1. Introduction
Metastatic large-cell neuroendocrine carcinoma (LCNEC) is a rare and aggressive type of cancer, with an age-adjusted incidence rate of 0.3/100.000 [
1]. Previously classified as a subtype of non-small cell lung cancer (NSCLC) by the World Health Organization, LCNEC was reclassified in 2015 to fall within the range of neuroendocrine neoplasms [
2]. Unlike advances in treatments for other neuroendocrine neoplasms [
3,
4,
5,
6,
7], such as neuroendocrine tumors (NETs) and small cell lung cancer (SCLC), therapeutic developments for metastatic LCNEC have been lagging and confined to chemotherapeutic drugs [
8,
9,
10].
The available prospective data on the efficacy of chemotherapy in patients with advanced LCNEC are limited to the phase 2 GFPC 0302 study, which demonstrated a survival benefit with the use of a platinum-etoposide combination [
11]. A decade later, in the DART study, the efficacy of the combination of nivolumab and ipilimumab was tested in high-grade neuroendocrine carcinomas, including high-grade neuroendocrine carcinomas of the lungs. Although the number of patients was small, a significant response to immunotherapy has been reported [
12]. Additional evidence from retrospective studies shows that a proportion of patients with LCNEC respond to immune checkpoint inhibitors (ICIs) [
13,
14,
15,
16]. However, these studies used various ICIs without a clear advantage of one drug over another or a suggested combination with chemotherapeutic drugs.
Atezolizumab (Tecentriq, F. Hoffmann-La Roche/Genentech) is a humanized monoclonal anti-programmed death ligand 1 (PD-L1) that has shown efficacy in the treatment of metastatic SCLC and has changed the standard of care after numerous negative trials [
6]. Recent studies examining the genetic and transcriptional profiles of LCNEC and SCLC have shown that, besides their significant differences, they share some common features, such as neuroendocrine phenotype, high mutation burden, and aggressive biological behavior [
17,
18]. Considering the overlapping biological features and parallel chemotherapy strategies for both SCLC and LCNEC, investigation of the efficacy of atezolizumab in patients with LCNEC is necessary.
In the LANCE study, we assessed the effectiveness of adding atezolizumab to chemotherapy for improving survival. Furthermore, we evaluated miR-375, a plasma marker that has been suggested to have diagnostic and prognostic value in patients with neuroendocrine carcinomas, as a potential biomarker [
19,
20].
2. Materials and Methods
Patient Selection and Pathology Review
In this non-randomized study, we prospectively enrolled all consecutive patients with histologically confirmed metastatic lung-derived LCNEC who presented to the 3rd Department of Medicine of the National and Kapodistrian University of Athens, Sotiria Chest Diseases Hospital, between November 2019 and August 2022. This study consisted of two cohorts and was conducted at a single institution. Enrolled patients provided written informed consent. Patient enrollment fulfilled the following inclusion criteria: i) histological diagnosis of lung-derived LCNEC by two independent pathologists, ii) histological or radiological confirmation of metastatic disease, and III) stable clinical condition (Eastern Cooperative Performance Status [ECOG PS] ≤2) to receive treatment upon physician evaluation. A second pathologist’s review was required after screening and blinded to the initial report. In the event of a discrepancy, a third pathologist’s review was requested, and two unanimous reports were required for the final diagnosis. Exclusion criteria included i) prior systemic treatment for their disease, ii) ECOG PS >2, and iii) contraindication to receive treatment with ICIs. Patients with brain metastases upon initial diagnosis were considered eligible for enrollment after receiving whole-brain radiotherapy. All patients were monitored from the initiation of their treatment until August 2022. The study protocol was approved by the Institutional Review Board of the Sotiria Chest Diseases Hospital (Athens, Greece; approval number: 4101/19) and was conducted in accordance with the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (NCT06049966).
Study Design
Since atezolizumab in combination with chemotherapy is not an approved regimen for the management of LCNEC in real-world clinical practice, applications were submitted to the national regulatory authorities (National Organization for the Provision of Health Services,EOPYY) for all patients to receive immunotherapy with atezolizumab (1200 mg on day 1), along with carboplatin (AUC5 on Day 1) and etoposide (100 mg/m² on Days 1-3) every 21 days for four cycles. Each request was evaluated individually by different members of EOPYY board. Patients who were granted approval for the regimen were stratified into the atezolizumab-chemotherapy (experimental) group and the rest into the chemotherapy (control) group. Patients in the experimental group continued with atezolizumab maintenance every 21 days after demonstrating a partial or complete response or stable disease according to RECIST 1.1, until disease progression or serious adverse events occurred. Patients of the control group were treated with only carboplatin and etoposide for up to six cycles.
In both arms, the patients continued with other treatments for disease progression based on the physician’s choice. Therapy with ICIs or participation in clinical trials was not permitted for any later lines of treatment. Assessment of response to treatment with computerized tomography (CT) and physical evaluation was scheduled once upon completion of the combination treatment and then every three–four months unless new onset of persistent symptoms or clinical deterioration of the patient indicated the need for early examination. The management of adverse effects and decisions regarding treatment discontinuation in patients receiving atezolizumab therapy followed the European Society of Medical Oncology (ESMO) guidelines for supportive and palliative care [
21].
Endpoints
The primary endpoints were overall survival (OS; time from treatment initiation to death from any cause) and progression-free survival (PFS; time from treatment initiation to disease progression according to RECIST or death from any cause, whichever occurred first). Patients with no events were censored on the date of their last follow-up. The censoring for Overall Survival (OS) and Progression-Free Survival (PFS) was applied as of the last date on which the survival status was verified for patients who had not experienced documented disease progression and remained alive. Other key secondary end points were the overall response rate (ORR; the percentage of patients with a CR or PR according to RECIST) the duration of response (DoR; the interval from response initiation, when either CR or PR is first determined, to progression or death, whichever occurred first), and the kinetics of miR357 in the plasma of patients
Statistical Analysis
Statistical analysis was performed using R software, version 4.3.2. PFS and OS were estimated using the Kaplan–Meier method with 95% confidence intervals (CIs). Survival distributions between the groups were compared using the log-rank test. For the estimation of hazard ratios, Cox Regression survival analysis was performed, including an assessment of the predictive ability/concordance index. All statistical tests were two-sided, with the significance level set at P < 0.05.
