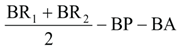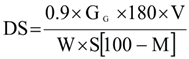1. Introduction
The development of latest and handy food products and the use of new technology have improved in current years (1). People recognition of the connection between a nutritious food regimen and good health is one of the important reasons for the popularity of functional foods that provide health advantages and lessen the chance of persistent sicknesses further to fundamental vitamins. Resistant starch (RS) that is identified as a type of dietary fibre is one of the most broadly used ingredients in functional foods. It has long been mentioned having a superb function among fibers for its several nutritional features that provide a couple of health benefits. Its consumption lowers the blood glucose levels after a meal, reduces cholesterol and triglyceride levels in blood, improve body's response to insulin and even reduce fat storage (2). Animal studies have shown that resistant starch have positive effect on gut health by helping to create a substance called butyrate. This can change the composition and balance of microorganisms in gut, which is the main reason of resistant starch becoming more popular as a food ingredient (3).
Resistant starch was discovered in 1980s as a component that resists to digestion by enzymes (4), and it has been defined as ’the sum of starch and products of starch degradation no longer absorbed in the small intestine of healthy individuals’ (5). Foods contain five main types of resistant starch. Resistant starch 1 (RS1) is a type of starch that the body cannot digest because of presence of whole, undamaged cell walls on grains, tubers, and seeds. It is used as an ingredient in a vast range of traditional foods due to its stability to heat during normal cooking processes (6). Because of their crystallinity, RS2 are natural, uncooked starch granules that are not greatly affected by hydrolysis. RS2 is found in raw starch granules like banana and potato and high amylose corn starches. RS3 is retrograded starches that were created during cooking and stored at room temperature or below (7). RS4 starches include cross-linked and starch ethers, which are chemically modified to develop resistance against enzymatic digestion (8). Amylose-lipid complexes, which occur when amylose and lipid come into contact, are where RS5 is created. Amylose-lipid complexes are created when lipids perforate the amylose chains found in numerous plant sources with high amylose concentration (9).
The mechanism that causes the starch chains to recrystallize after the gelatinized paste cools is known as retrogradation. The extent and rate of retrogradation of starch are determined by its features, such as its crystalline or molecular structure, and storage circumstances, such as duration, temperature, and water content (10).
The delayed digestion of resistant starch, which takes more than five to seven hours, results in a reduction in insulinemia and postprandial levels and may lengthen the duration of satiety (11). Its physiological actions are primarily responsible for its remarkable nutritional activity when compared to dietary fibre. When subclasses of fermentable dietary fibre sources are regularly consumed, there is a large synthesis of short chain fatty acids (SCFAs) and gut-related microbiota and immunological regulation occured(9). Resistant starch avoids the small intestine's digestive process and passes straight into the large intestine, where it ferments into short-chain fatty acids like acetate, propionate, and butyrate as well as gases like H2, CO2, and CH4 that have been produced by the large intestine's probiotic bacteria (12). The SCFA are absorbed via intestinal wall and transported into liver by portal circulation where mainly butyrate gets utilised by colonocytes (13). The SCFAs protects human body from metabolic disorders like obesity by providing energy to our brain muscles, and heart. It also increases the production of bile acids, mineral absorption and leptin production (14). Consuming resistant starch promotes the growth of beneficial bacteria like Lactobacillus, Bacteroides, and Bifidobacteria while inhibiting the growth of harmful bacteria like Firmicutes (3). Several methods have been described by RS to lower cholesterol levels, including increased excretion of bile in the faeces and a reduction in the influence of propionic acid on cholesterol production. In rats, SCFSs also lessen the synthesis of cholesterol in the gut and liver (15). Hence, It is suggested that at least 20g of resistant starch per day should be consumed to get various health benefits from it. In developing countries about 30 to 40 g/d of resistant starch is recommended (16) while in India and China RS consumption of about 10 and 18 g/d, respectively,is recommended indicating that , about 10 to 20% of daily carbohydrates consumption in the form of resistant starch is necessary to get health benefits from it (17).
Cooking and grinding of food products for longer period of time reduces its resistant starch content. During grinding, resistant starch content of rice and oats is reduced 12 to 5% and 16 to 3% respectively. Resistant starch formation in foods is also affected by water content, pH, heating processes (time, temperature, etc.), preservation methods (freezing, drying, storage, etc.), cooking methods, and the presence of components such as proteins, lipids, minerals, and inhibitors (6). Resistant starch content has been found to be impacted by both dry heating techniques including baking, frying, microwaving, and autoclaving, as well as wet cooking techniques like pressure cooking, steaming, and boiling. High baking temperature and time has been reported to increase the resistant starch content (18).
Considering the health benefits of resistant starch, the current study was designed to determine the manner in which cooking and storage temperatures affected the resistant starch in Basmati rice products and how that affected lipid profile and blood sugar levels. Dietary interventions using resistant starch may improve glucose metabolism and insulin sensitivity. Thus, products made from basmati rice were therefore selected because they represent an essential part of the diet of people from North India. We additionally examined at how well the resistant starch in basmati rice products worked in vivo to enhance lipid profiles and blood glucose levels in rats and humans.
2. Material and Methods
2.1. Procurement and Cooking
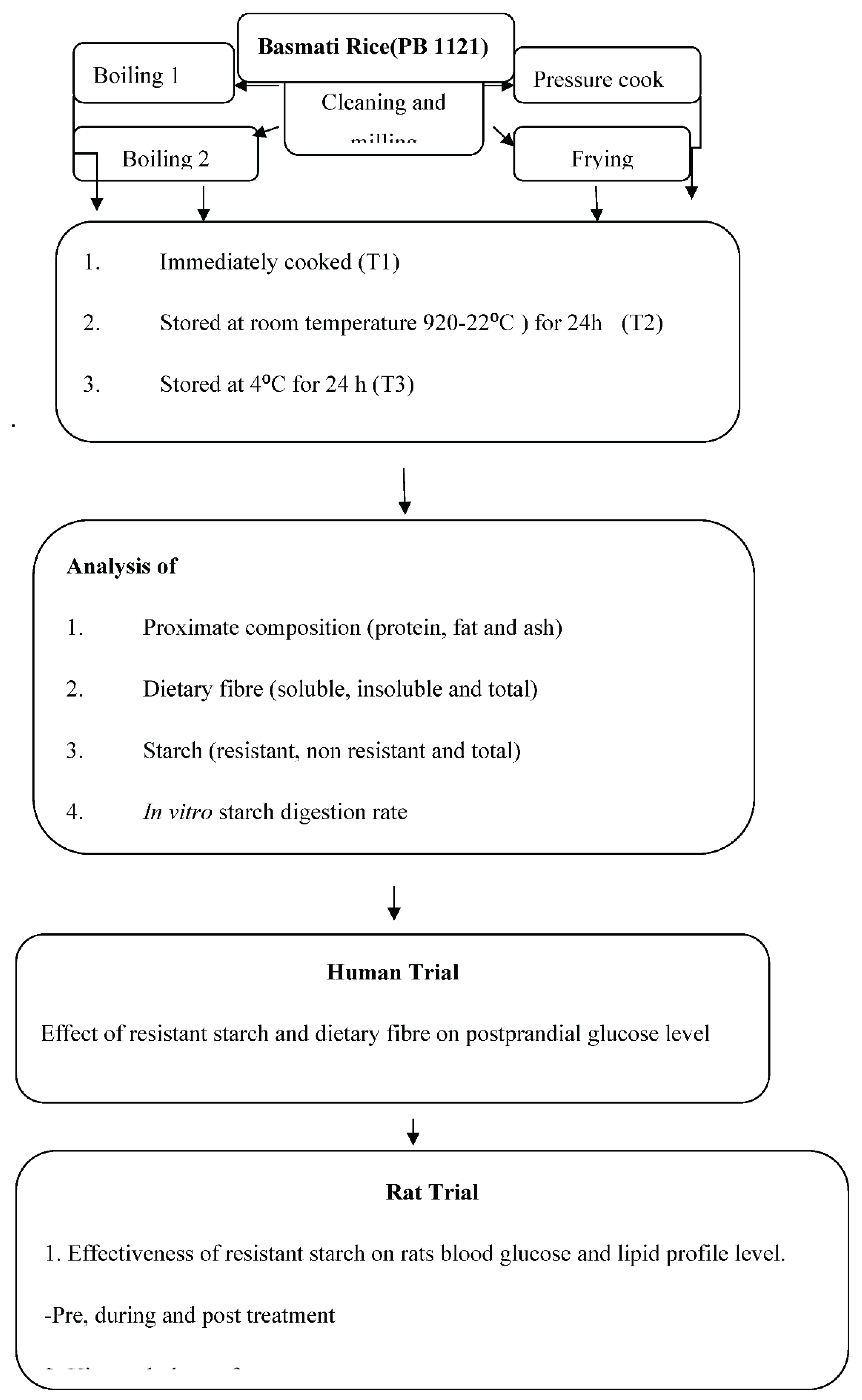
The most common form of Basmati rice (PB1121) was obtained by Punjab Agricultural University in Ludhiana's Department of Plant Breeding and Genetics. Because this variety has grown in large amounts in the fields of Punjab. The grains were cleaned and milled to remove the husk. Four common cooking methods namely Boiling 1 (Boiling by absorption), Boiling 2 (Boiled in extra amount of water), Frying (Fried rice) and Pressure cooking used by North Indians were chosen (
Table 1). These four food products were examined under four distinct storage conditions, or four treatments: they were prepared freshly (T1), kept at room temperature (20–22°C and 45–50% RH) for 24 hours (T2), stored at 4°C for 24 hours (T3), and then reheated after being kept at 4°C for 24 hours (T4) (
Figure 1). Every rice product was utilised three times during the trial. The samples were dried and used for nutritional analysis after the treatments.
