, the degree of detached cells other than plaques also varied from sample to sample. Therefore, we evaluated the antiviral activity of each sample using ImageJ to determine the area fraction of stained cells that remained on the bottom of the wells.
2.5. Measurement of Area Fraction of Damaged Cells
ImageJ was used to measure stained residual cells. First, a photograph of the resulting 6-well plate was taken. The photograph was imported into ImageJ. Image>Crop was then selected and cropped to the appropriate size. The scale was adjusted to match the bottom of the wells. The image was changed to 16-bit and adjusted to select the residual cell area after selecting Adjust>Threshold. This allowed us to measure the percentage of residual cells relative to the bottom of the well.
3. Results
3.1. Experimental Results for Control (Polyethylene Film: PE)
The vertical axis represents the percentage of residual cells in the area of the healthy bottom. The higher these percentages are, the fewer cells are infected with the virus. This means that the antiviral activity of the test material is high. The horizontal axis shows the results for the six test materials.
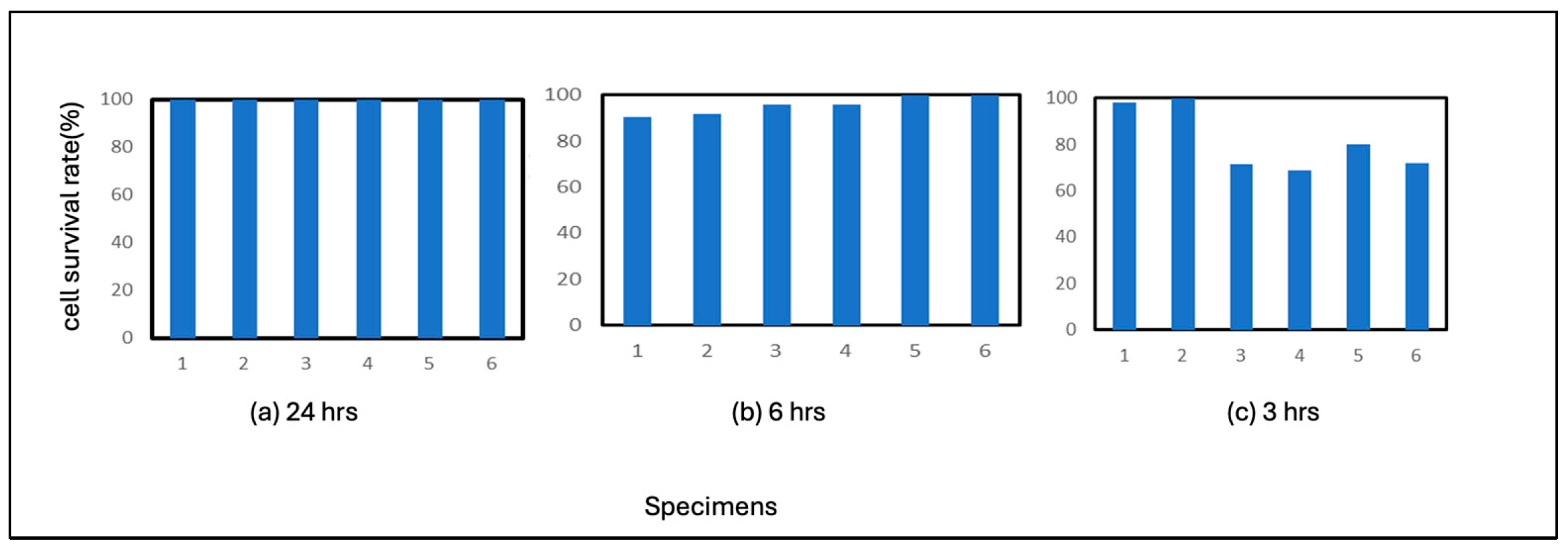
The results for the PE film used as a control are shown in
Figure 1. 24 hours of incubation resulted in 100% residual cells in all six results. The results for the 6-hour incubation period showed that, on average, about 90% of the cells remained uninfected, although some were almost entirely uninfected. The 3-hour results showed that more cells were infected with the virus than the 6-hour results, with about 80% of cells remaining. From this point of view, it can be considered that the virus is inactivated under the conditions of this experiment after 24 hours, even if there is no correlation.