The Shapiro-Wilk test was applied to evaluate the normality of miR375 expression levels. Subsequently, given the non-normal distribution, we employed the non-parametric Wilcoxon rank-sum test to compare miR375 levels between patients with large cell neuroendocrine carcinoma (LCNEC) and healthy controls. In addition, the Mann-Whitney U test was used as a complementary approach.
Spearman’s rank correlation coefficient was used to assess the correlation between changes in miR375 expression and disease progression.
Detailed patient data and the R code used for statistical analyses are available in the Supplementary Material.
miR357 Analysis
Plasma samples were collected from patients with LCNEC at two time points: immediately before the start of treatment (baseline) and upon imaging-confirmed disease progression, or at the end of the study follow-up period (follow-up).
The control samples were obtained from 13 healthy volunteers. These individuals had no history of neoplasia and underwent thorough physical examinations, including chest radiography and abdominal ultrasonography, which did not reveal any abnormalities that required further investigation. Additionally, women underwent mammography and gynecological screening, whereas men provided Prostate-Specific Antigen (PSA) values. Volunteers were matched to patients with LCNEC in terms of age and sex. However, there was no matching for comorbidities and chronic medications between the two groups.
All samples were centrifuged within one hour of collection at 1500 rpm for 15 minutes at 4 °C. The isolated plasma was stored at -80 °C. Sample analysis commenced only after all the patient samples were collected. The longest storage duration for all the samples was 26 months. Samples showing evidence of hemolysis after centrifugation were excluded from the analysis, and RNA was extracted from 200 μl of human plasma samples using the miRNeasy Serum/Plasma Kit (Qiagen) in accordance with the manufacturer’s instructions. cDNA synthesis was carried out with 8 μl of plasma RNA and the miRCURY LNA RT Kit in a 10 μl reaction, including the UniSp6 RNA spike-in (Qiagen). miRNA quantification was performed using the miRCURY LNA SYBR Green PCR Kit (Qiagen), hsa-miR-375-3p miRCURY LNA miRNA PCR Assay, and hsa-miR-103a-3p miRCURY LNA miRNA PCR Assay Kit (Qiagen), according to the manufacturer’s instructions.
The reference gene used in this study was miR103a-3p. UniSp6 miRCURY LNA miRNA PCR Assay was used to assess the consistency of cDNA synthesis and PCR amplification for all samples. All real-time PCR reactions were performed using a CFX96 Real-Time PCR Detection System (Bio-Rad). The expression levels of miRNAs were determined using the 2 − ΔΔCt method and reported relative to the control group.
This analysis was conducted at the Institute for Fundamental Biomedical Research (IFBR), Biomedical Sciences Research Center, ‘Alexander Fleming.’ This institution is responsible for all steps of sample processing, RNA extraction, cDNA synthesis, and miRNA quantification.
3. Results
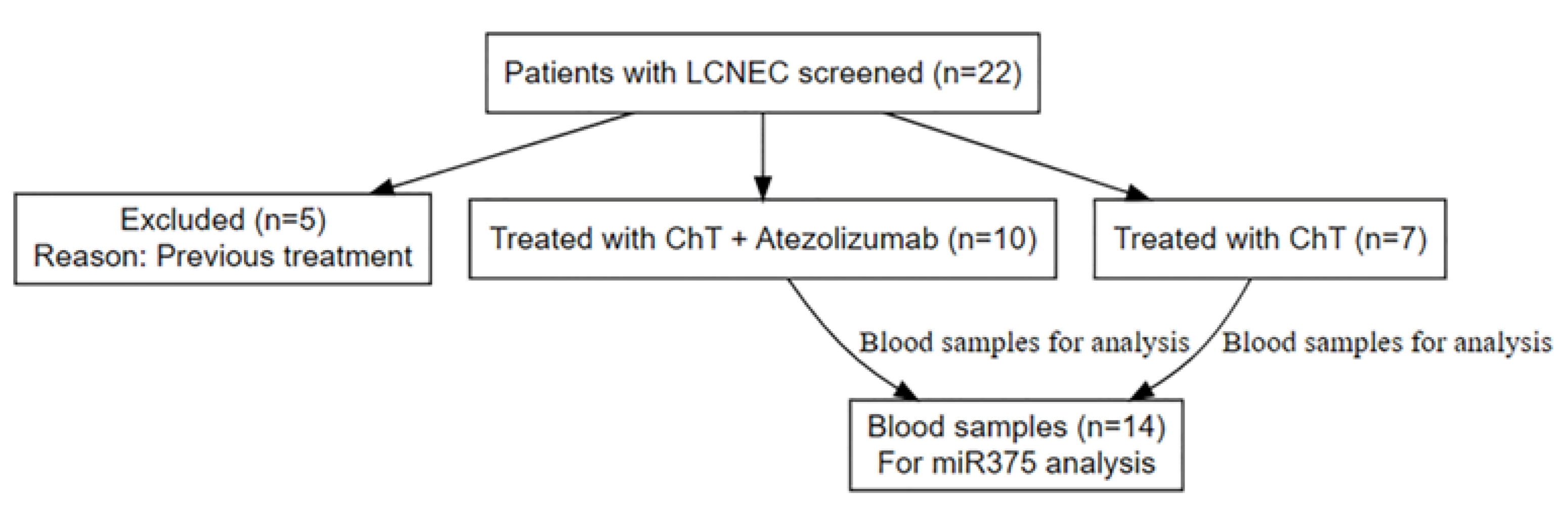
Between November 2019 and August 2022, 22 patients were prospectively screened for enrollment in the study. Of the 22 patients, five were excluded as ineligible because they had previously received chemotherapy and chest radiotherapy as they were initially diagnosed with limited-stage disease. The remaining 17 patients met all the eligibility criteria and were included in the study. The biopsies of the enrolled patients were reviewed by a second pathologist. In two patients, a third consultation was required to confirm the diagnosis of LCNEC. Of these patients, 10 were recruited to the experimental arm and treated with carboplatin, etoposide, and atezolizumab, while seven patients were treated with carboplatin and etoposide and served as the control arm (
Figure 1). The median follow-up duration was 23.3 months.
The baseline clinicopathological characteristics were well-balanced between the two treatment groups (
Table 1). The median age of patients in the experimental arm, receiving chemotherapy plus atezolizumab, was 68.5 years (range 54-82), compared to 76 years (range 53-85) in the control arm treated with chemotherapy alone. The majority of patients in both groups were male, accounting for 80% of the patients in the experimental arm and 85.7% in the control arm. Regarding smoking status, current smokers comprised 70% of the experimental group and 71.4% of the control group, with former and never-smokers accounting for a smaller proportion in both groups. All patients in both groups were Caucasians.