2.2. Nutritional Analysis
Using standardised techniques, the nutritional analyses of cooked samples were performed for crude protein, crude fat, and ash. Crude protein was measured using the macro-Kjeldahl technique. The (AOAC 2000) method was also used to measure the amount of crude fat and ash. (19)
2.2.1. Dietary Fiber
A megazyme total dietary fibre (K-TDFR-200A) kit was used to calculate the total amount of dietary fibre.The standard methodology provided by (19) was also utilised to analyse the contents of soluble and insoluble dietary fibre.The following formula was used to determine the dietary fibre:
R1 = residue weight 1 from m1, R2 = residue weight 2 from m2, m1 = sample weight 1,
m2 = sample weight 2, A = ash weight from R1,
p = protein weight from R
2 and
where:
BR = blank residue,
BP = blank protein from BR1, BA = blank ash from BR2.
2.2.2. Total Starch and Resistant Starch
Using a megazyme K-RSTAR assay kit provided by (20), the total and resistant starches were calculated. To find the total amount of starch, non-resistant (solubilized) starch and resistant starch were added.
2.2.3. In Vitro Starch Digestion Rate
The method described by (21), for determining the In vitro starch digestion rate, was used. 500 mg of the sample were incubated with 250 U porcine amylase in 1 ml of artificial saliva (carbonate buffer; Sigma A-3176 Type VI-B) for 15 to 20 seconds. A water bath at 37°C was used to incubate 5 ml of pepsin (1 ml per ml of 0.02 M aq. HCl; from gastric porcine mucosa; Sigma P-6887) for 30 minutes. Before correcting the pH to 6, the digesta was neutralised by adding 5 mL of 0.02 M aq. sodium hydroxide. (0.2 M C2H3NaO2 buffer, 25 mL) Two additional ingredients were added: 2 mg of pancreatin per ml of acetate buffer (Sigma P1750 from porcine pancreas) and 5 ml of amyloglucosidase (Sigma A-7420 from Aspergillus niger; 28 U per mL of acetate buffer). The solution was then incubated for 4 hours, during which the digesta's glucose concentration was periodically checked using an Accucheck glucometer.
2.2.4. Rapidly Digestible Starch and Slowly Digestible Starch
The following formula was used to translate the glucometer reading at 15 minutes to the percentage of starch digested.
Where:
GG = Reading of the glucometer (mM/L)
V = Digest volume (mL),
180 = glucose’s molecular weight W = sample weight (g)
S = sample’s starch content (g per 100 g dry sample)
M = moisture percentage in sample (g per 100 g sample)
0.9 = starch stoichiometric constant from glucose concentrations.
RDS% = percentage of starch digested at 15 min
SDS% = percentage of starch digested at 120 min – percentage of starch digested at 15 min.
2.3. Impact of Resistant Starch and Soluble Fibre Components on Postprandial Glucose Response by Measuring Glycemic Index
The formula for the Glycemic Index was computed by applying Goni's approach (22). Blood glucose levels were measured in ten healthy participants. In accordance with the Helsinki Declarations, the participants gave their explicit written consent. The participants were instructed to maintain a 12-hour fast over night. The test food was served in the morning, and participants had fifteen minutes to eat their meals. A finger prick was used to obtain blood samples using a Glucometer (Dr. Morphine). The blood glucose level was assessed at 0, 15, 30, 45, 60, 90, and 120 minutes following the ingestion of 50 g of cooked cereal product, which is equivalent to a fast. 50g of glucose were given to the control group in order to compare how cooked food affected their blood sugar levels. 150–300 ml of water could be drank by volunteers, depending on what they ate throughout the study. The following formula was used to determine the glycemic index:
Glycaemic load was calculated as:
2.4. Effectiveness of Resistant Starch on Blood Glucose Level and Lipid Profile in Rats
It was hypothesised that consuming foods high in RS might benefit the treatment of diabetes and hyperlipidemia, either by increasing insulin secretion or decreasing its sensitivity, or by lowering the synthesis of cholesterol. We performed a rat experiment to gather accurate, true, and unbiased data on this. Furthermore, rats and humans share a great deal of similarities in terms of biology and genetics, as well as behavioural traits.
2.4.1. Animal Collection
35 Wistar albino rats weighing between 180 and 220 g and 2 to 3 months old were acquired from the Akal College of Pharmacy and Technical Education Mastuana sahib, Sangrur (Registered breeder of CCSEA) animal house and breeding centre (AHBC). The Institutional Animal Ethics Committee granted authorization for the experiment to be carried out (IAEC no.: GADVASU/2023/1AEC/68/12). The animals were kept in cages, given commercial pellets to eat, and had unlimited access to water.
2.4.2. Induction of Diabetes
Intraperitoneal injections of freshly produced 230 mg/kg nicotinamide (NA) with buffer saline NaCl 0.9% were administered to the wistar albino rats. The rats were again given an intraperitoneal injection of approximately 60 mg/kg of streptozotocin (STZ) after a quarter of an hour. After injection, rats were given 5% glucose water to avoid hypoglycemia. Their blood samples were obtained five days into the induction process, and the levels of lipids, insulin, and blood glucose were assessed. More than 200 mg/kg of blood glucose was a sign of diabetes in the rats. Blood glucose levels were monitored during the first, third, and last weeks of the 28-day treatment period for the rats. The first and concluding weeks of the trial were devoted to measuring the lipid profile and insulin.
2.4.3. Treatment Protocol
1. Group-I: (Normal control) Normal rats were fed a normal diet for a period of 28 days.
2. Group-II: (Diabetic control) was fed a regular diet for 28 days following the onset of diabetes.
3. Group-III Diabetic rats were given FBR (Freshly prepared boiled basmati rice) diet for 28 days.
4. Group-IV (Treatment group) Diabetic rats were given 4BR (Stored at 4⁰C for 24 h boiled basmati rice) for 28 days.
5. Group-V (Treatment group) Diabetic rats were given RehBR (Reheated basmati rice after being stored at 4⁰C for 24h) for 28 days.
Only rice prepared by boiling 1 method were given to the rats as limited numbers of rats were available to us. Moreover, boiling 1 cooking method is the common way of cooking of rice in north Indian population and rice prepared by this method was found to have high amount of resistant starch content and lower glycmic index than any other cooking method. The rice that was kept at room temperature for the entire day (T2) was also removed from the treatment since the product developed microorganisms as a result of the storage.
2.5. Histopathological Study of Rat Organs
2.5.1. Preparation of Samples
After the termination of the experiments, the pancreas and livers were examined for histopathological studies. These organs were cut into thin-sectioned pieces (1 mm × 1 mm × 1 mm). Samples of tissue were gathered and preserved with 10% formalin. After that, the samples were placed in alcohol at successive concentrations—70%, 80%, 95%, and pure alcohol—in order to extract the water from the tissue. Subsequently, the material was purified using xylol and then embedded in block paraffin. Next, a microtome was used to cut the paraffin blocks into 5μm thick sections in order to prepare the tissue for sectioning. The tissue portion was then left on the hot plate set at 50°C for 15 minutes (23).
2.5.2. Hematoxylin-Eosin (HE) Staining
Hematoxylin-Eosin staining was used in a number of procedures. The tissue sample was first deparaffinized by dipping it into xylol I, xylol II, and xylol III for three minutes each. Subsequently, the tissue samples underwent rehydration with the addition of ethanol concentrations in increments of 100%, 95%, 80%, and 70% for a duration of two minutes each. After soaking the samples in Harri's Hematoxylin for ten minutes, they were washed for ten minutes with tap water. Additionally, the samples were submerged in eosin for ten minutes before being serially dehydrated with ethanol concentrations ranging from 70% to 100%. Xylol I, II, and III were used to hold the samples during the cleaning procedure. Following the colouring procedure, Canada balsam glue was dripped, covered in a glass cover, and allowed to dry. All organs were then carefully investigated under a microscope (SZX16 Olympus) using 600 ×magnification. (23,24).
2.6. Statistical Analysis
Data was analysed using descriptive statistics, analysis of variance and post hoc tests with SAS software. Factorial completely randomised design was used to examine the quantity and effect of cooking and storage temperature on proximate composition, total starch and dietary fibre content of cereal products. One way analysis of variance was used to compare the average of glycemic index, glycemic load and pre, during and post treatments in rats blood glucose levels. t- test analysis was performed to examine average effect of resistant starch rich products on insulin and lipid profile of rats.
3. Results
3.1. Proximate Composition
It was discovered that Basmati rice had the highest crude protein level (9.54%) which was prepared using Boiling 1 method followed by Frying (fried rice,8.28 %), Boiling 2 (8.22%) and pressure cooking (7.49%) (
Table 2). The protein content of the products held at different temperatures showed a significant variation (≤0.001*), with T3 having the greatest content (9.47%), followed by T2 (8.32%), T1 (8.03%), and T4 (7.39%). Compared to protein, the crude fat content of fried rice (10.86%) and boiled 1 (2.18%) was found to be higher than that of the other cooked products due to the additional oil used during the frying process. Out of all the storage temperatures, T3 had the greatest crude fat content (4.03%), followed by T2 (4%), T4 (3.92%), and T1 (3.78%). The ash content of basmati rice was found highest in boiling 1 (1.86%), followed by frying (1.85 %), boiling 2 (1.75%) and pressure cooking (1.46%). There was no noticeable difference in the ash concentration (≤0.001*) between the rice products stored at various temperatures.