3.2. Results for Some Metal Specimens
3.2.1. Pure Iron
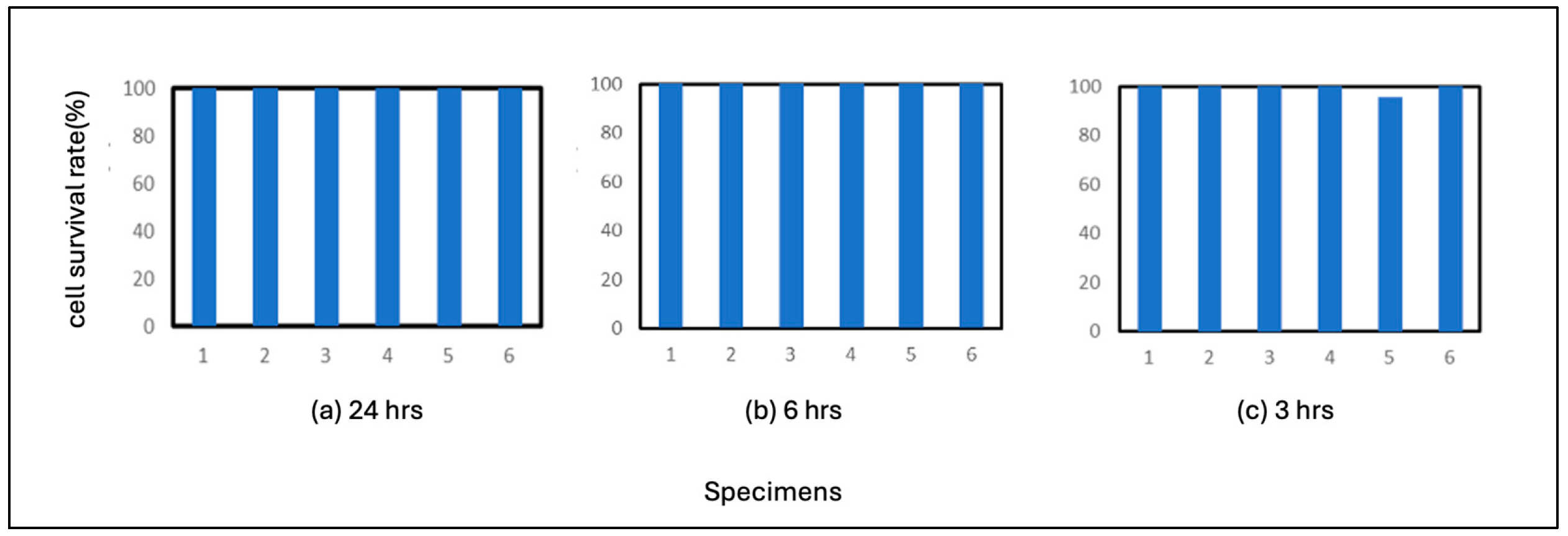
The results for pure iron are shown in
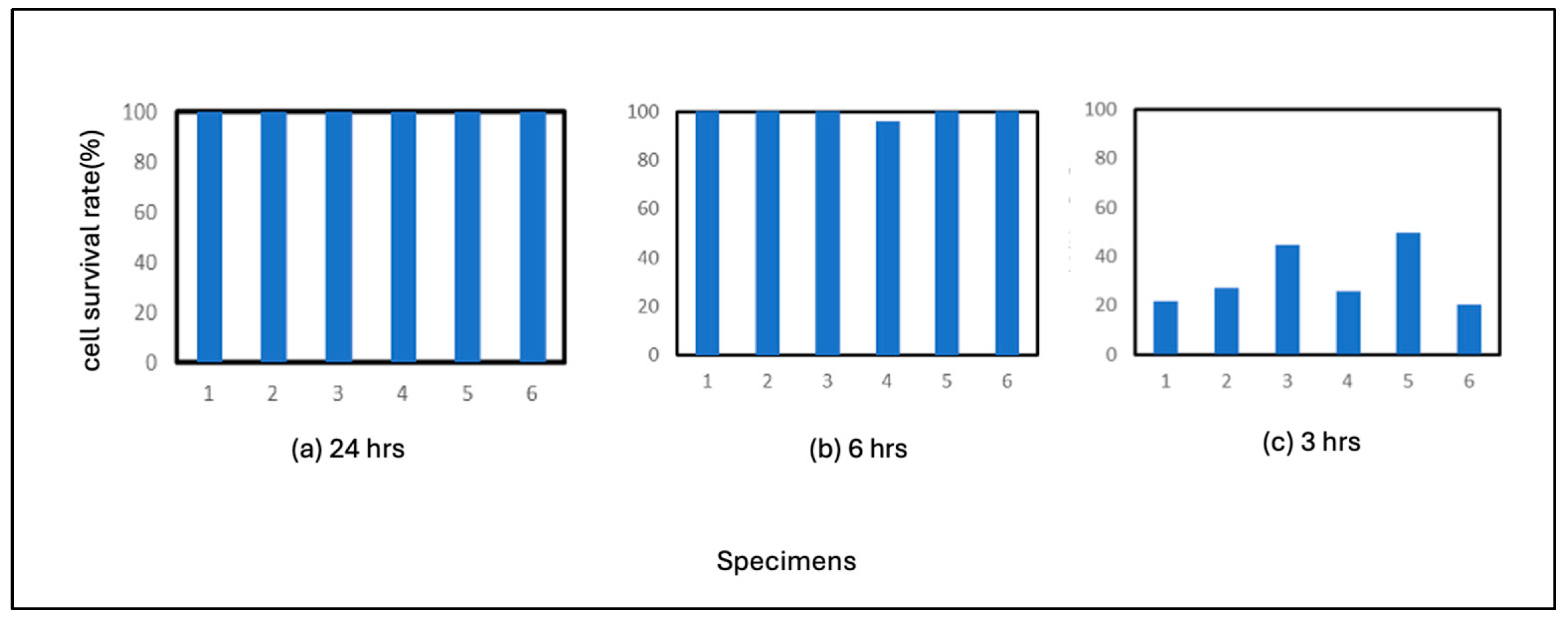
Figure 2. The average number of residual cells in the 3-hour incubation period was 99%. These results indicate that pure iron is highly antiviral compared to the above-mentioned control test results and that the virus is inactivated after 6 hours of adhesion on Fe. The results also showed that the virus was sufficiently antiviral after 3 hours of adhesion. This suggests that pure iron strongly correlates with the virus.
3.2.2. Commercial Carbon Steel (JIS SS400)
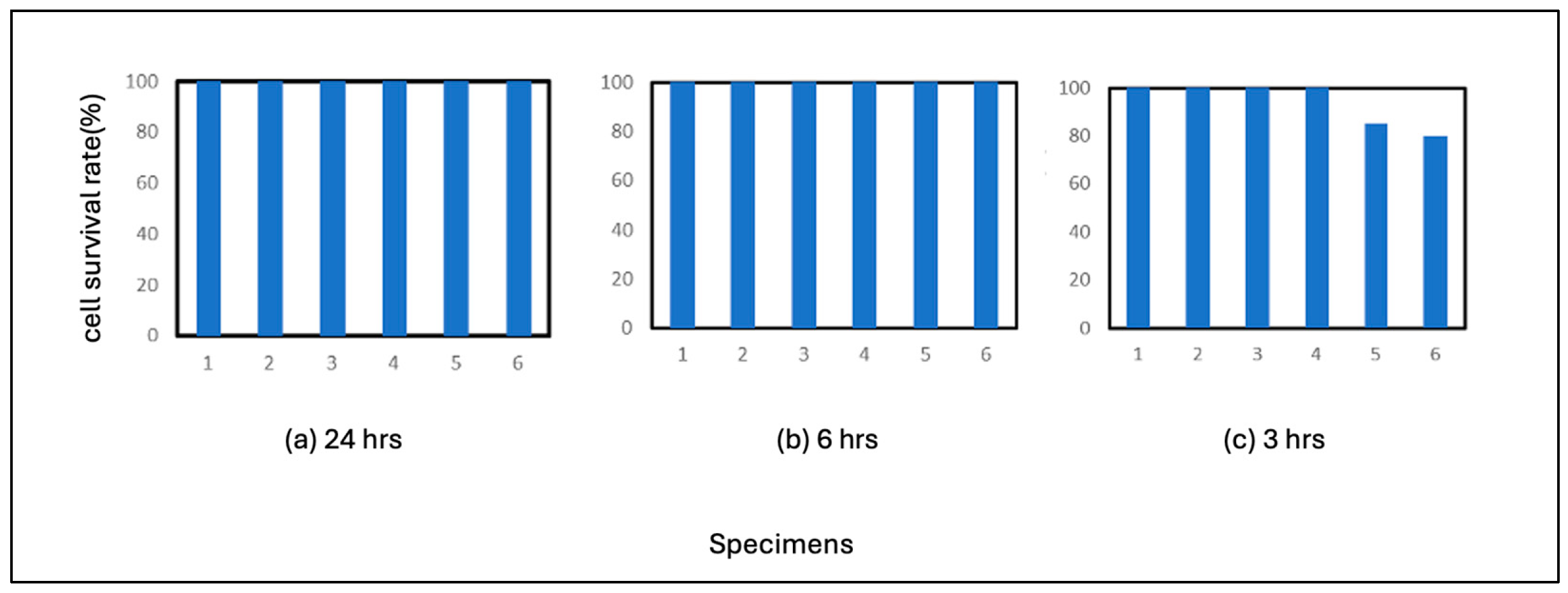
The results of SS400 are shown in
Figure 3. SS400 also showed high antiviral activity, with no plaque formation after 24 hours of incubation and 6 hours of incubation as in the case of Fe. The average of residual cells after 3 hours of incubation was 94%, which was slightly less antiviral than Fe. This result does not necessarily make it clear, but it can be concluded that the correlation with the virus is either equal to that of pure iron or slightly weaker than that of pure iron. If the latter view is correct, the production of iron ions was somewhat lower than that of pure iron under the conditions of this experiment. In the case of metallic materials, the reaction of ions with biologically relevant polymers is considered to be responsible for many of the phenomena.