The time from diagnosis to treatment initiation was 35 and 26 days in the experimental and control arms, respectively. The incidence of brain metastases was slightly higher in the control group (28.6%) than in the experimental group (20%). A similar pattern was observed for liver metastases (28.6% in the control group and 20% in the experimental group). As for the Eastern Cooperative Oncology Group (ECOG) Performance Status, both groups had similar distributions across categories 0, 1, and 2. TP53 positive immunohistochemistry (IHC) was observed in 80% of the experimental group and 71.4% of the control group. RB1 positive IHC was present in 40% of the experimental group and 42.9% of the control group. Previous studies have shown that mutations in TP53 were detected in 78% of LCNEC tumors, whereas the second most frequently mutated gene was RB1 (38%)[
18]. Finally, the median Ki67% was 75% in the experimental arm and 70% in the control arm.
Effectiveness of Atezolizumab
Response Rate and Duration of Response
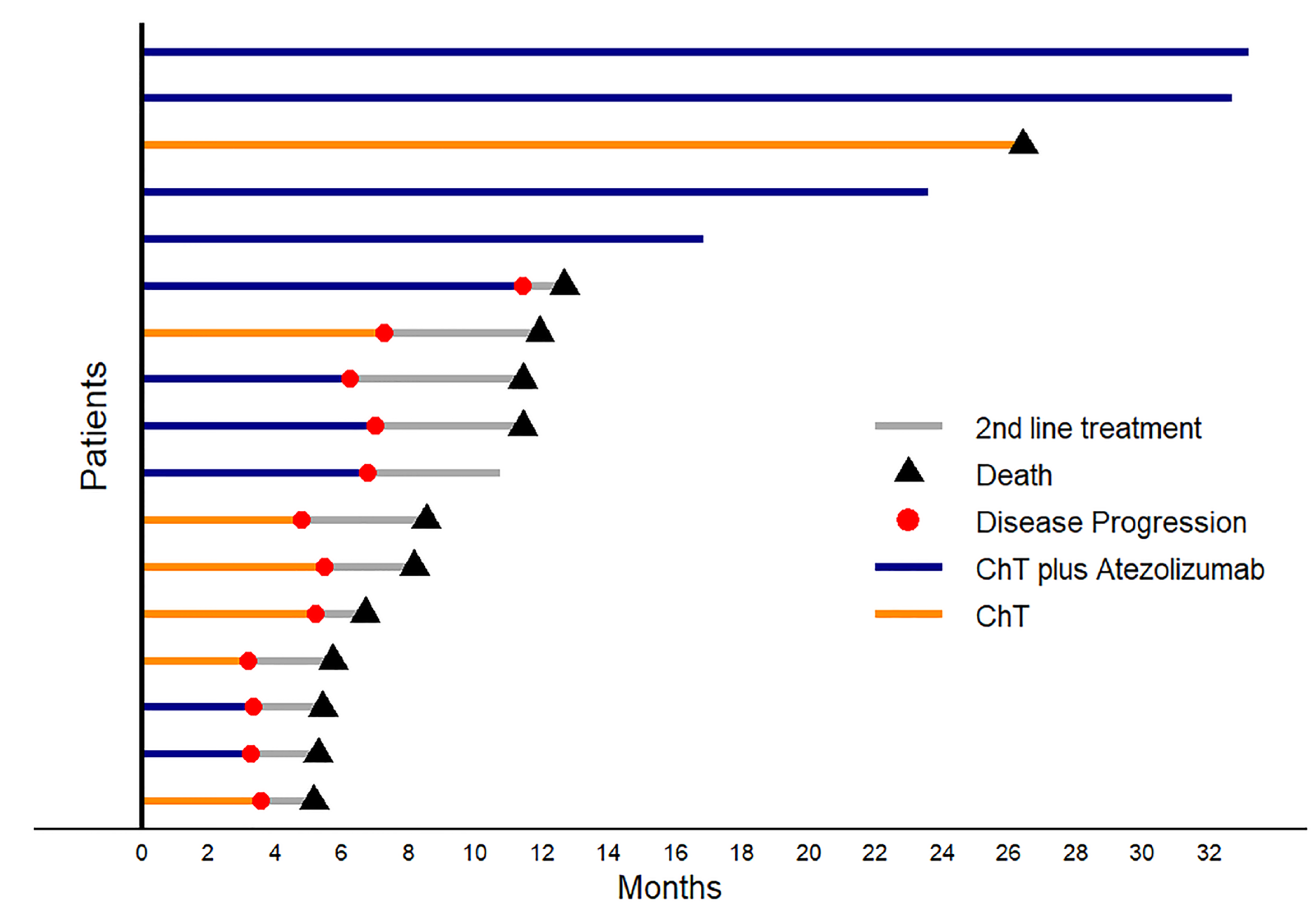
The Objective Response Rate (ORR) was 50% for the chemotherapy plus atezolizumab group (ChT plus Atezo) and 42.9% for the chemotherapy alone (ChT) group. By the end of the study follow-up period, 40% of the patients in the experimental arm demonstrated a durable clinical benefit in contrast to the control arm, where no patients survived (
Figure 2).