3.2. Dietary Fibre
The soluble dietary fibre content of basmati rice was found higher in pressure cooked rice (0.92%), followed by boiling 2 (0.89%), boiling 1 (0.87%) and frying (0.78%). Among storage conditions it was found higher in freshly prepared i.e T1 (1.08%) and lesser in T3 (0.61%). The insoluble dietary fibre was highest in boiling 1 (2.80%) followed by boiling 2 (2.47%), pressure cooking (T4) and frying (2.25%). It was found to be higher in T3 (2.88%) and lower in T1 (2.14%) among the storage temperatures. The amount of dietary fibre in the prepared food products was impacted by temperature changes during storage. The amount of insoluble and total dietary fibre in basmati rice increased as the length for time and when stored at low temperatures. The highest amount of dietary fibre was found in products made by boiling 1 (3.67%) and kept at 4ᵒC (T3) (3.49%) for 24 hours. The highest amount of soluble fibre was found in fresh food samples (T1) (
Table 3).
3.3. Resistant Starch, Non Resistant Starch and Total Starch
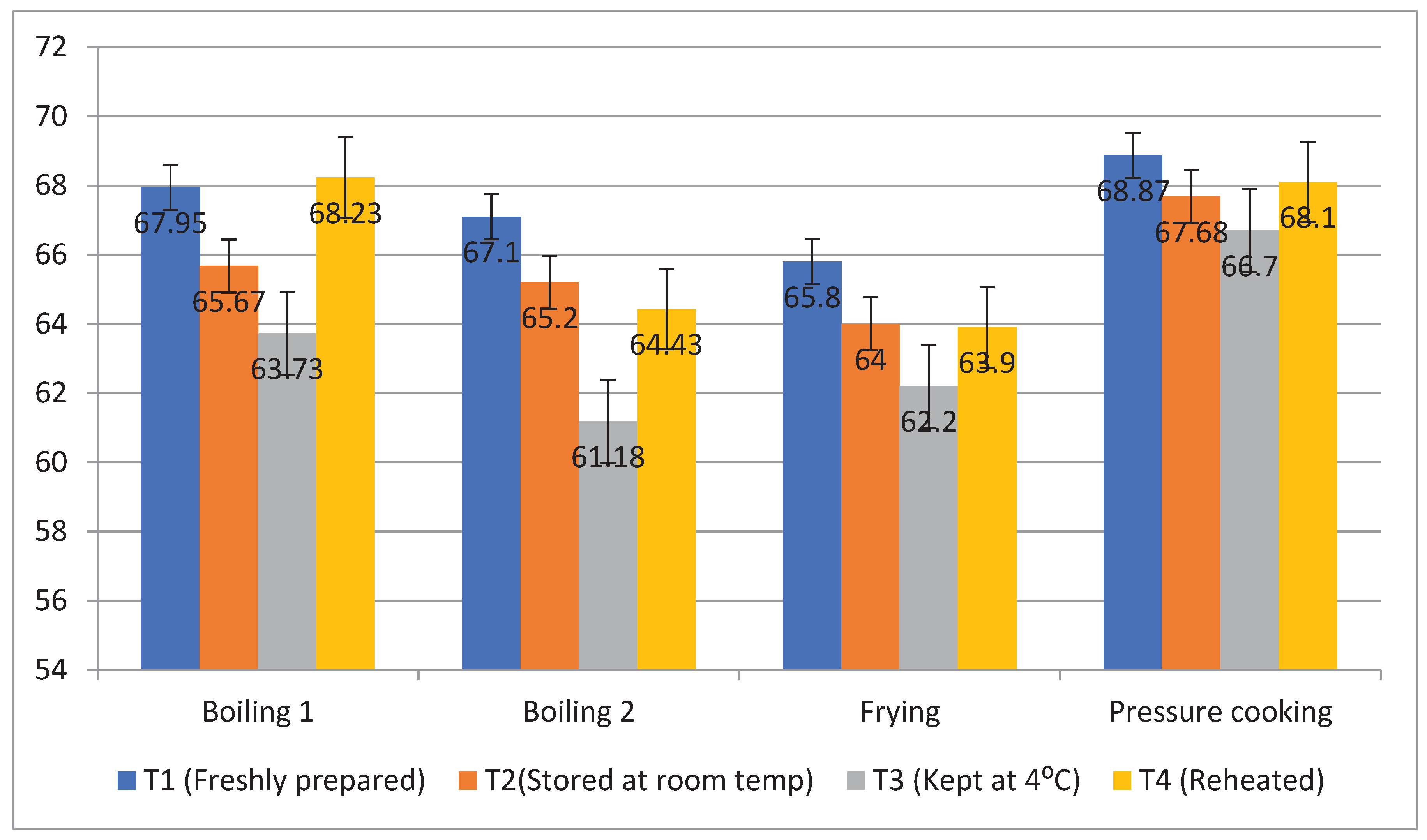
Rice is considered as a starchy food product. The total starch content of rice products was found to range from 73.2 to 79.2%, with boiling 1 having the greatest level of total starch and boiling 2 having the lowest level (
