Submitted:
09 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
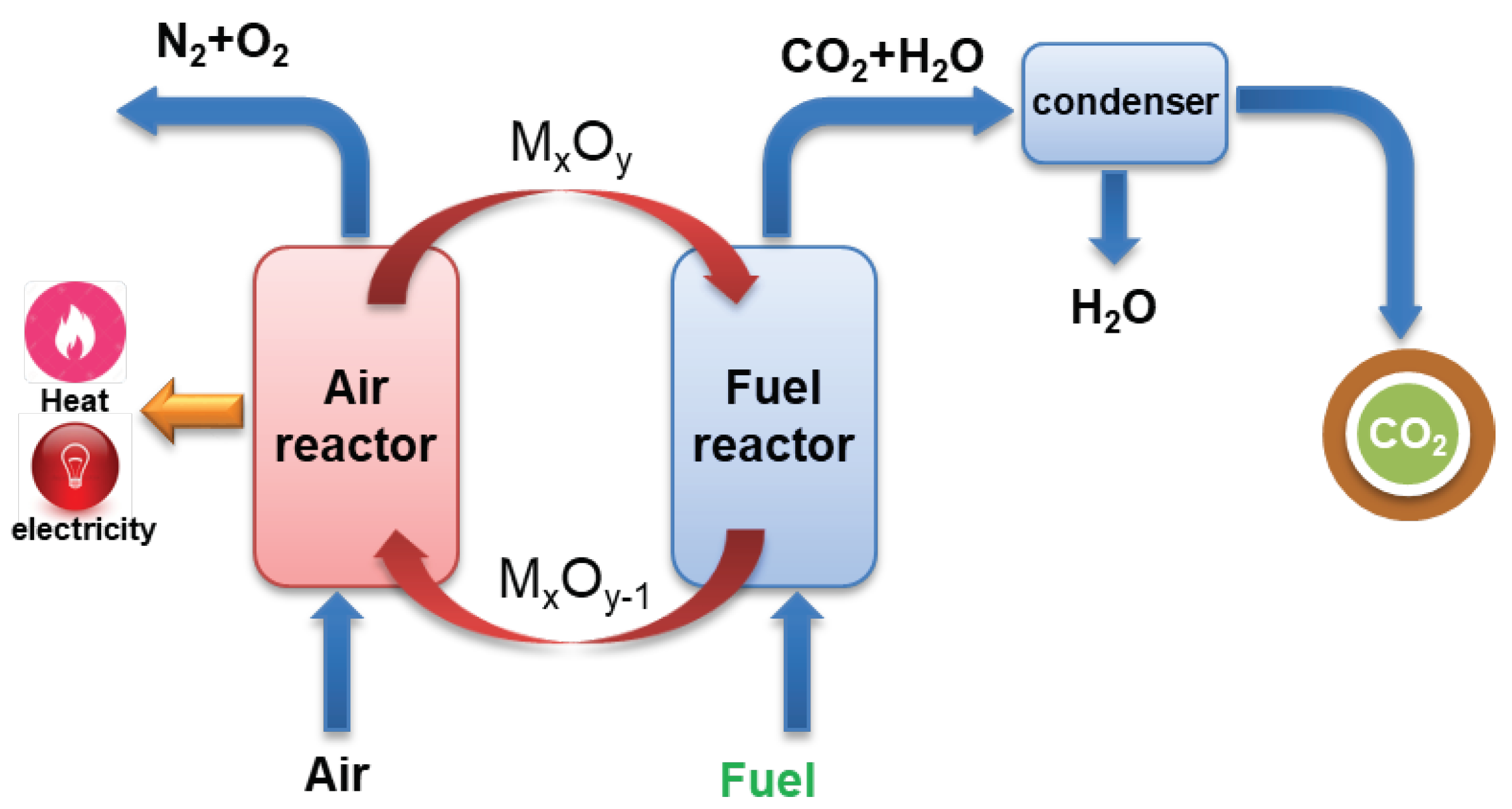
2. Methods
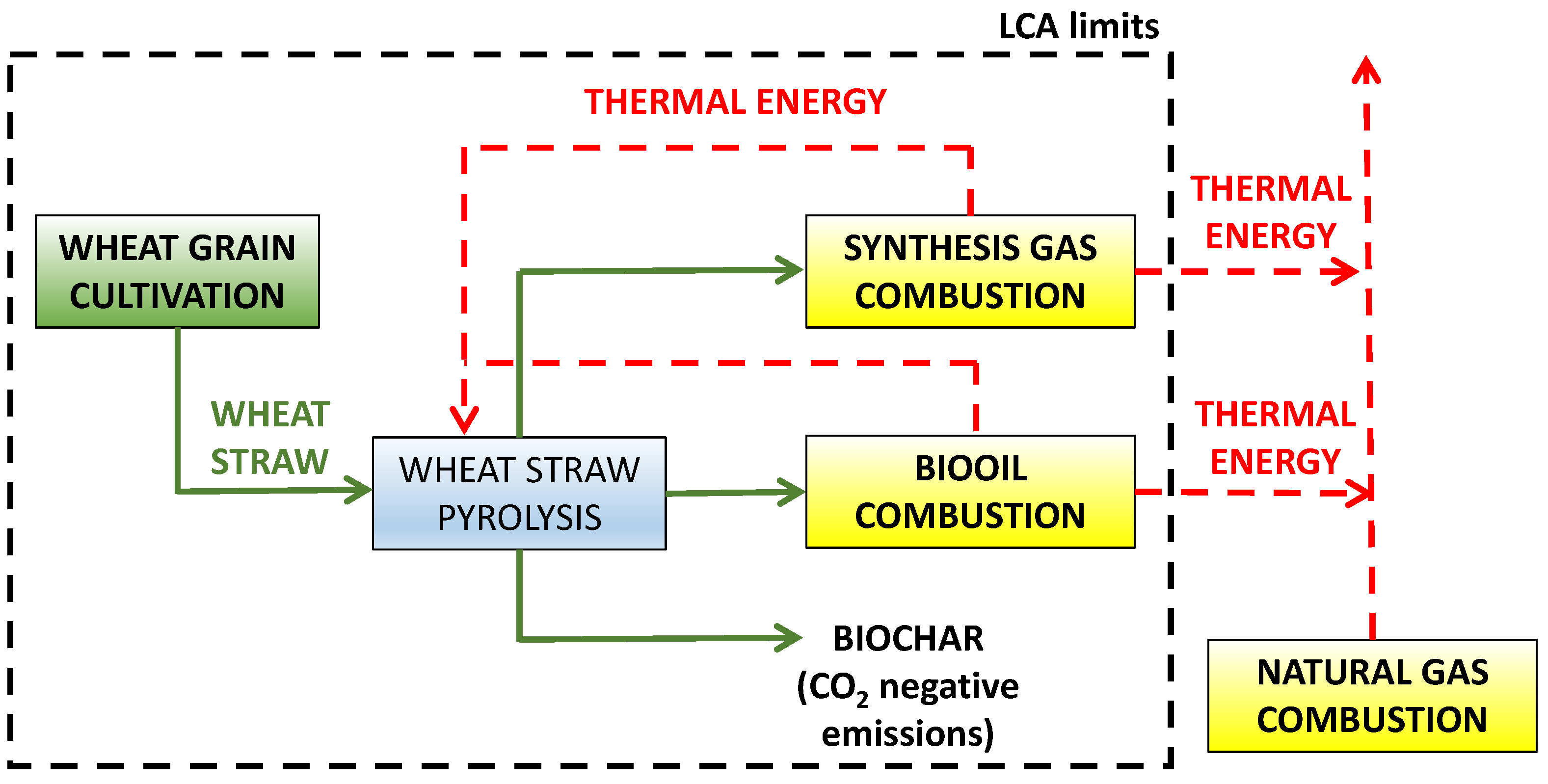