Progression-free Survival (PFS)
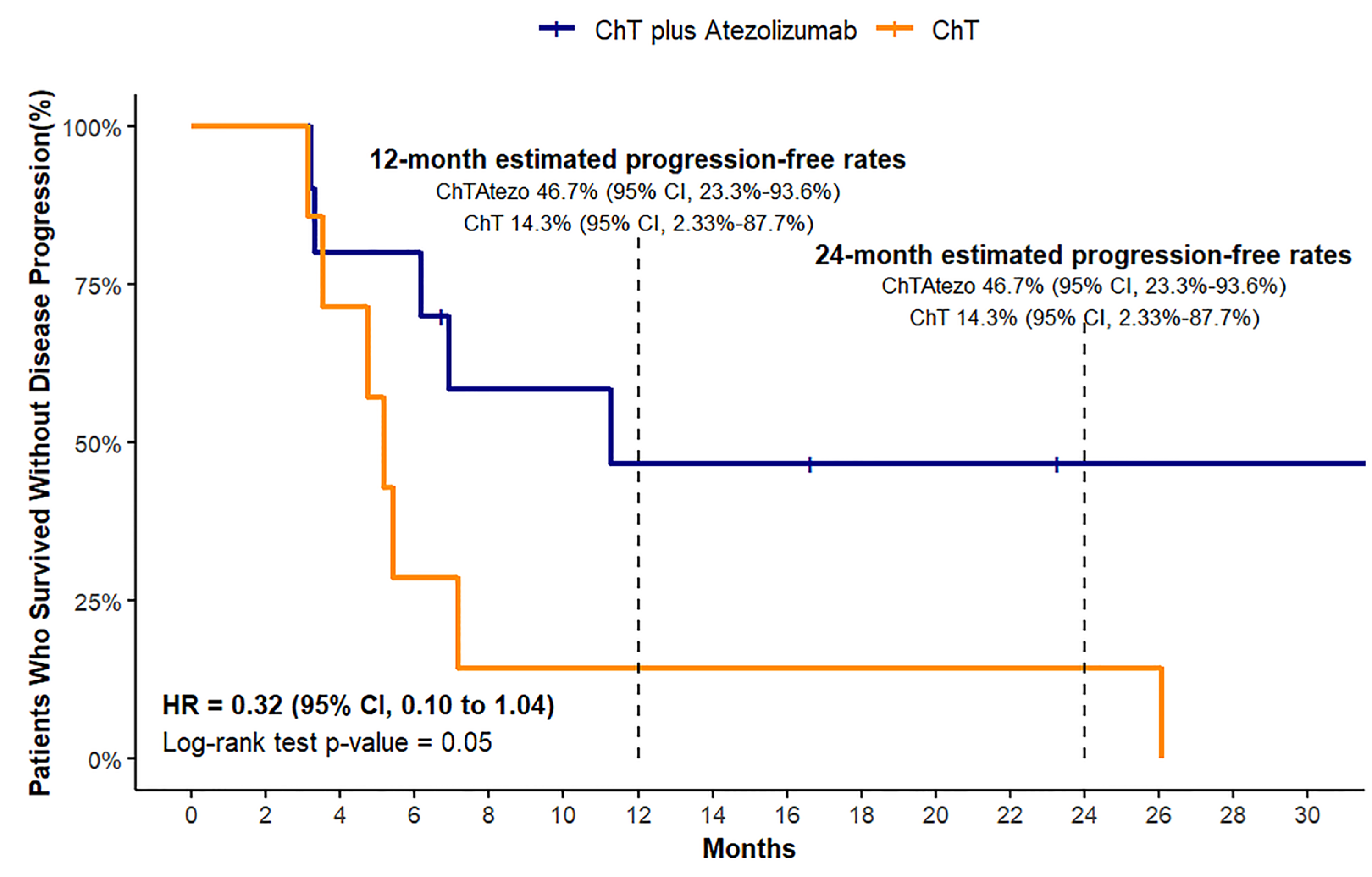
In our analysis of the median PFS for both treatment groups, we encountered a limitation owing to the small sample size and lack of sufficient events. As a result, we were unable to calculate the definitive median PFS for either treatment group. Given these constraints, we chose to present survival data at specific time points, namely, at 12 and 24 months. This approach allowed us to provide a more accurate and meaningful interpretation of the available data, focusing on the survival rates at these time intervals.
The PFS analysis revealed a 12-month estimated progression-free rate of 46.7% for the ChT plus Atezo group (95% CI, 23.3%-93.6%), which was significantly higher than the 14.3% observed in the ChT group (95% CI, 2.33%-87.7%). The 24-month progression-free rates remained unchanged from the 12-month estimates in both the treatment arms (
Figure 3). A log-rank test for PFS indicated a statistically significant difference between the two treatment groups (chi-square = 3.9, df = 1, p = 0.05). The adjusted hazard ratio for PFS was 0.32 (95% CI, 0.10 to 1.04).
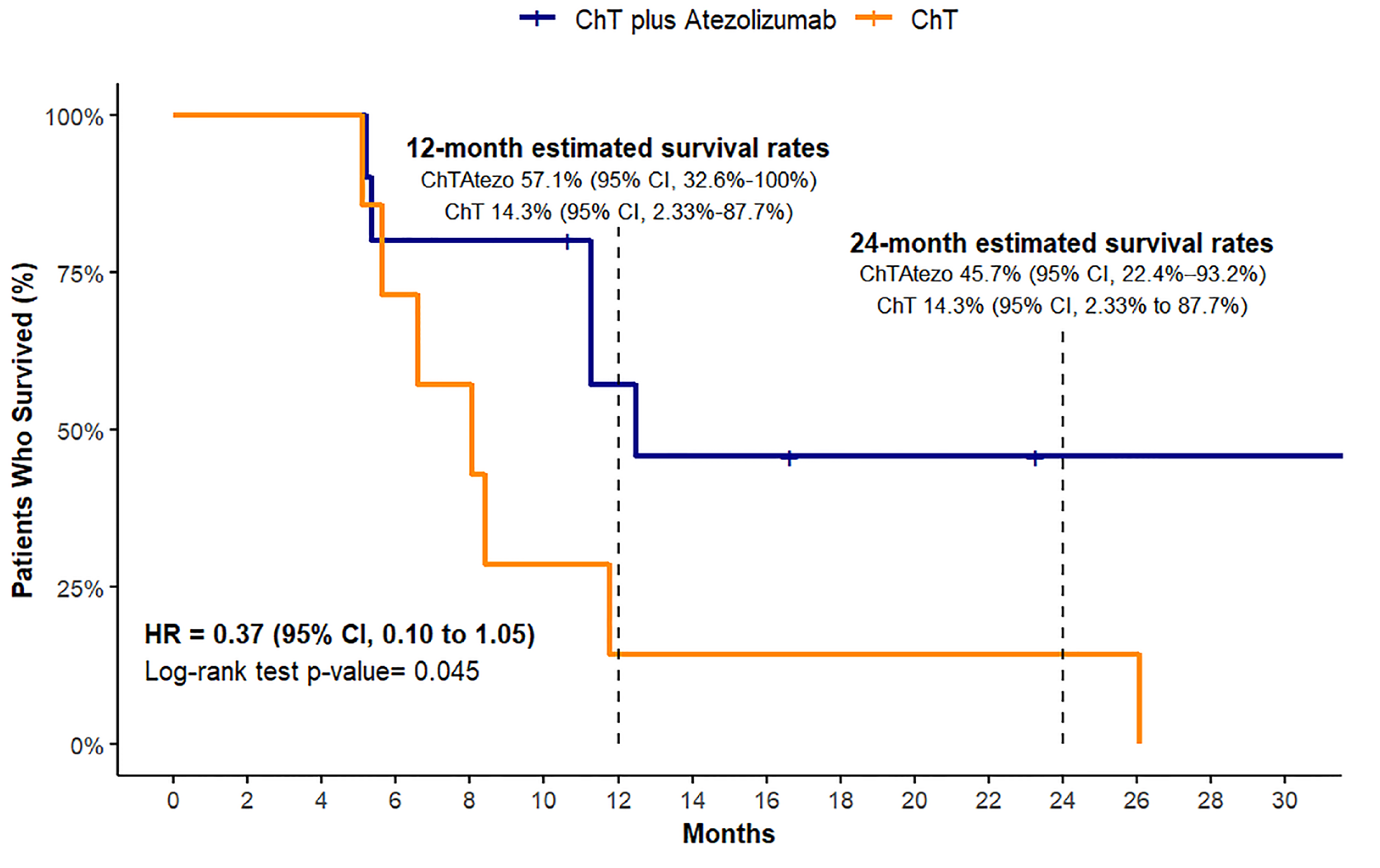
Overall Survival (OS)
Our analysis failed to establish definitive median survival times for the experimental treatment group, likely because of the small sample size and inadequate event occurrence during the observation period. The OS analysis indicated that the ChT plus Atezo group had an estimated 12-month survival rate of 57.1% (95% CI, 32.6% - 100%) and a 24-month survival rate of 45.7% (95% CI, 22.4%-93.2%). In contrast, the ChT group had an estimated 12- and 24-month survival rate of 14.3%, with a 95% CI ranging from 2.33% to 87.7% (