Figure 2). The RS content of raw basmati rice was found to be which increased/ decreased after applying different cooking methods. After all cooking methods, the resistant starch percentage of raw basmati rice increased to 2.27%. The resistant starch content of basmati rice was found highest after boiling 1 method (12.81%) followed by frying (11.67%), boiling 2 (8.72%) and pressure cooking (7.57%). The RS content of all the rice products decreased during storing at different temperatures. Products that were held at T3 had the highest level of resistant starch content (11.76%), followed by T2 (10.53%) and T4 (9.64%). Conversely, freshly made products T1 (8.84%) showed the lowest amount of RS after cooking for 15 minutes at 100°C. However, the non-resistant starch content of basmati rice was found to be higher during T1 (67.42%) compared to T3 (63.45%) and higher during pressure cooking (67.83%) and boiling 1 (66.39%), respectively. The results showed that the cooking technique and the storage temperature greatly influences the structure of starch leading to change in its physical and nutritional characteristics.
3.4. In Vitro Starch Digestion Rate
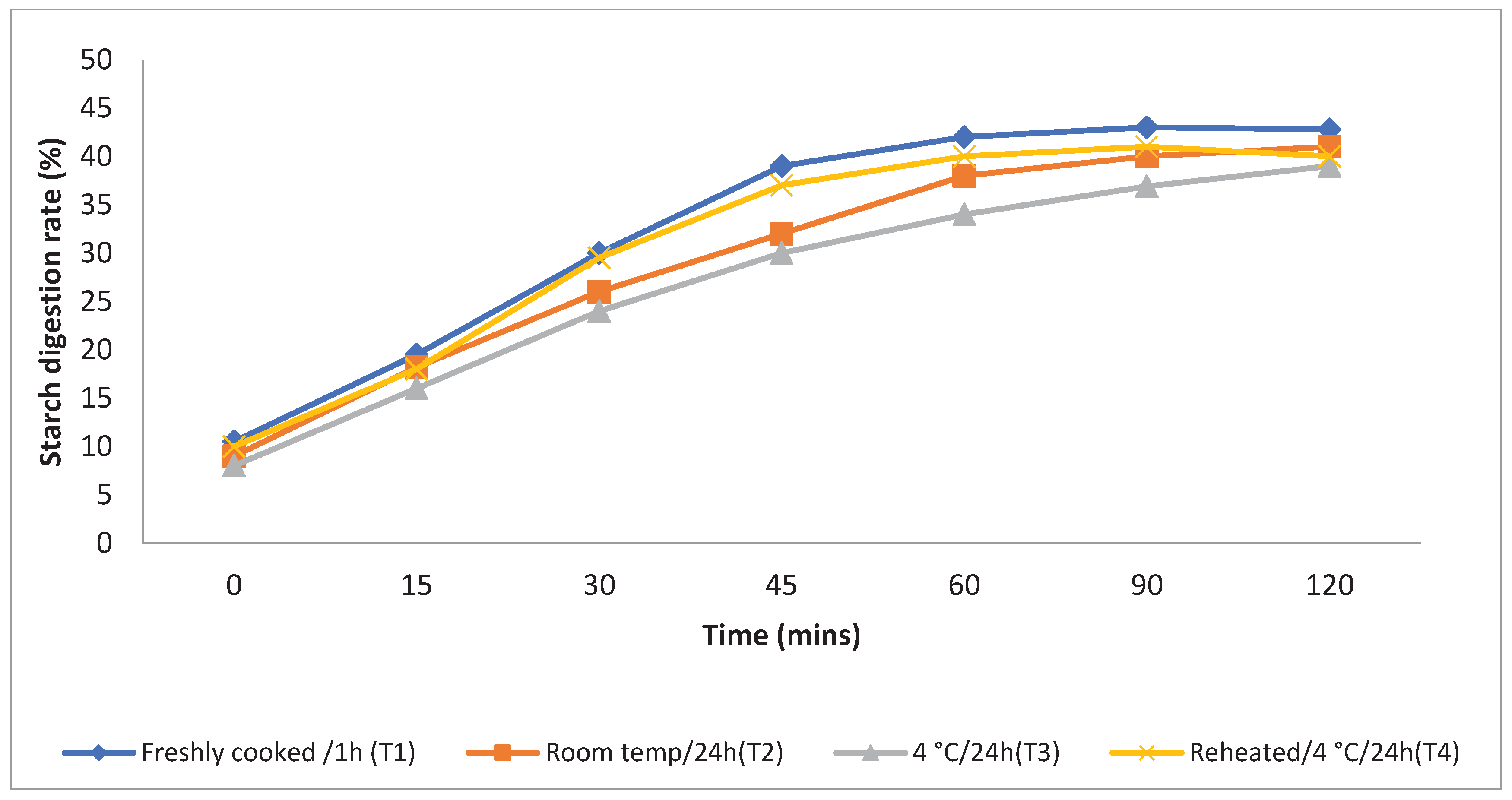
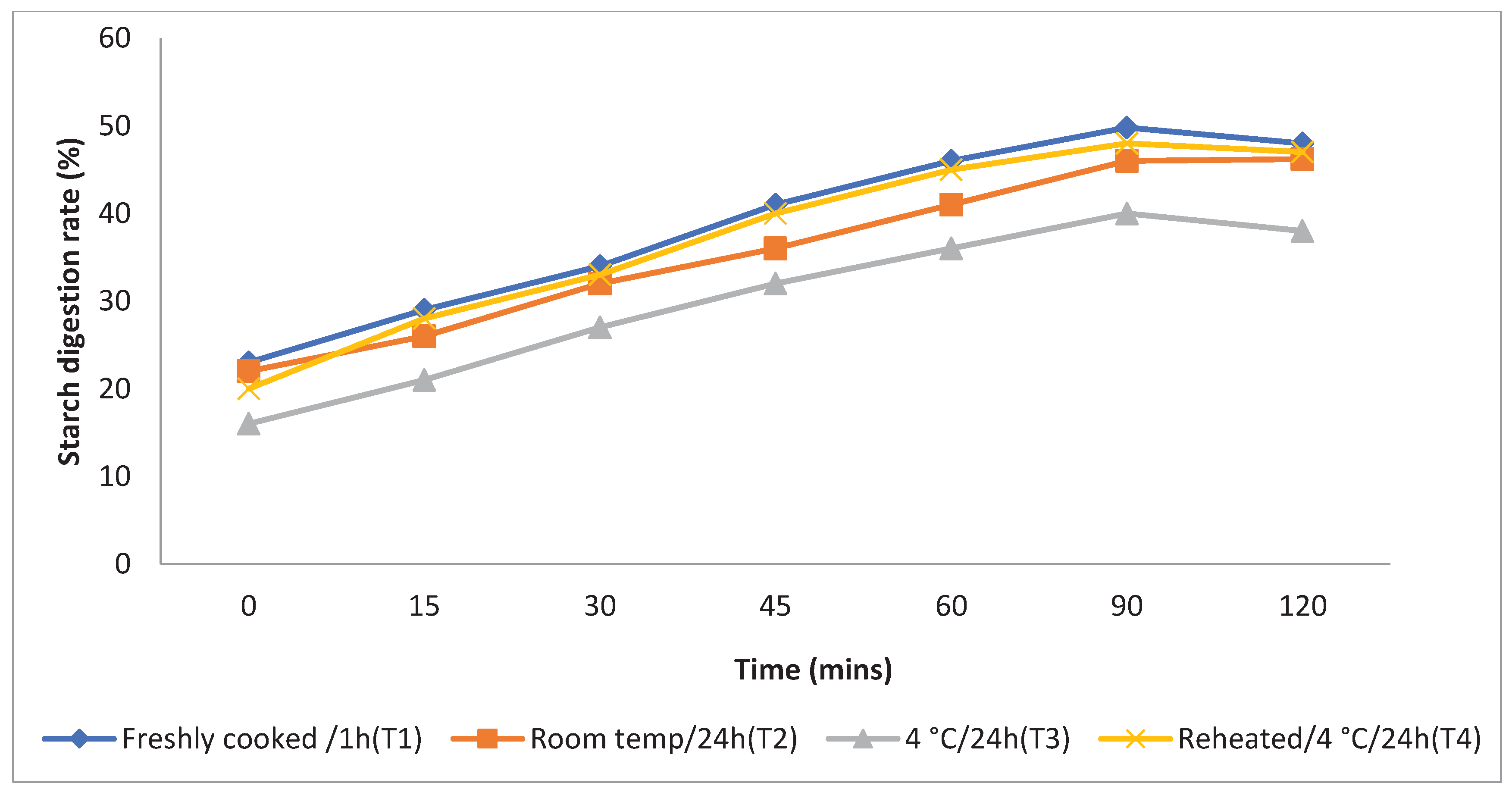
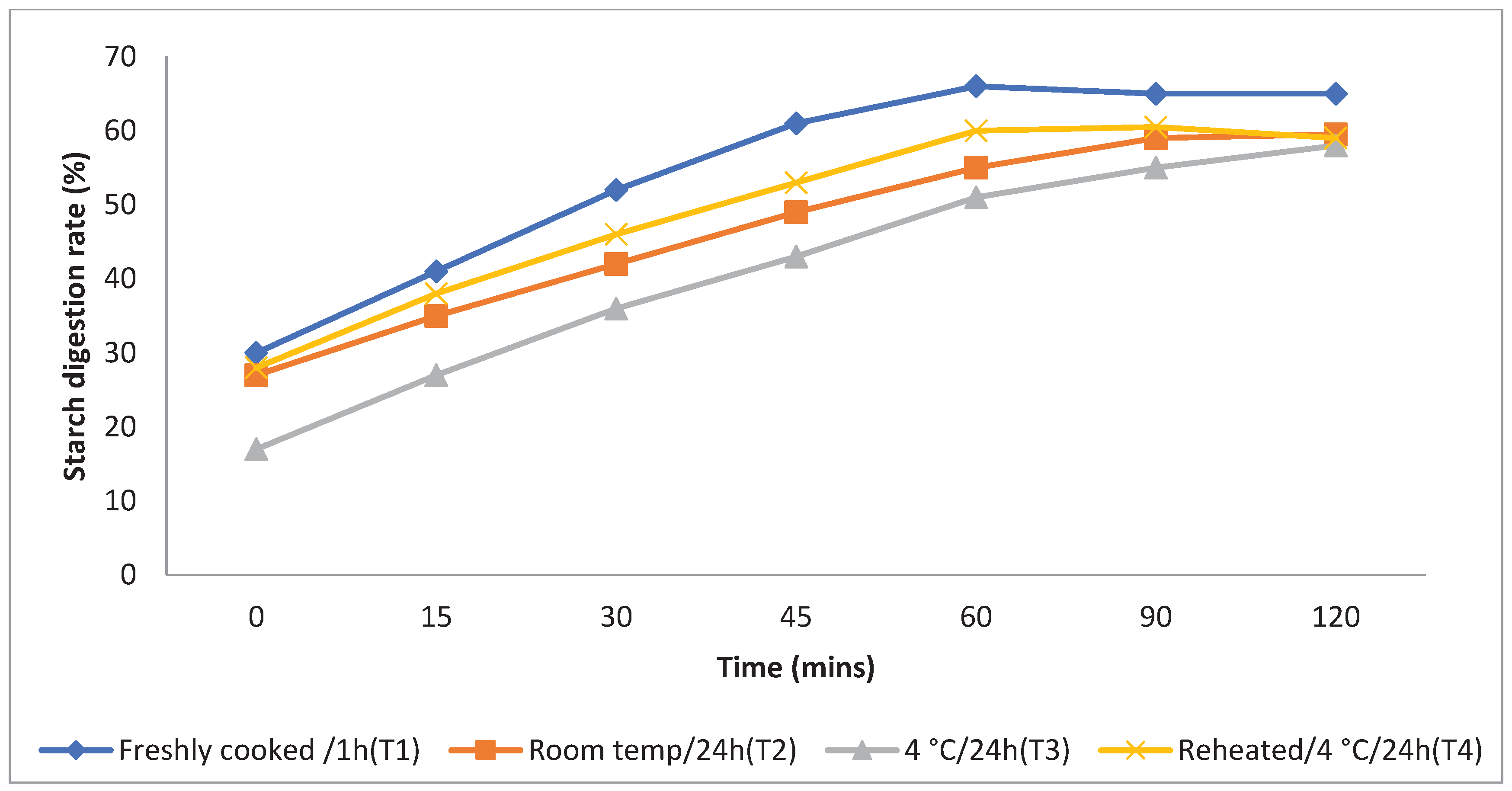
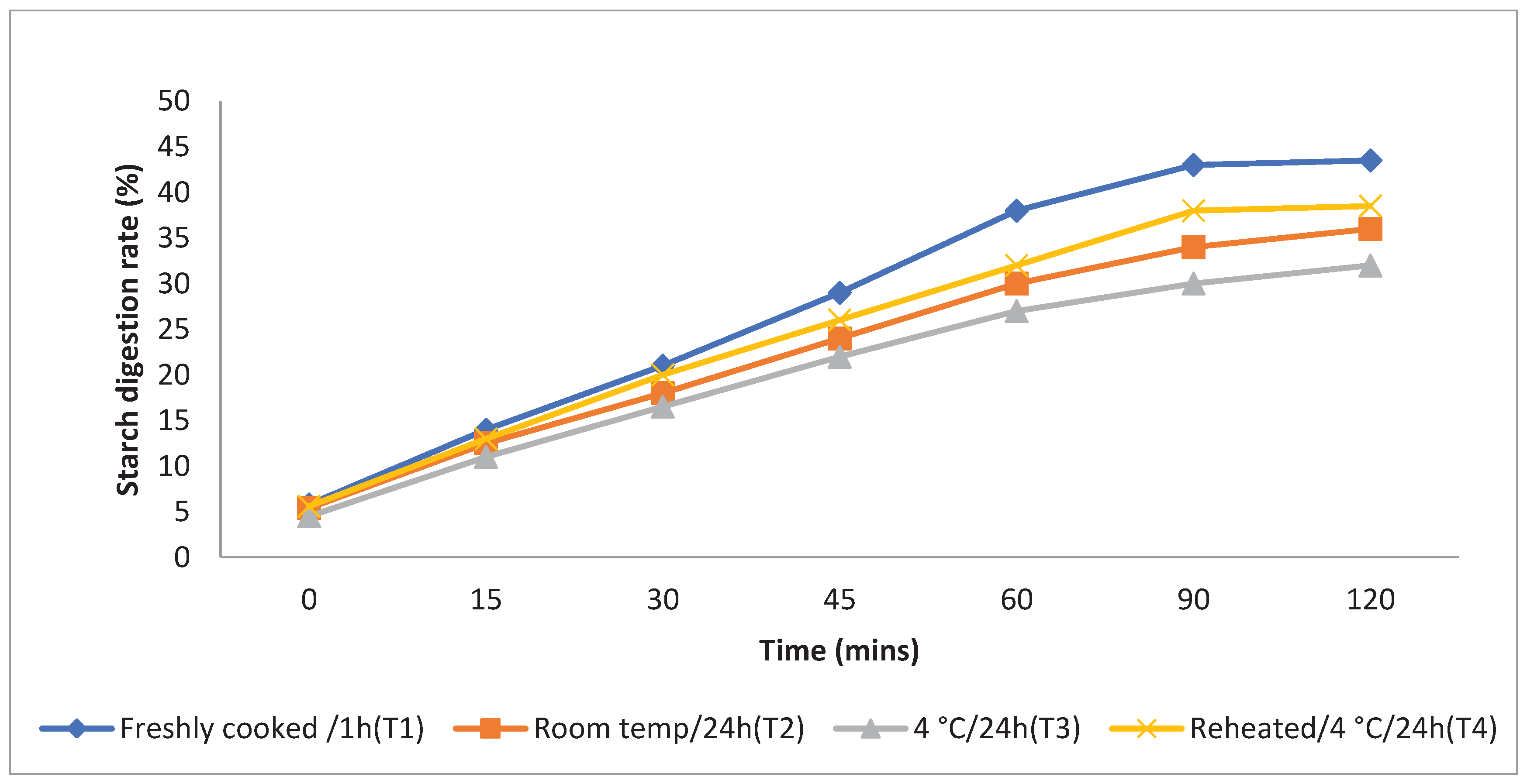
The rate at which starch is metabolised in vitro is a crucial factor in assessing a food product's ability to elevate a person's blood glucose levels. Different storage temperatures had an impact on the in vitro starch digestion rate of basmati rice products (