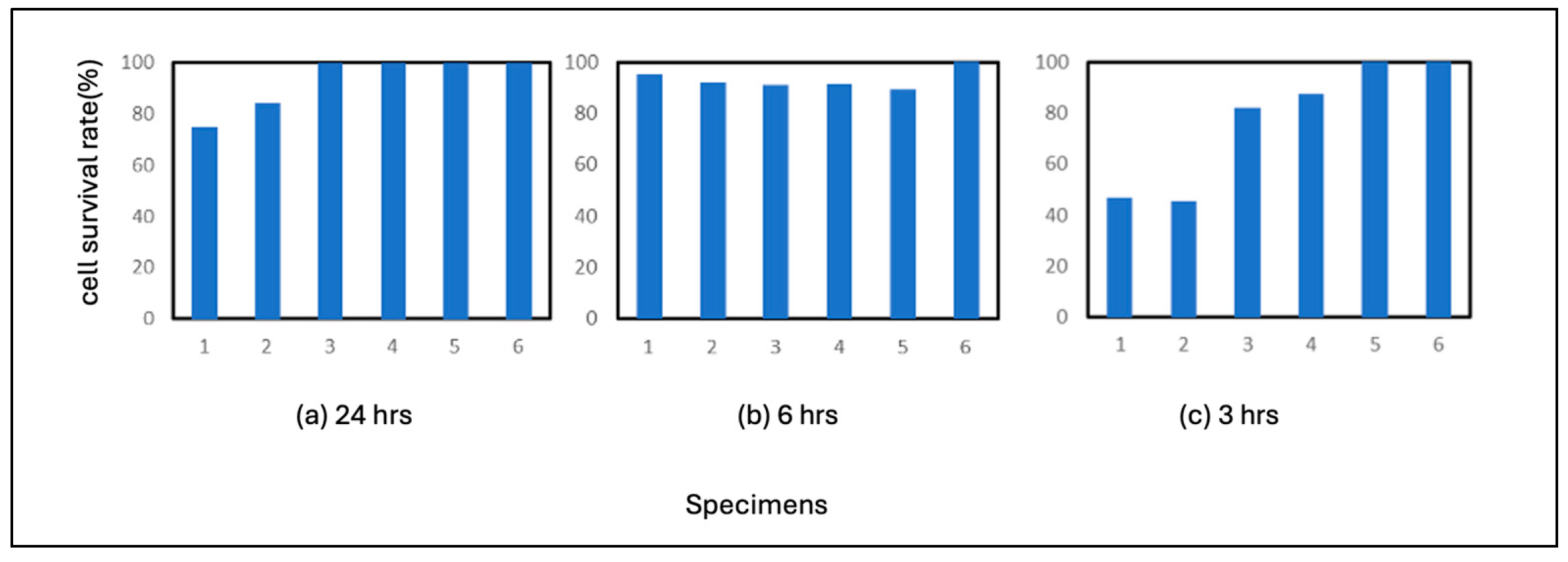
3.2.3. Pure Nickel
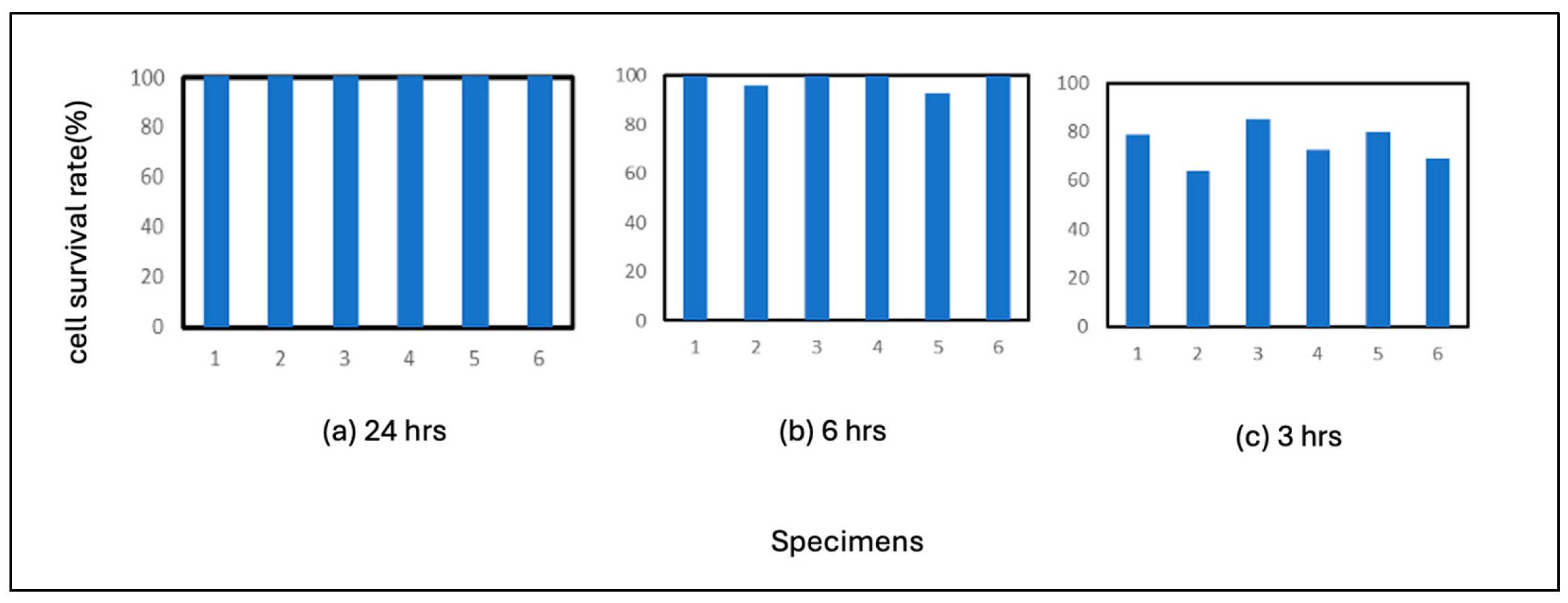
The results of the pure nickel test are shown in
Figure 4. As shown in the figure, no plaques were formed on pure nickel after 24 hours of incubation, and the virus was inactivated on nickel after 24 hours of incubation. This indicates that pure nickel is more susceptible to virus inactivation than pure Fe or SS400. In the 3-hour incubation period, plaques formed in all wells. The average residual cell count was 75%. In terms of correlation with a virus, the correlation between nickel ions and the virus is estimated to be lower than that of iron ions.
3.2.4. Pure Titanium
The results of the pure titanium test are shown in
Figure 5. Pure titanium also showed no plaque formation after 24 hours of incubation, and viruses were inactivated after 24 hours of incubation. Fewer plaques were formed in the 6-hour incubation period than in the pure nickel incubation period. 3-hour incubation resulted in the formation of many plaques in all six wells. These results indicate that pure titanium maintains a high level of viral activity during the 3-hour incubation period. This may mean that titanium does not react with the virus, that titanium does not ionize easily under the conditions of this experiment, or both.