Figure 4). A log-rank test for OS showed a statistically significant difference in survival between the two treatment groups (chi-square = 4, df = 1, p = 0.04). Overall survival univariate HR for the two treatment groups based on the log-rank method showed a borderline significant benefit for the atezolizumab-treated group (HR= 0.37, 95%CI, 0.10-1.05).
Safety of Atezolizumab
Two patients who experienced grade 3 immunotherapy-related adverse events (AEs) were required to discontinue treatment and were administered prednisolone (1 mg/kg) until their symptoms improved to grade 1. Following resolution of their side effects, both patients were able to continue treatment with atezolizumab. The first patient developed a grade 3 skin reaction, specifically exfoliative dermatitis, on the dorsal and palmar surfaces of their upper limbs and on their trunk and thighs after the 10th cycle of maintenance treatment with atezolizumab. Lesions on the trunk and thighs had a less aggressive appearance than those on the upper limbs. Treatment with prednisolone resulted in resolution of the lesions to grade 1, and the patient was able to continue treatment. However, by the end of the follow-up period, the toxicity remained at grade 1 but did not fully remit. The second patient experienced pneumonitis after six months of treatment with atezolizumab. Prednisolone treatment for two months resulted in the complete resolution of pneumonitis. No other Grade 3 or 4 immune-related events were observed in the study follow-up period
Survival Analysis Using Cox Proportional Hazards Model
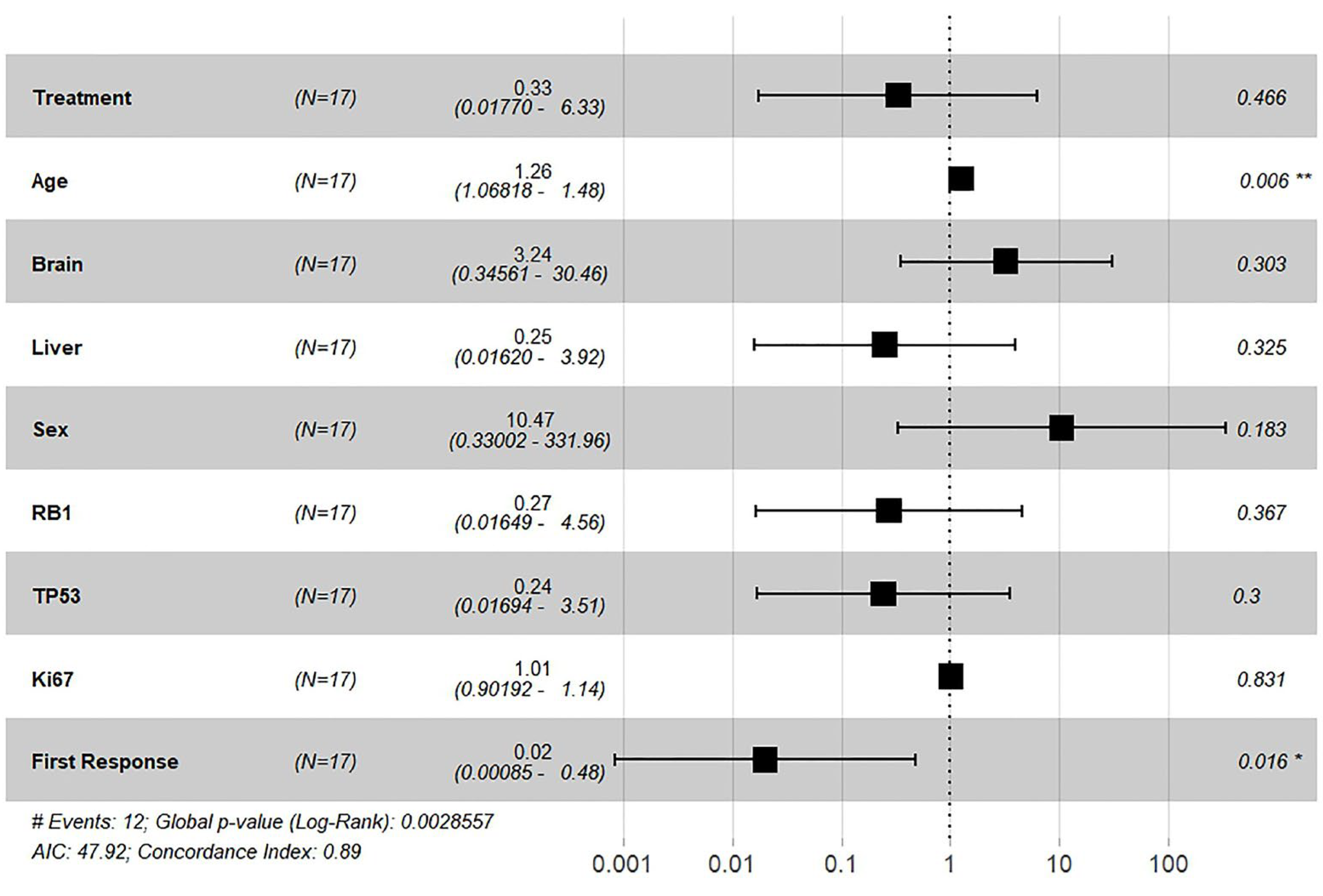
In a Cox proportional hazards regression analysis, the treatment variable showed an estimated adjusted hazard ratio (HR) of 0.33 (95% CI: 0.0177–6.33). The unadjusted HR for the treatment effect was 0.32 (95%CI, 0.10- 1.05) p = 0.06) (
Figure 5).