boiling 1, boiling 2, frying, and pressure cooking). This rate was measured 120 minutes after the food sample had finished digesting (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). The basmati rice boiled by absorption (Boiling 1) and stored at T3and T2 had a slower digestion rate of 39 and 41% at 120 minutes compared to T1 and T4 rice that had completed digestion with 43 and 41% at the end of 90 minutes In basmati rice boiled in an extra amount of water (Boiling 2), the rate of starch digestion was lower inT3 and T2 products with 38 and 42.2% at 120 minutes as compared to T1 as well as all other rice products. The starch digestion rate of fried and pressure cooked basmati rice was also high in T1 and T4 products compared to stored at low temperature.Therefore, the results indicated that the rice cooked using Boiling 1 method followed by storing with T3 had the slowest
in vitro starch digestion rate at the end of 120minutes of digestion indicating the presence of higher amount of RS and dietary fibre in the product.
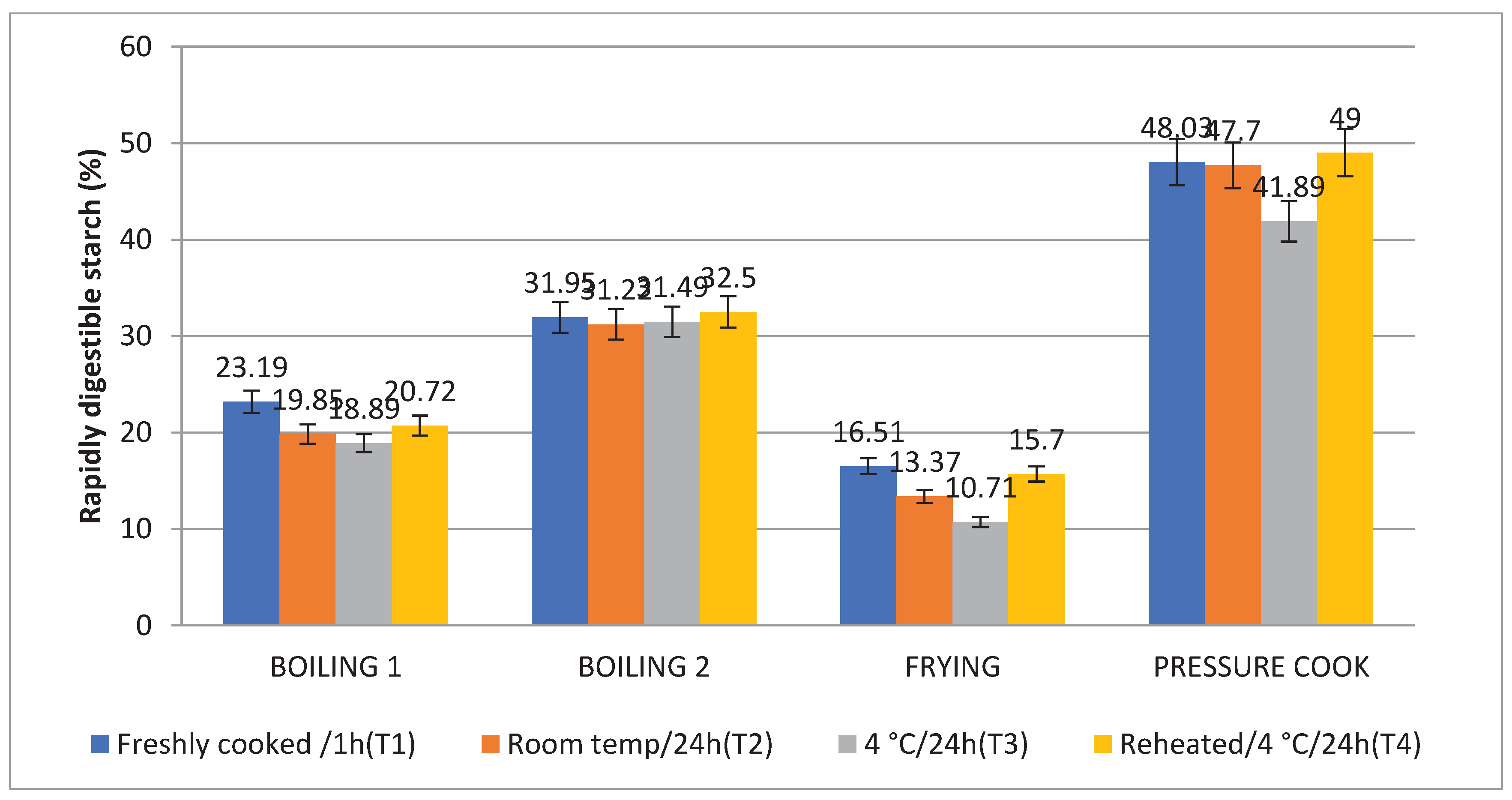
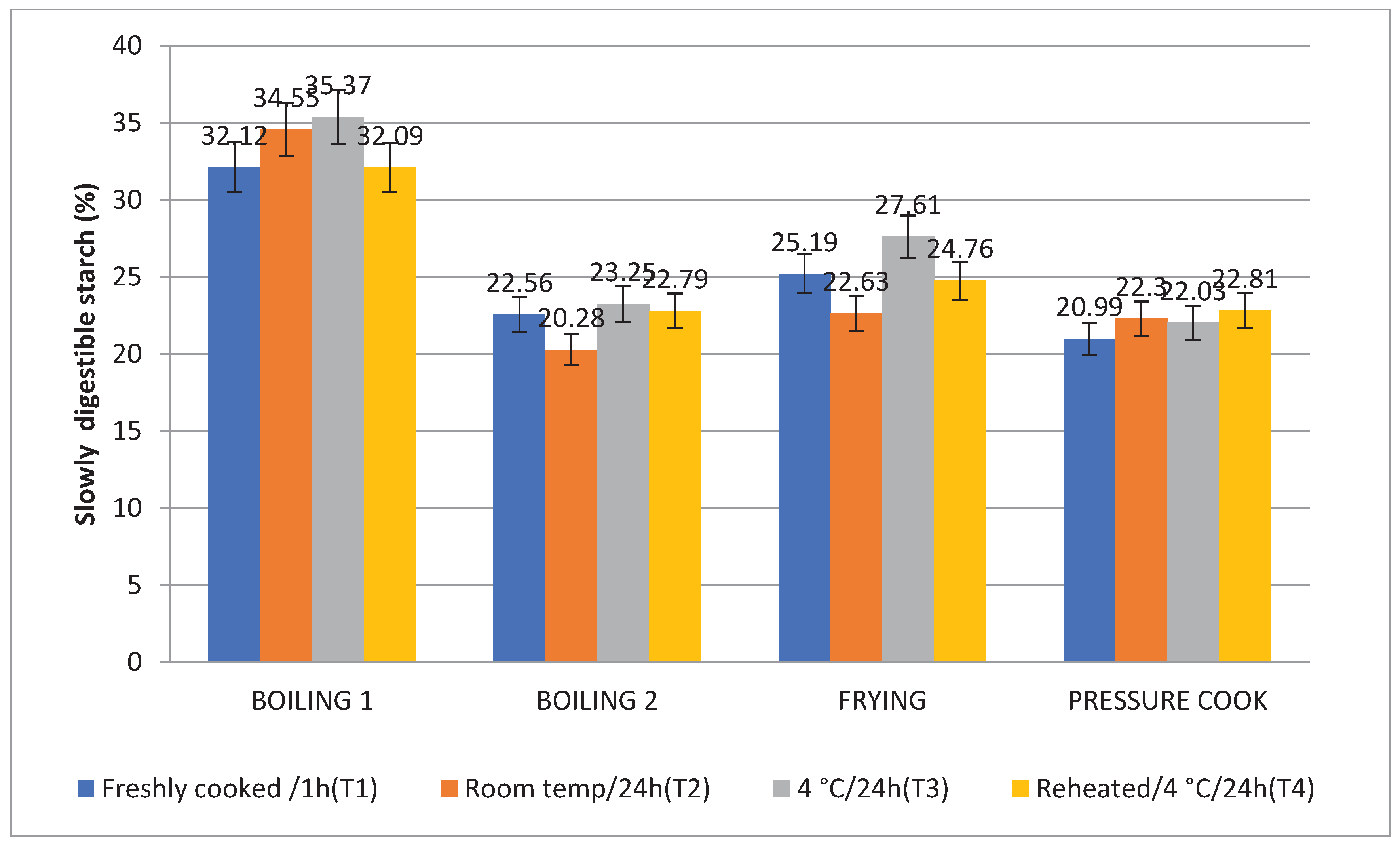
3.5. Rapidly Digestible Starch (RDS) and Slowly Digestible Starch (SDS)
The amount of SDS in Boiling 1 (35.37%) was highest, followed by Frying (26.71%), Boiling 2 (23.25%), and Pressure Cooking (22.03%), which is comparable to the in vitro starch digestion rate. Following T2, T4, and T1, treatment 3 (T3) raised the level of SDS to its highest point. Consequently the SDS content is higher (
Figure 7 and