3.2.5. Austenitic Stainless Steels
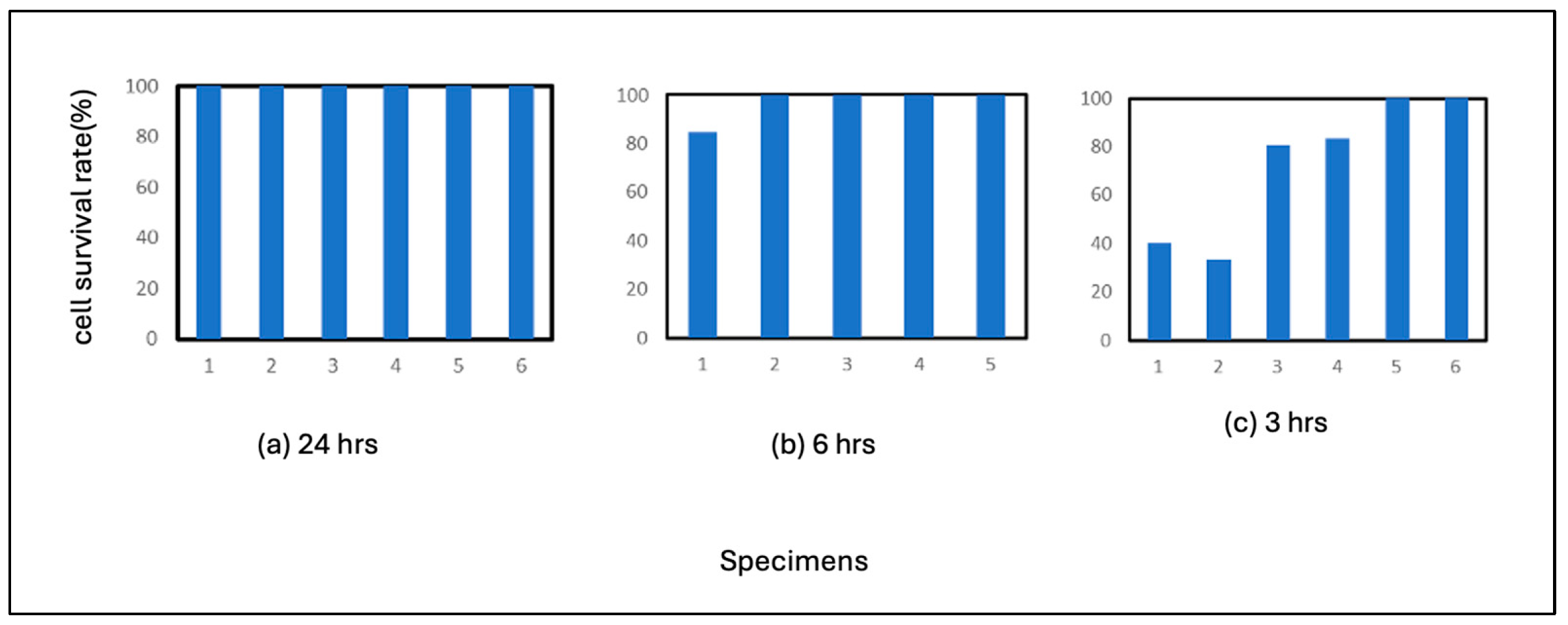
JIS SUS 304 and SUS 316 were investigated in this experiment. The results for SUS 304 are shown in
Figure 6. 24 hours of incubation resulted in the formation of plaques in two wells, which was not significantly different from the results for 6 hours of incubation. The results suggest that SUS 304 steel may have less effect on virus activity than pure iron or carbon steel, even though they are the same steel materials. 3 hours of incubation also resulted in the formation of more plaques in the case of SUS 304 steel, indicating that inactivation of the virus was less likely to occur than with pure iron or carbon steel. This suggests that virus inactivation was less likely to occur with SUS 304 steel than pure iron or carbon steel. If the correlation between the formation of iron ions and polymers as virus components is responsible for the inactivation of the virus in steel materials, this is because the high corrosion resistance coating of stainless steel prevents the formation of iron ions, which are considered to have a slightly weaker correlation with the virus than iron, a component of the virus. The same may be true for nickel ions, which have a slightly weaker correlation with viruses than iron.
The results for SUS316L are shown in
Figure 7. 24 hours of incubation resulted in no plaque formation, while 6 hours of incubation resulted in plaque formation in a few wells. 3 hours of incubation resulted in plaque formation in many wells. Comparing these results with those of SUS 304, which is also an austenitic stainless steel, it can be assumed that inactivation of the virus is slower in the case of SUS 316 steel. This is because the percentage of cells remaining after 24 hours tends to be slightly higher for SUS 304 and lower for SUS 316. This means that inactivation of the virus is delayed in SUS316. The significant difference between the alloying elements of SUS316 and SUS316 steel is molybdenum. The corrosion resistance of SUS316 steel is usually higher, and the dissolution of iron ions tends to be more inhibited in SUS316 steel. From this point of view, viral inactivation is more inhibited in SUS316 steel than in SUS304 steel. In addition, molybdenum decreases virus activity in the form of a single substance, oxide, or complex. Therefore, the inhibitory effect of molybdenum itself may be related to this.
3.3. Comparison of Results in the Case of Influenza Virus and Feline Calicivirus
The average of the time of incubation for each sample is shown in Figure 13, and the results for feline calicivirus after 6 hours of incubation are shown in Figure 14. 24 hours of incubation resulted in no plaque formation for the samples other than SUS304. As shown in Figure 14, most of the viruses used in this study, influenza A virus (H3N2) ATCC VR-1680, were inactivated after 24 hours or more of incubation on the metal material. The 3-hour incubation period showed significant differences among the samples. The 3-hour incubation period showed significant differences among the samples, with the pure titanium sample showing plaque formation overlapping the entire well. There was no significant difference in the average number of residual cells between the 24-hour and 6-hour incubations, whereas plaques were formed in all samples at 3 hours of incubation. These results indicate that the time the virus remains attached to the sample surface significantly affects viral activity.