Further subgroup analysis was performed for baseline clinicopathological parameters using the Cox proportional hazards model. In the multivariate analysis, brain metastasis was associated with a more than threefold increase in the hazard ratio HR=3.24, p = 0.303. Similarly, liver metastasis, sex, RB1 and TP53 mutations, and Ki67 levels were not significantly associated with survival outcomes.
“First response” was defined as response or not based on the RECIST criteria after completion of four cycles of treatment and was characterized as either: 1) complete response (CR), partial response (PR), stable disease (SD) and 2) disease progression (PD). The first response to treatment was a strong predictor of better survival, with a 98% reduction in the hazard ratio (HR, 0.02; 95% confidence interval [CI], 0.00085–0.48; p = 0.016), indicating that patients who responded (CR/PR/SD) during the first 4 cycles of treatment had significantly improved survival rates. The model demonstrated good predictive ability with a concordance index of 0.89 and a global p-value of 0.002 from the log-rank test.
miR-375
Samples deemed sufficient for miR375 analysis were collected from 14 of the 17 patients included in the study. In two patients, blood was collected only before the start of treatment and not subsequently, as the patients died within the first few cycles of treatment. In the remaining 12 patients, plasma was collected before and at the time of disease progression or at the end of the study follow-up interval. The miR375 concentrations in samples collected from 13 male volunteers were used for comparison.
Comparison of mi375 baseline plasma levels between patients and healthy volunteers did not show a statistically significant difference in the median levels of miR-375 between the two groups (p = 0.16)
We then examined whether the change in plasma miR375 concentration values in patients with LCNEC was associated with disease progression. To assess the relationship between the increase in miR375 levels and disease progression, we performed Spearman’s rank correlation analysis. The analysis yielded a Spearman’s rho value of 0.52, suggesting a moderate positive correlation. However, the p-value of 0.07 suggests that this correlation is not statistically significant at the level of 0.05. Further investigation with a larger sample size or additional variables may be necessary to fully understand the potential predictive value of miR375 levels for disease progression in patients with LCNEC
4. Discussion
To the best of our knowledge, this study represents the first prospective, real-world study on the efficacy of combining atezolizumab with chemotherapy for the treatment of patients with metastatic LCNEC. Immune checkpoint inhibitors have revolutionized the treatment of various types of cancer, including metastatic SCLC, and their efficacy has been evaluated in neuroendocrine tumors with promising results [
22,
23]. However, there are limited data available on their efficacy in LCNEC, with most existing studies being retrospective in nature. Recent publications indicate that chemotherapy is the most commonly used treatment option for LCNEC, followed by surgery and radiotherapy [
24].
The LANCE trial was a prospective, non-randomized pilot study that enrolled patients with histologically confirmed metastatic lung-derived LCNEC. These patients were treated with either chemotherapy in combination with atezolizumab, or chemotherapy alone. Despite the absence of randomization, the participants’ baseline characteristics were well balanced. The results of the study showed that patients who were administered combination chemotherapy with atezolizumab demonstrated a significantly greater likelihood of achieving progression-free survival and overall survival at 12 and 24 months than those who received chemotherapy alone. Specifically, the 12-month progression-free rate for the combination therapy was 46.7%, which was significantly higher than that in the chemotherapy-only group (14.3 %). This trend continued at the 24-month follow-up. Similarly, the 12-month overall survival rate was 57.1% in the combination group versus 14.3% in the chemotherapy group, with a comparable pattern at 24 months. The hazard ratios indicated a strong trend toward better outcomes with the addition of atezolizumab, suggesting its effectiveness in improving patient survival.
By the end of the follow-up period, 40% of the patients in the ChT plus Atezo arm demonstrated a durable clinical benefit, in contrast to the control arm, where no patients survived. This difference underscores the potential of combining Chemotherapy with Atezolizumab to significantly improve patient outcomes, in terms of both PFS and OS.
No new irAEs were observed in this study. In particular, two patients (20%) in the experimental arm discontinued immunotherapy because of grade 3 immune-related AEs. One patient presented with grade 3 dermatitis and one patient presented with grade 3 pneumonitis. In both cases, the patients were treated for an extended period (approximately two months) with prednisone and treatment was restarted when the AE was downgraded to Grade 1. Those findings are in line with anticipated atezolizumab irAEs in other studies
miR-375 is associated with neuroendocrine lung neoplasms and is detected at high concentrations in these cancers [
20,
25,
26,
27]. Reduced miR-375 expression inhibits neuroendocrine cell differentiation and carcinogenesis, both in vitro and in vivo. Studies have found that miR-375 has prognostic and predictive values in LCNEC. Increased miR-375 expression has been associated with poor prognosis in LCNEC, with a median survival of 8-12 months [
28,
29,
30]. These studies assessed the predictive value of mir375 as a biomarker for chemotherapy-treated patients.
The LANCE trial evaluated plasma samples from 14 of the 17 enrolled patients to determine miR375 levels. The analysis found that the median miR375 concentrations did not significantly differ between the patient cohort with LCNEC and the healthy control group. Despite this, a moderate positive association was observed between increased miR375 levels and the progression of LCNEC, although this correlation was not statistically significant. Further large-scale studies are necessary to determine the utility of miR375 as a reliable biomarker for disease progression.
The present study had several limitations. First, the sample size was relatively small, and the fact that the study was conducted at a single center without enrolling patients with different demographic characteristics may limit the generalizability of the results. However, to our knowledge, the 17 patients with metastatic LCNEC included in this study represent one of the largest series of immunotherapy studies on this rare disease, highlighting the challenges of conducting large-scale studies on rare diseases, and emphasizing the significance of our findings. Additionally, the absence of a randomization process in this study may have influenced the results; however, there were no significant differences in the baseline or pathological characteristics of the patients or their tumors. While these limitations are acknowledged, they can be addressed in future large multicenter studies. Overall, this pilot study demonstrated prospective data supporting the significant survival benefit associated with the addition of atezolizumab immunotherapy in metastatic LCNEC patients receiving platinum-based chemotherapy.