Figure 8). When stored with treatment 3, frying (10.71%) and boiling 1 (18.89%) were found to have the lowest RDS, followed by boiling 2 (31.49%) and pressure cooking (41.89%).
3.6. Impact of Resistant Starch and Soluble Fibre Components on Postprandial Glucose Response
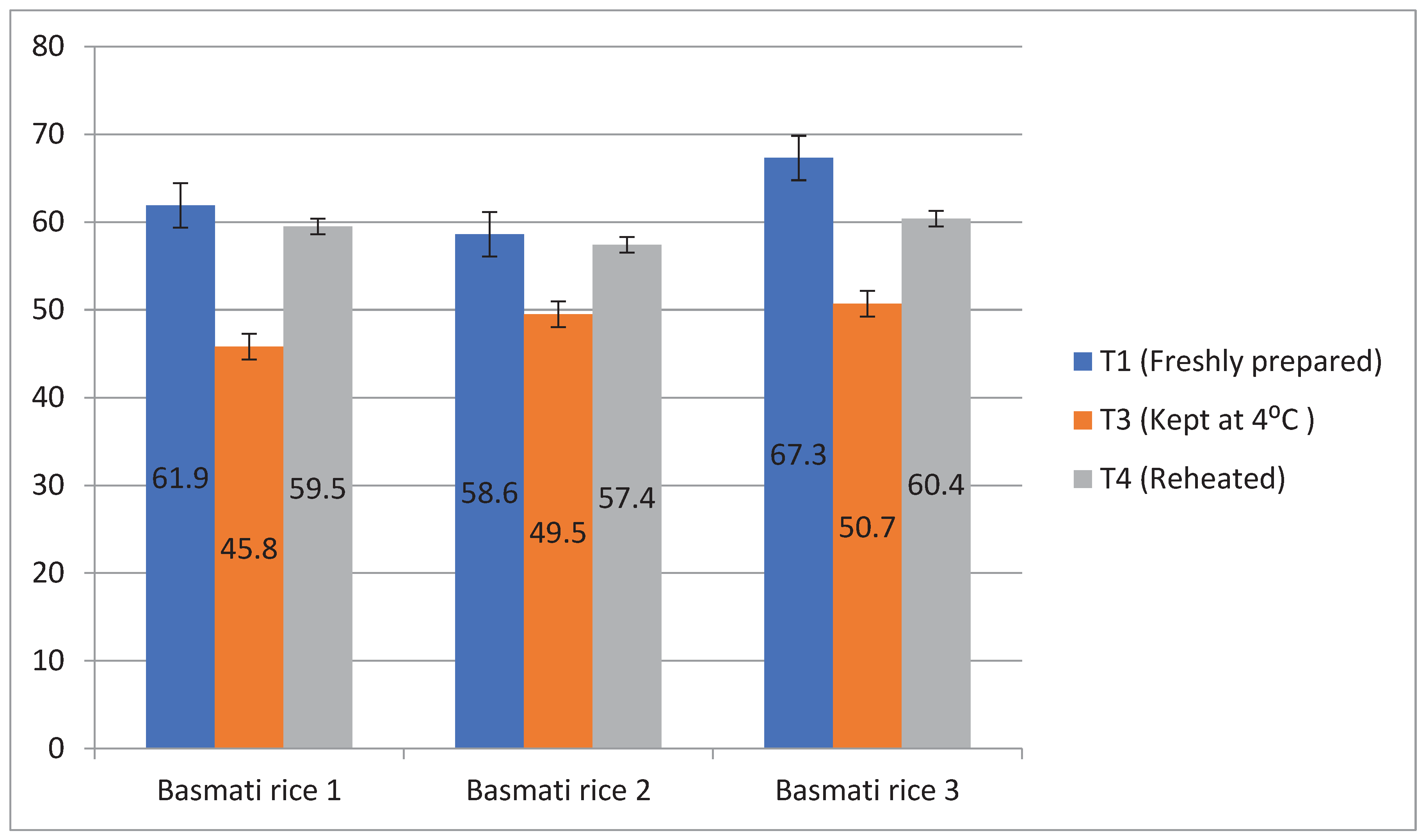
Ten healthy human participants were given rice products made by boiling 1, boiling 2, and pressure cooking that had a high RS content in order to test the glycemic response. Rice prepared using frying was not fed to the subjects as they denied its consumption claiming it as a fried product with higher amount of oil. Also, basmati rice products that received treatment 2 was not offered to the subjects as it was stored at room temperature and this temperature in summer season led to the microbial growth in the product. The lowest glycemic index was observed after the consumption of basmati rice prepared by boiling 1 (45.8%) having T3 followed by boiling 2 (49.5%) and pressure cooked rice (50.7%). Treatment 3 was determined to have the lowest glycemic index storage conditions, which may help those suffering from diabetes (
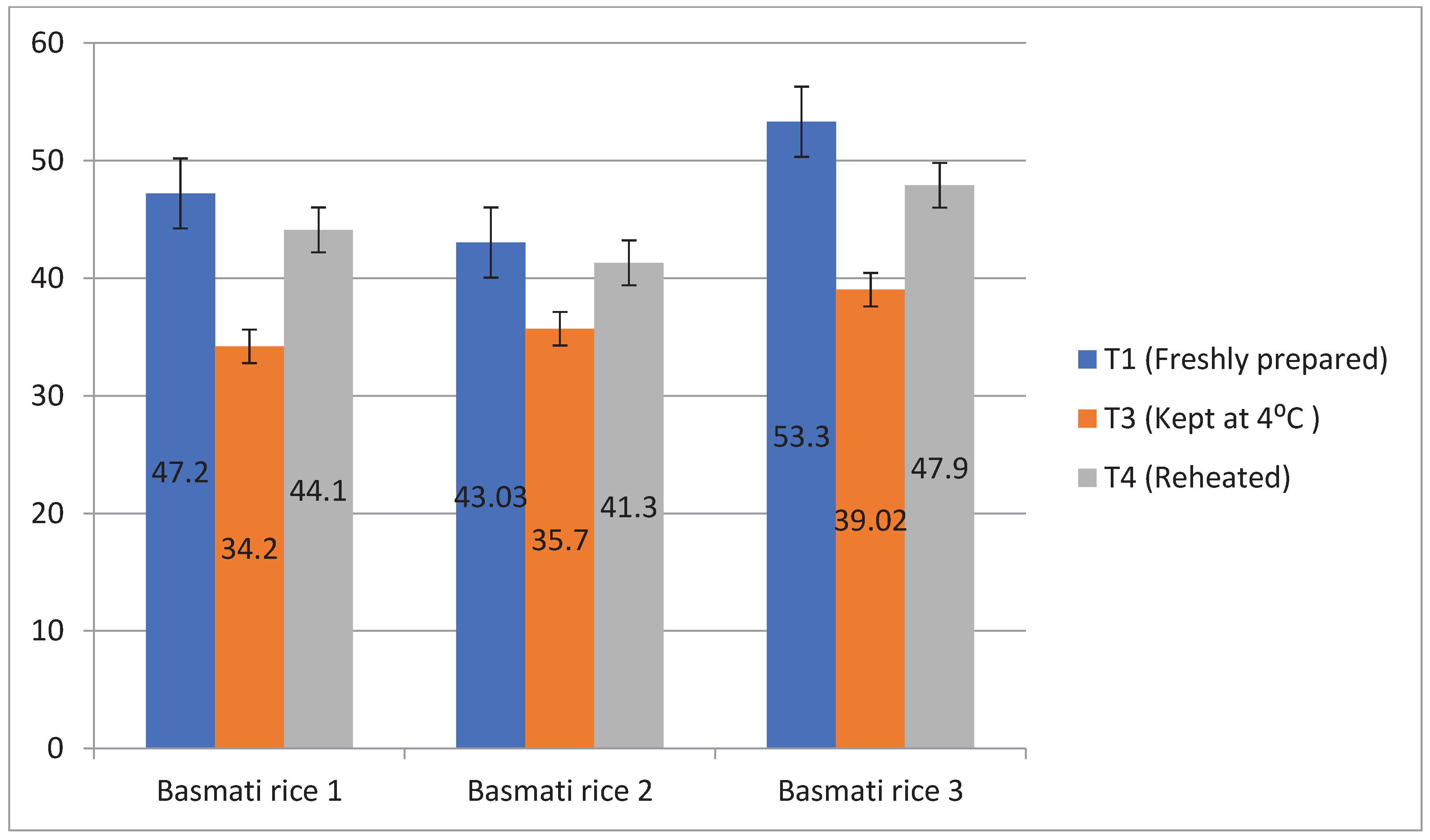
Figure 9). Similarly, glycemic load was found high in pressure cooked rice ( 53.3%), followed by boiling 1 (47.2%) and boiling 2( 43.03%). But after storing at T3, the glycemic load was decreased and it was found lowest in boiling 1 ( 34.2%) than other cooking methods (
Figure 10).