The 6-hour incubation results were used to compare influenza virus and feline calicivirus. The trend for each feline calicivirus sample was similar to that of the influenza virus. Iron ions were the most antiviral, resulting in the highest cell survival rate. Carbon steel showed slightly lower antiviral activity, followed by pure nickel, and pure titanium showed even lower antiviral activity. Stainless steel was also more antiviral than pure titanium but less so than pure iron and carbon steel. This suggests that, as a trend, the type of ionized metal is the dominant factor in antiviral binding to biogenic polymers rather than differences in viral structure, but further investigation is needed. Although only a comparison of the 6-hour results is shown in this experiment, detailed differences could be further elucidated by varying the time of incubation and virus concentration.
4. Discussion
The ions formed on the surface of the metallic material are thought to react with the polymers in the outer layer of the virus to form a complex. Some metal ions are thought to be incorporated into the virus and react with nucleic acids and capsids. Therefore, it is thought that the ease with which metals dissolve as ions and react with the polymers of viral components positively influences viral inactivation, i.e., antiviral activity. This can be discussed in more detail as follows.
The first is the ease or speed of solubilization as metal ions. Metal ions that quickly form complexes with polymers in the viral outer layer or materials readily soluble as metal ions and dissolve at a high rate tend to promote viral inactivation. Of particular importance in this regard are the iron elements. The affinity of iron ions for the polymers that make up the virus is extremely high, probably because they bind to the polymers and form complexes with them[
23]. This is also the case for biofilms, where polymers derived from bacteria and bacterial cells are essential components[
24]. It is well known that iron is an essential element in living cells in general and that it readily binds to polymers derived from living organisms. Nickel and molybdenum are also trace elements in living organisms, and although not to the same extent as iron, they are metals that can bind to polymers of biological origin. Titanium, on the other hand, generally does not bind to biogenic polymers. Therefore, we speculate that the solubilization of iron as an ion is the most dominant factor influencing viral inactivation.
The second factor influencing inactivation is the dissolution rate of iron, nickel, and molybdenum (in the case of this experiment), which are easily converted to ions. Stainless steel was used in this experiment. The surface of this steel has a surface film composed not only of iron but also of oxides of these metals by chromium, nickel, and molybdenum, which is denser and more stable than an oxide or passive film composed only of iron. Therefore, the formation rate of iron ions, which are the main element, is significantly reduced. The same is true for other metals, such as nickel and molybdenum. Although we could not conduct experiments on chromium in this study, we assume that the same is valid for chromium. Therefore, the inactivation of stainless steel, where the rate of ion formation is slower due to a dense surface film on the material, may be delayed compared to pure iron or carbon steel.
In any case, once the metal ions are formed and react with the polymer, inactivation of the virus occurs, which in the case of this experiment was found to occur within a few hours. After a more extended period (24 hours), all viruses were inactivated, suggesting that an appropriate time frame is necessary to evaluate the steel material accurately.
5. Conclusions
In this study, the antiviral properties of pure iron, carbon steel, pure nickel, pure titanium, and two types of austenitic stainless steels against influenza virus and feline calicivirus were evaluated by combining the film covering method and plaque assay to determine the area fraction of cells not damaged by the virus (residual The antiviral activity was evaluated by measuring the area fraction where cell damage by the virus does not occur (residual area fraction) using a combination of the film covering method and plaque assay. The relationship between the type of metal ions and the virus inactivation rate was then examined, and the mechanism and other effects of metal ions formed on the surface of metal materials on virus inactivation were discussed. The results suggest that metal ions promote virus inactivation by forming a complex with the outer polymer of the virus or by being incorporated into the interior of the virus and reacting with nucleic acids and capsids. Further studies are expected to elucidate the mechanism of virus inactivation by metal ions and to develop more effective antiviral materials.