5. Conclusions
The conclusions drawn from the LANCE trial underscore the potential of atezolizumab, in combination with chemotherapy, as a promising therapeutic avenue for patients suffering from metastatic Large Cell Neuroendocrine Carcinoma of the lung. Our study, albeit pilot in nature, reveals a notable improvement in progression-free survival and overall survival rates among patients treated with the combination therapy compared to those receiving chemotherapy alone. This indicates not only the therapeutic efficacy of atezolizumab when paired with chemotherapy but also highlights the necessity for further exploration into the integration of immunotherapy within standard treatment protocols for metastatic LCNEC.
Furthermore, the exploratory analysis regarding the predictive value of plasma miR-375 levels for disease progression, despite not reaching statistical significance, opens new avenues for research into biomarkers that could guide treatment decisions and prognosis in LCNEC. Given the rarity and complexity of this disease, the findings from the LANCE trial contribute valuable insights into the treatment landscape of metastatic LCNEC, advocating for the pursuit of larger, randomized studies to validate these preliminary outcomes and to further refine the role of miR-375 as a potential biomarker. Moving forward, it is imperative to build upon this foundational work, aiming to enhance patient outcomes through innovative and evidence-based treatment strategies.
Author Contributions
G.E.: Conceptualization, Investigation, Methodology, Software, Formal analysis, Writing - original draft, Supervision, I.T.: Writing - original draft, Resources; I.G.: Data curation, Validation, I.V.: Resources, Investigation, C.P.: Data curation, Investigation M.G.: Resources, G.G.: Resources, I.T.: Resources, I.V.: Resources, M.A.: Data curation, Resources; V.K.: Investigation; Methodology; K.S.: Conceptualization; Project administration.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Sotiria Chest Diseases Hospital of Athens (protocol ID 4101/19).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset derived from the LANCE study and comprising patient outcomes, such as overall and progression-free survival, demographic information (age and gender), Performance Status, treatment regimens, and immunohistochemical markers (TP53, RB1, Ki67%), has been deposited in
https://data.mendeley.com/datasets/64strcdfjh/1. Additionally, the dataset included pre- and post-treatment miR375 plasma levels and R scripts for data analysis.
Acknowledgments
The authors would like to express their gratitude to the medical professionals at the 3rd University Clinic and Homonymous Laboratory of the National and Kapodistrian University of Athens for their invaluable assistance and support. Additionally, we extend our sincere appreciation to the patients and their families for their cooperation and contributions to this study.
Conflicts of Interest
G.E. reports receiving support for attending meetings from ΙPSEN, REGENERON, and institutional fees from Merck and IPSEN. KS reports receiving honoraria from Bristol and Amgen, and consulting fees from AstraZeneca and MSD. The other authors have no conflicts of interest to declare.
References
- Deng, C., S.G. Wu, and Y. Tian, Lung Large Cell Neuroendocrine Carcinoma: An Analysis of Patients from the Surveillance, Epidemiology, and End-Results (SEER) Database. Med Sci Monit, 2019. 25: p. 3636-3646. [CrossRef]
- Travis, W.D., E. Brambilla, A.G. Nicholson, Y. Yatabe, J.H.M. Austin, M.B. Beasley, L.R. Chirieac, S. Dacic, E. Duhig, D.B. Flieder, et al., The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol, 2015. 10(9): p. 1243-1260.
- Strosberg, J., G. El-Haddad, E. Wolin, A. Hendifar, J. Yao, B. Chasen, E. Mittra, P.L. Kunz, M.H. Kulke, H. Jacene, et al., Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine, 2017. 376(2): p. 125-135. [CrossRef]
- Paz-Ares, L., M. Dvorkin, Y. Chen, N. Reinmuth, K. Hotta, D. Trukhin, G. Statsenko, M.J. Hochmair, M. Özgüroğlu, J.H. Ji, et al., Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet, 2019. 394(10212): p. 1929-1939.
- Yao, J.C., N. Fazio, S. Singh, R. Buzzoni, C. Carnaghi, E. Wolin, J. Tomasek, M. Raderer, H. Lahner, M. Voi, et al., Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. The Lancet, 2016. 387(10022): p. 968-977. [CrossRef]
- Horn, L., A.S. Mansfield, A. Szczęsna, L. Havel, M. Krzakowski, M.J. Hochmair, F. Huemer, G. Losonczy, M.L. Johnson, M. Nishio, et al., First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New England Journal of Medicine, 2018. 379(23): p. 2220-2229. [CrossRef]
- Caplin, M.E., M. Pavel, J.B. Ćwikła, A.T. Phan, M. Raderer, E. Sedláčková, G. Cadiot, E.M. Wolin, J. Capdevila, L. Wall, et al., Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. New England Journal of Medicine, 2014. 371(3): p. 224-233. [CrossRef]
- Atieh, T. and C.H. Huang, Treatment of Advanced-Stage Large Cell Neuroendocrine Cancer (LCNEC) of the Lung: A Tale of Two Diseases. Frontiers in Oncology, 2021. 11. [CrossRef]
- Corbett, V., S. Arnold, L. Anthony, and A. Chauhan, Management of Large Cell Neuroendocrine Carcinoma. Front Oncol, 2021. 11: p. 653162. [CrossRef]
- Ferrara, M.G., A. Stefani, M. Simbolo, S. Pilotto, M. Martini, F. Lococo, E. Vita, M. Chiappetta, A. Cancellieri, E. D’Argento, et al., Large Cell Neuro-Endocrine Carcinoma of the Lung: Current Treatment Options and Potential Future Opportunities. Frontiers in Oncology, 2021. 11. [CrossRef]