3.7. Effectiveness of Resistant Starch on Blood Glucose Level in Rats
Similar to human experiment, treatment 2 for Basmati rice was not considered for rat trial. Only basmati rice prepared by boiling 1 method were given to rats due to their limited number available to us and considering the fact that boiling 1 preparation has high RS content and is a common cooking method used by the North Indian population. The findings showed that blood glucose concentrations of resistant starch from various diet groups tended to decrease, but the mean values did not differ from those of the control or diabetic control groups. In diabetic rats, blood glucose levels increased dramatically from (114.3±6.9) mg/100ml in normal rats to (286.5±16.5) mg/100ml. Nevertheless, following the administration of the treatment diet, the pre-treatment groups' levels dramatically restored to the range of the normal range for each treatment group (G3–G5). After administering basmati rice T1, the blood glucose level dropped from (286.3±16.to 244.2±11.0) in G3. Basmati rice stored at T3 was administered to G4, and it was discovered that the blood glucose level decreased from (282.1±18.4 to 198.3±18.1), and that the blood glucose level decreased from (276.6±30.1 to 232.5±25.8) in G5 (
Table 4). The diets given in G3, G4 and G5 resulted in 14.7, 29.7 and 15.9% decrease in blood glucose level of the rats. Therefore, rice cooked with absorption techniques (Boiling 1) and stored at T3 resulted in maximum decline in the blood glucose levels of the experimental rats.
3.8. Effect of Resistant Starch on Lipid Profile in Rats
The diabetes group had significantly (<0.001) higher levels of triglycerides, total cholesterol, and low density lipoprotein (LDL) than the normal control and treatment groups. In contrast, the diabetic group had lower levels of high density lipoprotein (HDL). The maximum reduction in cholesterol was found in G4 with 39.9% followed by G5 (33.5%) and G3 (32.5%) respectively (
Table 5). Similarly, the triglyceride and LDL levels were reduced maximum in G4 (31.3%, 30.5%) followed by G5 (21%, 17.7%) and G3 (20.4%, 11.34%) respectively. The HDL levels were increased in G4 (54.7%) followed by G5 (50.3%) and G3 (48.9%) (Table 9). The data shows the glucose and lipid lowering potential of T3 diet given to G4 of rats.
4. Histopathological Study of Rat Organs
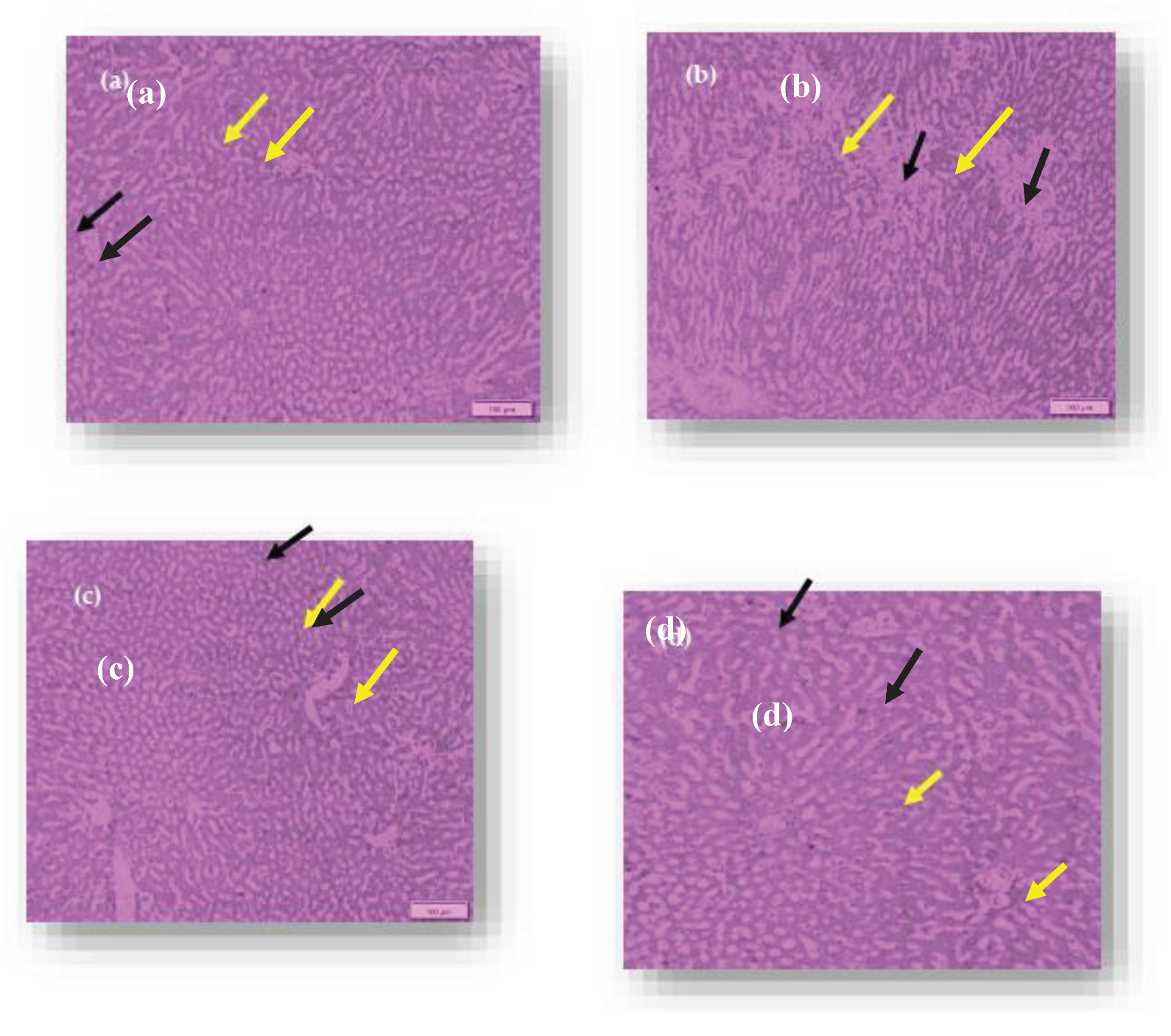
The blood glucose and lipid levels of rats were totally changed after consumption of RS3. Microscopic observation of liver of the rats is showen in
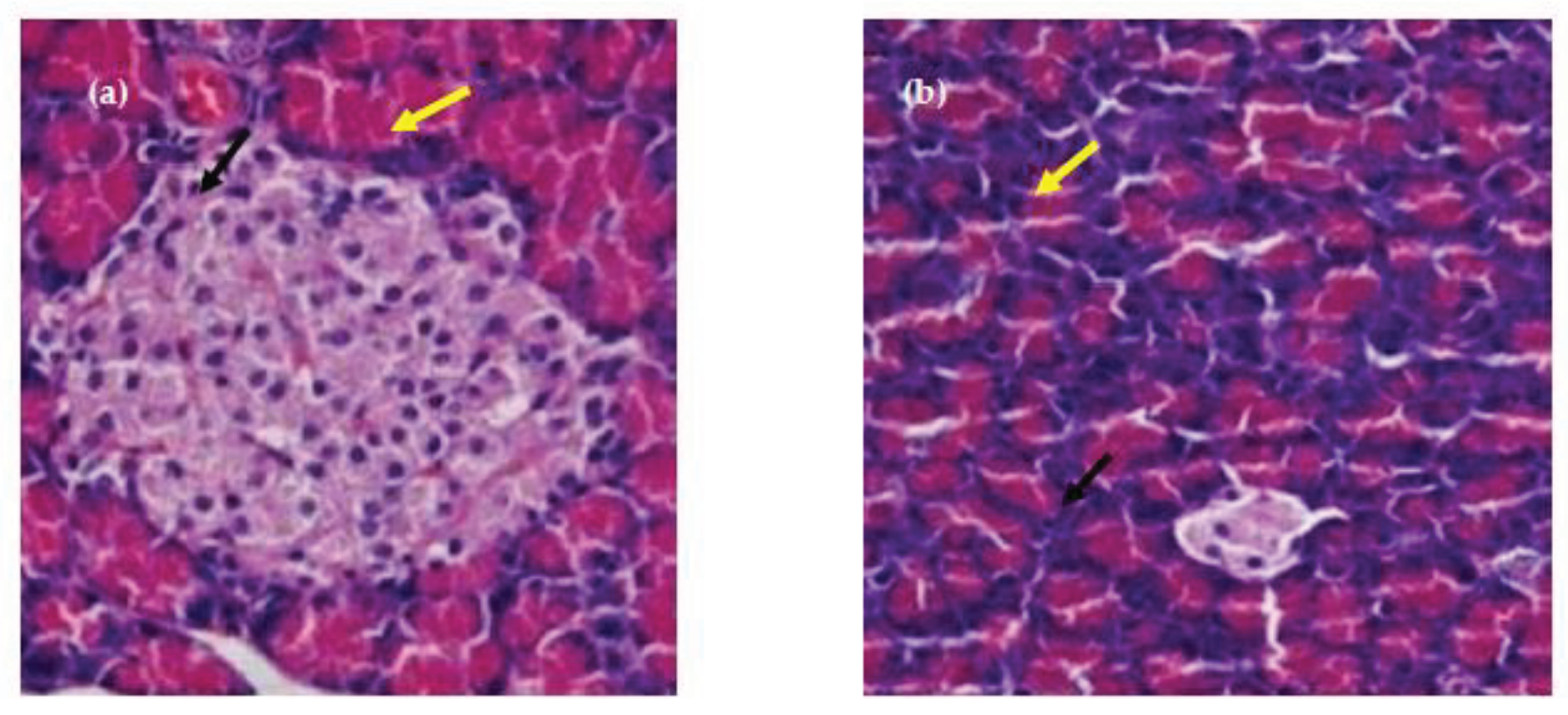
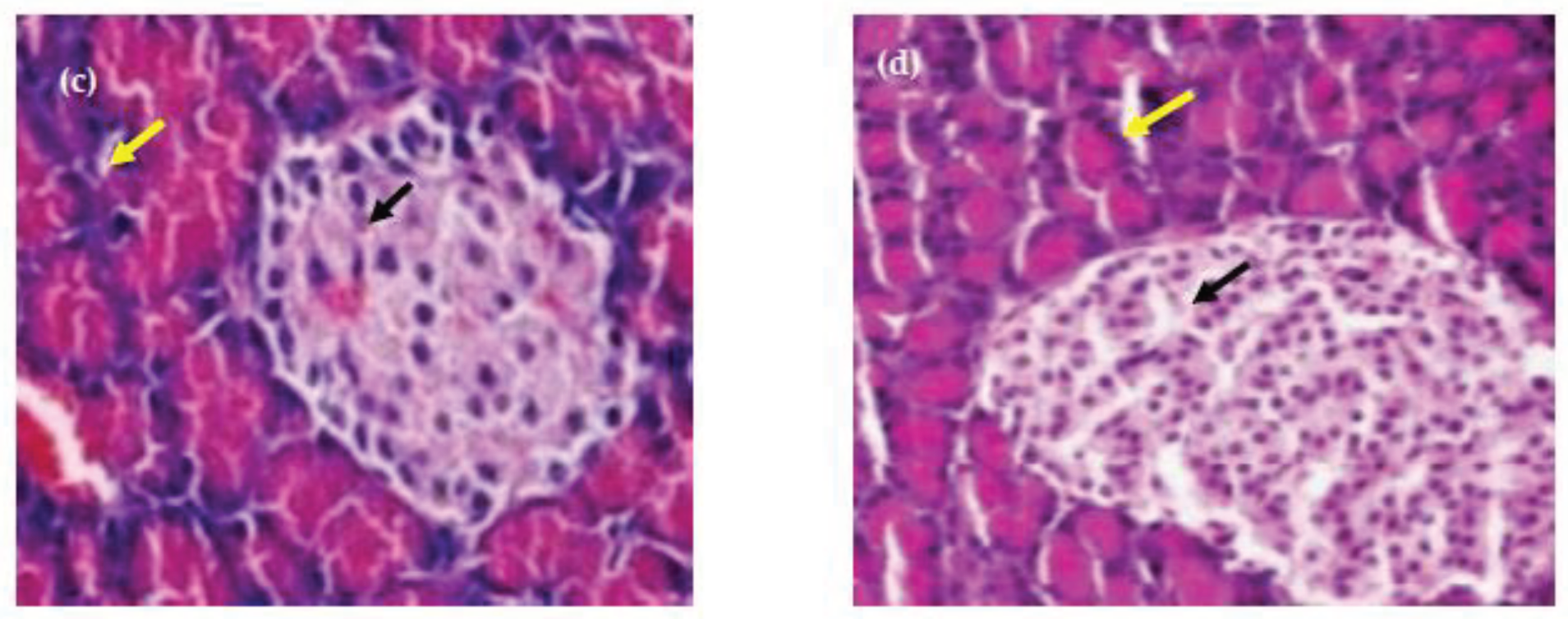
Figure 11. The tissue staining in control showed the normal architecture of liver with normal hepatocytes and sinusoidal layer. In Diabetic control, due to induction of diabetes, the liver showed the hepatocytes degeneration and inflammatory cellular infilteration with fatty liver. However, in treatment controls, the liver showed the mild degeneration of hepatocytes. The rat group(G4) consumed basmati rice T3 showed less degeneration compared to group consumed basmati rice stored at T1. On the other hand, while observing pancreas the tissue staining in control group showed the normal architecture of pancreas with normal range of Langerhans islands and beta cells (