2.1. Scope Definition
2.1.1. Functional Unit and Base Case
- (i)
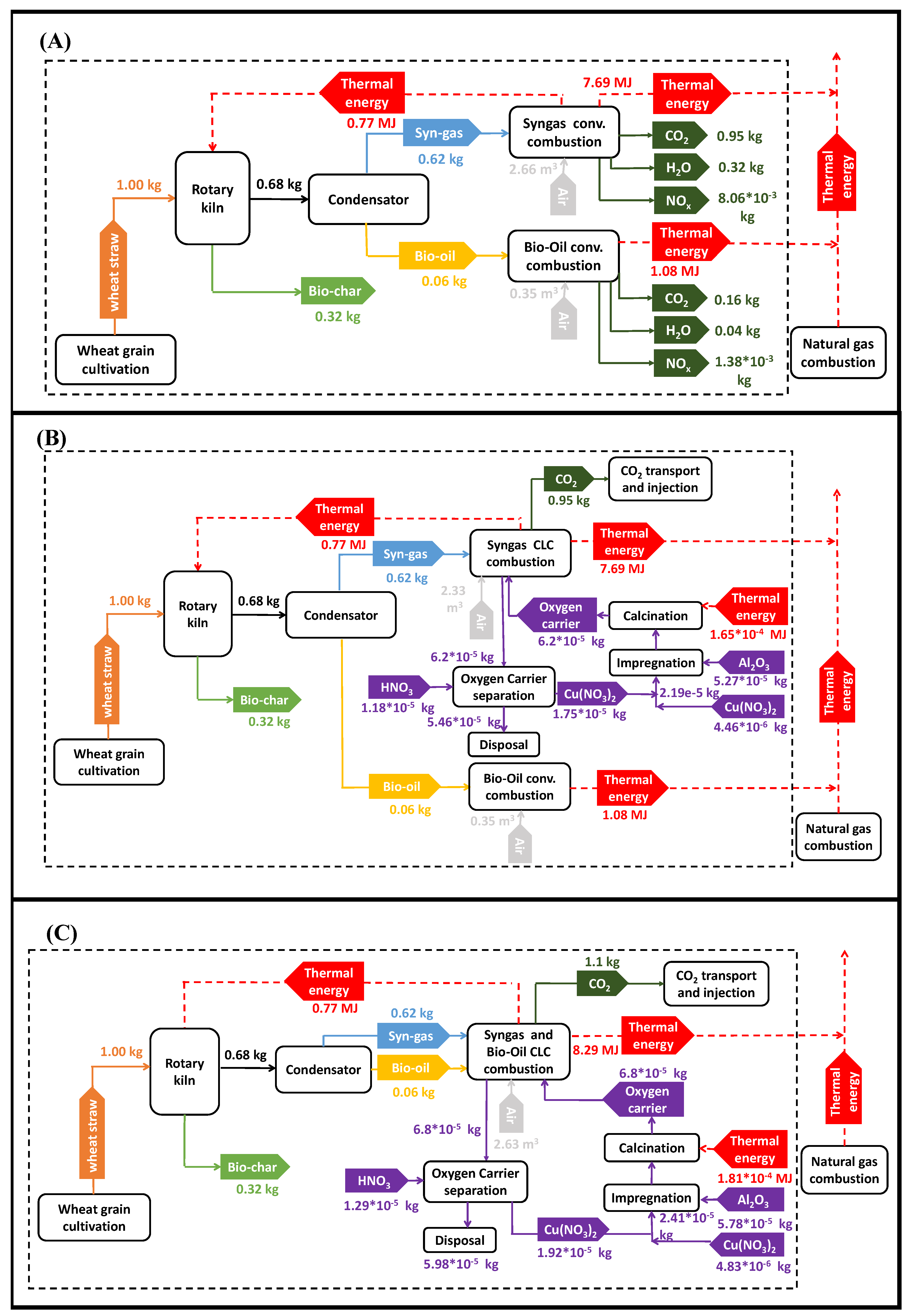
- Case I- Conventional combustion gas fraction
- (ii)
- Case II- Gas fraction CLC combustion and bio-oil conventional combustion.
- (iii)
- Case III- Gas fraction and bio-oil CLC combustion.