Author Contributions
Writing—original draft preparation, T.T..; Data curation, T.T., H.M., A.O., H.E., H.K., Formal analysis, T.T., H.M., A.O. , T.H and R.I.; Project administration, H.K., H.M., H.N., E.N., Y.I., R.I., and D.M.B. Writing—review and editing, D.M.B. and H.K.; Funding, N.H. and H.M.; Investigation, T.T., A.O., H.M., E.N., Y.I., N.H., T.K., T.H., H.E., D.M.B., Methodology, E.N., Y.I., T.K., R.I., H.E., D.M.B and H.K.; Supervision, H.K., D.M.B, T.H., R.I., T.K., E.N., Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
JSPS KAKENHI (Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, Grant Number 23K04465 and 21K12739) supported this work. A part of this work was supported by the GEAR 5.0 Project of the National Institute of Technology (KOSEN) in Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate The Society of International Sustaining Growth for Antimicrobial Articles (SIAA) for their helpful advice and information about biofilms.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study’s design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Barry, D.M.; Kanematsu, H. Studies to Combat COVID-19 using Science and Engineering; Barry, D.M., Ed.; Springer: Singapore, 2022. [Google Scholar]
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, ü.; Ganbarov, K.; Pagliano, P.; Espsoito, S.; Kafil, H.S. Protection and disinfection policies against SARS-CoV-2(COVID-19. Le Infezioni in Mediciana 2020, n.2, 185–191. [Google Scholar]
- WHO Europe. Coronavirus disease (COVID-19) pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 2022).
- Bloom, J.D.; Chan, Y.A.; Baric, R.S.; Bjorkman, P.J.; Cobey, S.; Deverman, B.E.; Fisman, D.N.; Gupta, R.; Iwasaki, A.; Lipsitch, M. Investigate the origins of COVID-19. Science 2021, 372, 694–694. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.; Mao, L.; Wang, S.; Xue, K.; Yang, L. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bishop, P. Microbiology - and infection control for health professional, 6th Edition ed.; 2015.
- Lostroh, P. Molecular and Cellular Biology of Viruses; Garland Science: New York, USA, 2019. [Google Scholar]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and perspective of antiviral protective material. Advanced Fiber Materials 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Liang, L.; Ahamed, A.; Ge, L.; Fu, X.; Lisak, G. Advances in antiviral material development. ChemPlusChem 2020, 85, 2105–2128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, W.; Sun, Y.; Chen, W.; Zhang, Y. Application of antiviral materials in textiles: A review. Nanotechnology Reviews 2021, 10, 1092–1115. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Rama Krishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: chemistry and biological activity toward tackling COVID-19-like pandemics. ACS Pharmacology & Translational Science 2020, 4, 8–54. [Google Scholar]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Communications Materials 2021, 2, 53. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K.K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustainable Materials and Technologies 2020, 25, e00203. [Google Scholar] [CrossRef]
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene films containing plant extracts in the polymer matrix as antibacterial and antiviral materials. International Journal of Molecular Sciences 2021, 22, 13438. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Campo, K.N.; Arns, C.W.; Gabriel, L.P.; Webster, T.J.; Lopes, É.S. From bulk to nanoparticles: an overview of antiviral materials, its mechanisms, and applications. Particle & Particle Systems Characterization 2021, 38, 2100044. [Google Scholar]
- Seifi, T.; Kamali, A.R. Antiviral performance of graphene-based materials with emphasis on COVID-19: A review. Medicine in Drug Discovery 2021, 11, 100099. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D. Viral Behaviors on Materials and the International Standard Between Materials and Microbial/Viral Environments. In Studies to Combat COVID-19 using Science and Engineering; Springer, 2022; pp. 39–52. [Google Scholar]
- Popescu, C.; Alain, S.; Courant, M.; Vardelle, A.; Denoirjean, A.; Cavarroc, M. Thermal spray copper-based coatings against contamination of thermoplastic surfaces: A systematic review. Engineering Science and Technology, an International Journal 2022, 101194. [Google Scholar] [CrossRef]
- Popescu, C.; Alain, S.; Courant, M.; Vardelle, A.; Denoirjean, A.; Cavarroc, M. Thermal spray copper-based coatings against contamination of thermoplastic surfaces: A systematic review. Engineering Science and Technology, an International Journal 2022, 101194. [Google Scholar] [CrossRef]
- Verch, T.; Trausch, J.J.; Shank-Retzlaff, M. Principles of vaccine potency assays. Bioanalysis 2018, 10, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.; Kehn-Hall, K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. JoVE (Journal of Visualized Experiments) 2014, e52065. [Google Scholar]
- Crichton, R.R. Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function; Elsevier, 2012. [Google Scholar]
- Hideyuki, K.; Barry, D.M.; Ikegai, H.; Mizunoe, Y. Biofilm control on metallic materials in medical fields from the viewpoint of materials science - from the fundamental aspects to evaluation. International Materials Review 2022. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).