- Le Treut, J., M.C. Sault, H. Lena, P.J. Souquet, A. Vergnenegre, H. Le Caer, H. Berard, S. Boffa, I. Monnet, D. Damotte, et al., Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol, 2013. 24(6): p. 1548-52. [CrossRef]
- Patel, S.P., M. Othus, Y.K. Chae, F.J. Giles, D.E. Hansel, P.P. Singh, A. Fontaine, M.H. Shah, A. Kasi, T.A. Baghdadi, et al., A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res, 2020. 26(10): p. 2290-2296. [CrossRef]
- Dudnik, E., S. Kareff, M. Moskovitz, C. Kim, S.V. Liu, A. Lobachov, T. Gottfried, D. Urban, A. Zer, O. Rotem, et al., Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung. Journal for ImmunoTherapy of Cancer, 2021. 9(2): p. e001999. [CrossRef]
- Komiya, T. and E. Powell, Role of immunotherapy in stage IV large cell neuroendocrine carcinoma of the lung. Journal of Clinical Oncology, 2020. 38(15_suppl): p. 9060-9060. [CrossRef]
- Song, L., F. Zhou, T. Xu, L. Zeng, Q. Xia, Z. Wang, L. Deng, Y. Li, H. Qin, H. Yan, et al., Clinical activity of pembrolizumab with or without chemotherapy in advanced pulmonary large-cell and large-cell neuroendocrine carcinomas: a multicenter retrospective cohort study. BMC Cancer, 2023. 23(1): p. 443. [CrossRef]
- Agar, C., M. Geier, G. Léveiller, R. Lamy, J.-L. Bizec, M. Tiercin, C. Bernier, G. Robinet, H. Léna, C. Ricordel, et al., Brief Report on the Efficacy of Nivolumab in Patients With Previously Treated Advanced Large-Cell Neuroendocrine Cancer of the Lung. JTO Clinical and Research Reports, 2021. 2(4). [CrossRef]
- Kim, C., J.E. McGrath, J. Xiu, M. Nagasaka, P.C. Ma, J.J. Nieva, G. Lopes, H. Borghaei, C. Ikpeazu, T.K. Owonikoko, et al., Genomic and immunologic characterization of large-cell neuroendocrine carcinoma of the lung. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 8535-8535. [CrossRef]
- Rekhtman, N., M.C. Pietanza, M.D. Hellmann, J. Naidoo, A. Arora, H. Won, D.F. Halpenny, H. Wang, S.K. Tian, A.M. Litvak, et al., Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res, 2016. 22(14): p. 3618-29. [CrossRef]
- Wong, J.J.M., P.S. Ginter, K. Tyryshkin, X. Yang, J. Nanayakkara, Z. Zhou, T. Tuschl, Y.T. Chen, and N. Renwick, Classifying Lung Neuroendocrine Neoplasms through MicroRNA Sequence Data Mining. Cancers (Basel), 2020. 12(9). [CrossRef]
- Yang, X., J. Nanayakkara, D. Claypool, S. Saghafinia, J.J.M. Wong, M. Xu, X. Wang, C.J.B. Nicol, I.P. Michael, M. Hafner, et al., A miR-375/YAP axis regulates neuroendocrine differentiation and tumorigenesis in lung carcinoid cells. Scientific Reports, 2021. 11(1): p. 10455.
- Haanen, J., M. Obeid, L. Spain, F. Carbonnel, Y. Wang, C. Robert, A.R. Lyon, W. Wick, M. Kostine, S. Peters, et al., Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology, 2022. 33(12): p. 1217-1238. [CrossRef]
- Halperin, D.M., S. Liu, A. Dasari, D. Fogelman, P. Bhosale, A. Mahvash, J.S. Estrella, L. Rubin, A.C. Morani, M. Knafl, et al., Assessment of Clinical Response Following Atezolizumab and Bevacizumab Treatment in Patients With Neuroendocrine Tumors: A Nonrandomized Clinical Trial. JAMA Oncology, 2022. 8(6): p. 904-909.
- Owen, D.H., B. Benner, L. Wei, V. Sukrithan, A. Goyal, Y. Zhou, C. Pilcher, S.A. Suffren, G. Christenson, N. Curtis, et al., A Phase II Clinical Trial of Nivolumab and Temozolomide for Neuroendocrine Neoplasms. Clin Cancer Res, 2023. 29(4): p. 731-741. [CrossRef]
- Khan, J., A.Q.K. Yasinzai, S. Matosz, M. Khan, S. Heneidi, H. Mesa, A. Chauhan, J. Del Rivero, N.A. Karim, and A. Ullah, Pulmonary large cell neuroendocrine carcinoma (LCNEC): a population-based study addressing recent molecular-genetic advances and emerging therapeutic approaches. Clin Exp Med, 2023. [CrossRef]
- Korotaeva, A., D. Mansorunov, N. Apanovich, A. Kuzevanova, and A. Karpukhin, MiRNA Expression in Neuroendocrine Neoplasms of Frequent Localizations. Noncoding RNA, 2021. 7(3). [CrossRef]
- Detassis, S., V. Del Vescovo, M. Grasso, S. Masella, C. Cantaloni, L. Cima, A. Cavazza, P. Graziano, G. Rossi, M. Barbareschi, et al., miR375-3p Distinguishes Low-Grade Neuroendocrine From Non-neuroendocrine Lung Tumors in FFPE Samples. Front Mol Biosci, 2020. 7: p. 86. [CrossRef]
- Nanayakkara, J., K. Tyryshkin, X. Yang, J.J.M. Wong, K. Vanderbeck, P.S. Ginter, T. Scognamiglio, Y.-T. Chen, N. Panarelli, N.-K. Cheung, et al., Characterizing and classifying neuroendocrine neoplasms through microRNA sequencing and data mining. NAR Cancer, 2020. 2(3). [CrossRef]
- Zatelli, M.C., E.M. Grossrubatscher, E. Guadagno, C. Sciammarella, A. Faggiano, and A. Colao, Circulating tumor cells and miRNAs as prognostic markers in neuroendocrine neoplasms. Endocr Relat Cancer, 2017. 24(6): p. R223-r237. [CrossRef]
- Roesel, C., S. Welter, K.-O. Kambartel, G. Weinreich, T. Krbek, M. Serke, M. Ibrahim, Y. Alnajdawi, T. Plönes, and C. Aigner, Prognostic markers in resected large cell neuroendocrine carcinoma: a multicentre retrospective analysis. Journal of Thoracic Disease, 2020. 12(3): p. 466-476. [CrossRef]
- Szczyrek, M., P. Bitkowska, M. Jutrzenka, and J. Milanowski, The Role of the Selected miRNAs as Diagnostic, Predictive and Prognostic Markers in Non-Small-Cell Lung Cancer. Journal of Personalized Medicine, 2022. 12(8): p. 1227. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).