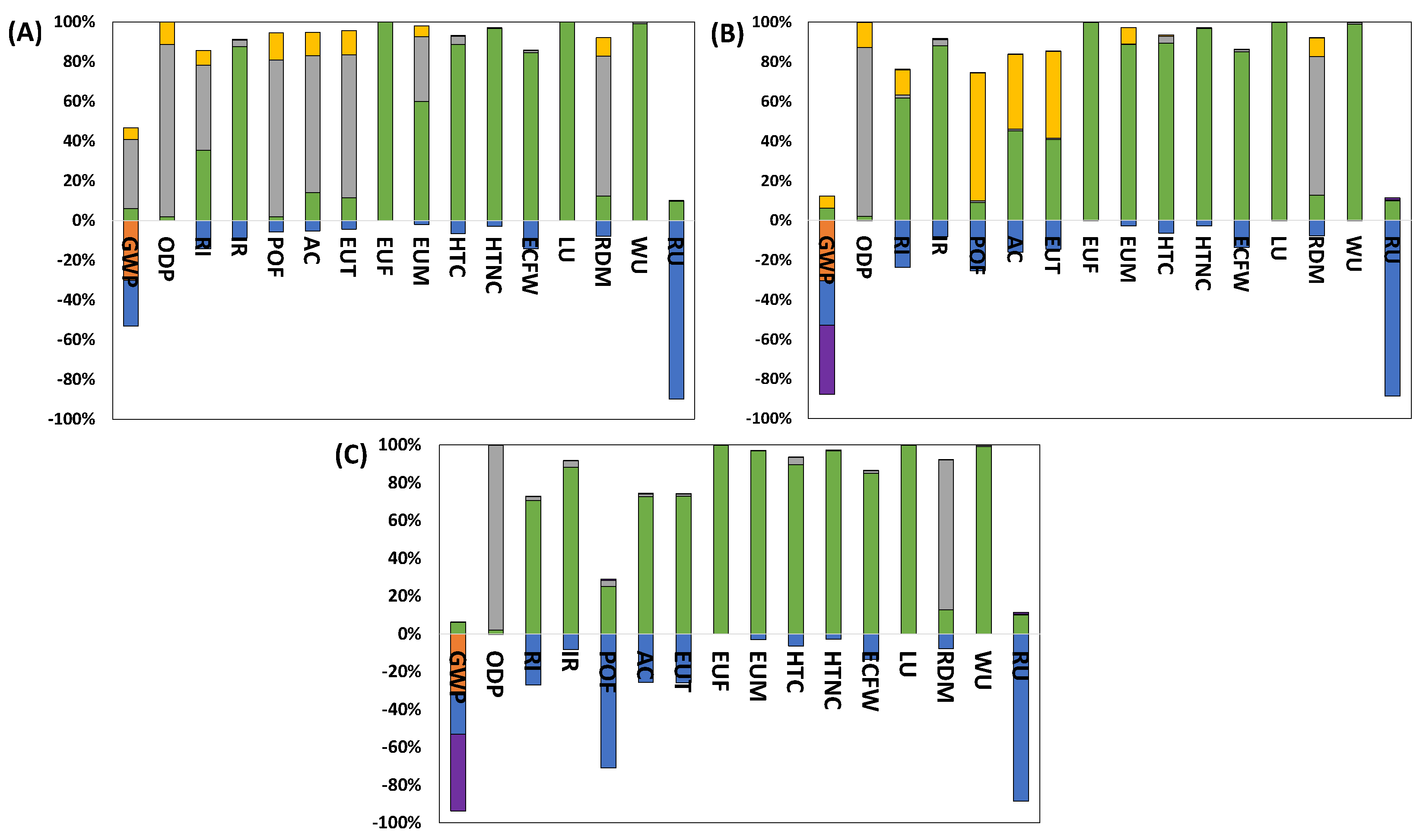
2.1.2. Impact Categories
2.2. Life Cycle Inventory
3. Results
3.1. Study of the CO2 Avoided
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ONU United Nations Framework Convention for Climate Change. The Paris Agreement. Available online: http://unfccc.int/paris_agreement/items/9485.php.
- Shukla, P.; Skea, J.; Reisinger, A.; Slade, R.; Fradera, R.; Pathak, M.; Al Khourdajie, A.; Belkacemi, M.; van Diemen, R.; Hasija, A.; et al. IPCC, 2022: Summary for Policymakers. In Climate Change 2022: Mitigation of Climate Change.; Cambridge University Press, C., UK and New York, NY, U. (Ed. )., Ed.; 2022.
- Shafawi, A.N.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Recent Advances in Developing Engineered Biochar for CO<inf>2</Inf> Capture: An Insight into the Biochar Modification Approaches. J. Environ. Chem. Eng. 2021, 9. [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A Critical Review of the Production and Advanced Utilization of Biochar via Selective Pyrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2020, 312. [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel Oil Quality of Biomass Pyrolysis Oils - State of the Art for the End Users. Energy and Fuels 1999, 13, 914–921. [CrossRef]
- Mattisson, T.; Keller, M.; Linderholm, C.; Moldenhauer, P.; Rydén, M.; Leion, H.; Lyngfelt, A. Chemical-Looping Technologies Using Circulating Fluidized Bed Systems: Status of Development. Fuel Process. Technol. 2018, 172, 1–12. [CrossRef]
- Lyngfelt, A. Chemical Looping Combustion: Status and Development Challenges. Energy and Fuels 2020, 34, 9077–9093. [CrossRef]
- Adánez, J.; Abad, A. Chemical-Looping Combustion: Status and Research Needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [CrossRef]
- Lyngfelt, A.; Leckner, B. A 1000 MW<inf>th</Inf> Boiler for Chemical-Looping Combustion of Solid Fuels – Discussion of Design and Costs. Appl. Energy 2015, 157, 475–487. [CrossRef]
- Navajas, A.; Uriarte, L.; Gandía, L. Application of Eco-Design and Life Cycle Assessment Standards for Environmental Impact Reduction of an Industrial Product. Sustainability 2017, 9, 1724. [CrossRef]
- Navajas, A.; Echarri, I.; Gandía, L.M.; Pozuelo, J.; Cascarosa, E. Life Cycle Assessment in Higher Education: Design and Implementation of a Teaching Sequence Activity. Sustainability 2024, 16. [CrossRef]
- EC-JRC Recommendations Based on Existing Environmental Impact Assessment Models and Factors for Life Cycle Assessment in European Context. ILCD Handbook. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC61049/jrc61049_ilcd handbook final.pdf.
- Hofbauer, H. Niedertemperatur Drehrohrpyrolyse Als Vorschaltprozess Für Die Co-Verbrennung von Unkonventionellen Brennstoffen in Thermischen Anlagen, Technischen Universität Wien, 2010.
- He, F.; Yi, W.; Bai, X. Investigation on Caloric Requirement of Biomass Pyrolysis Using TG-DSC Analyzer. Energy Convers. Manag. 2006, 47, 2461–2469. [CrossRef]
- Habib, M.A.; Mokheimer, E.M.A.; Sanusi, S.Y.; Nemitallah, M.A. Numerical Investigations of Combustion and Emissions of Syngas as Compared to Methane in a 200 MW Package Boiler. Energy Convers. Manag. 2014, 83, 296–305. [CrossRef]
- Basu, P. Pyrolysis. Biomass Gasification, Pyrolysis and Torrefaction 2013, 147–176. [CrossRef]
- Forero, C.R.; Gayán, P.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Adánez, J. Syngas Combustion in a 500 Wth Chemical-Looping Combustion System Using an Impregnated Cu-Based Oxygen Carrier. Fuel Process. Technol. 2009, 90, 1471–1479. [CrossRef]
- Cabello, A.; Abad, A.; Mendiara, T.; Izquierdo, M.T.; de Diego, L.F. Outstanding Performance of a Cu-Based Oxygen Carrier Impregnated on Alumina in Chemical Looping Combustion. Chem. Eng. J. 2023, 455. [CrossRef]
- Navajas, A.; Mendiara, T.; Goñi, V.; Jiménez, A.; Gandía, L.M.; Abad, A.; García-Labiano, F.; de Diego, L.F. Life Cycle Assessment of Natural Gas Fuelled Power Plants Based on Chemical Looping Combustion Technology. Energy Convers. Manag. 2019, 198. [CrossRef]
- Saskatchewan province web Harvesting Surplus Cereal Straw Available online: https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/field-crops/cereals-barley-wheat-oats-triticale/harvesting-surplus-cereal-straw (accessed on 9 November 2023).
- Sphera Process Data Set: Electricity from Photovoltaic; AC, Technology Mix of CIS, CdTe, Mono Crystalline and Multi Crystalline; Production Mix, at Plant; 1kV - 60kV Available online: https://sphera.com/2023/xml-data/processes/5c5c2277-8df1-4a73-a479-9c14deec9bb1.xml (accessed on 10 November 2023).
- International Energy Agency Greenhouse Gas R&D Programme 2007 Capturing CO2; 2007;
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A Review on Biomass as a Fuel for Boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [CrossRef]

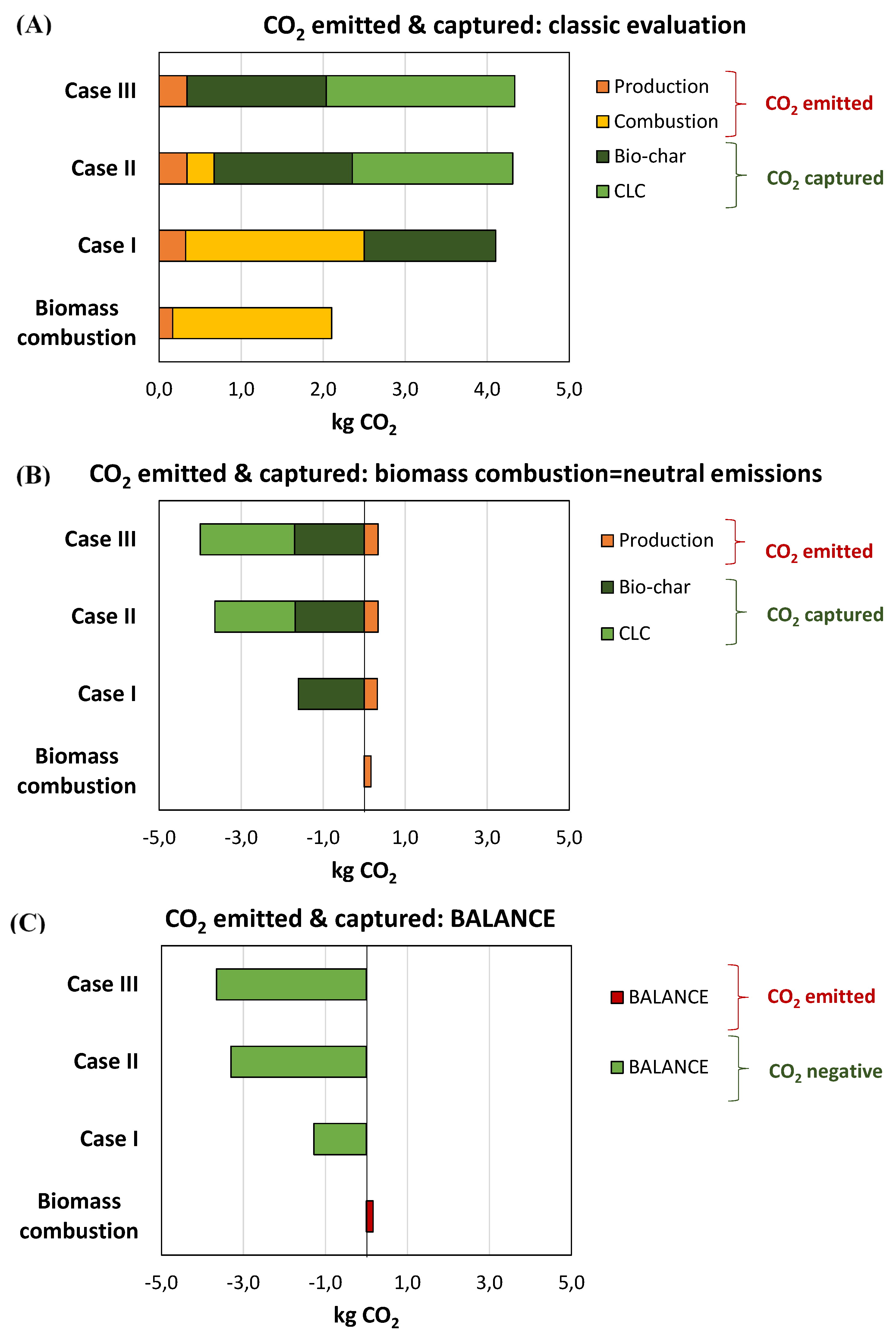
| Biomass combustion | Case I | Case II | Case III | |
|---|---|---|---|---|
| Biomass amount (kg) | 1.00 | 1.95 | 2.05 | 2.06 |
| CO2 emissions (kg) | ||||
| Production | 0.165 | 0.322 | 0.338 | 0.340 |
| Combustion | 1.938 | 2.178 | 0.332 | 0.000 |
| CO2 captured (kg) | ||||
| Bio-char | 0.00 | 1.605 | 1.685 | 1.696 |
| CLC | 0.000 | 0.000 | 1.956 | 2.302 |
| Total emitted (kg) | 2.103 | 2.501 | 0.670 | 0.340 |
| Total captured (kg) | 0.000 | 1.605 | 3.641 | 3.998 |
| Balance (kg CO2) | 2.103 | 0.896 | -2.971 | -3.657 |
| Net balance (kg CO2) | 0.165 | -1.283 | -3.303 | -3.657 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