Figure 12). Section from the pancreas of diabetic rat depicted cytoplasmic degeneration of islets of langerhans . While Group 3 and 4 (Treatment groups ) showed the reduction in number of beta cells and degranulated cytoplasm of most cells compared to control group, but far better than diabetic group. Group 4 in which basmati rice stored at 4⁰C for 24 h were given showed improved architecture of pancreas with increment in the formation of beta cells and regeneration of islets of langerhans compared to group 3. Results revealed that consumption of high RS rice products resulted in better insulin sensitivity, and delayed glucose absorption leading to improvement in blood glucose level. Similarly, fecal bile excretion and reduced cholesterol synthesis by SCFA were the main reason found that controlled the triglyceride and cholesterol levels in the experimental rats.
5. Discussion
The goal of the current study was to determine the ideal method of cooking and storage conditions for raising the resistant starch content of the basmati rice products that are often consumed in India. It was discovered that the boiling 1 approach had a higher protein content than the boiling 2 method. This might have happened because of the effect of soaking and cooking as in boiling 2, cooking denatures protein and soaking solublize some protein content that lead to its reduction during draining of water after cooking (25). In a study, boiling was found to be superior (4.99%) among other heat treatments like microwave (2.49%) and autoclaving (3.5%) to increase the protein content as the kinetic energy of all the techniques is mainly responsible to increase or decrease the protein content of rice while cooking (26).
Fat is important to increase the palatability of the food. Fried rice had higher fat content because of the addition of extra oil and more absorption. Compared to frying, other cooking methods like boiling 1, boiling 2 and pressure cooking were found to give a low in fat content due to . Fat hydrolysis that occurred in the presence of water and heat The ash content was found higher in boiling 1 and frying method compared to boiling 2 and pressure cooking because of higher amounts of protein, fat and fibre content (27). Boiling 2 had low ash content; that could be because of leaching out of macro and micro elements during soaking and draining while reduction of fibre content could be a reason for lower ash content observed in the pressure cooked rice sample.
Human digestive enzymes cannot break down dietary fibre. In addition to managing large intestine functions, it has a major physiological impact on glucose, lipid, and mineral metabolism (28). The solubility of dietary fibre in water is primarily determined by its soluble or insoluble nature. Like a magnet, soluble dietary fibre attracts body water into the digestive tract (29). Soluble fibre in the stomach creates a gel-like structure that is water soluble and facilitates easy food movement (30). Water insoluble fibres include lignin, cellulose, hemicellulose, and resistant starch. These fibres do not dissolve in water. Insoluble fibre helps move faeces out of the body by raising intestinal pressure (31). With the exception of frying, the amount of soluble dietary fibre in each cooking method was found to be similar in the current study. After cooking, the amount of soluble dietary fibre increased, but this is related to the temperature and cooking time. The primary cause of T1's higher soluble fibre content than T3 is heat treatments of cereals, which increase the viscosities of water extract, thereby converting insoluble dietary fibre into soluble form (32). Boiling methods were shown to have significant amounts of insoluble dietary fibre. Maillard's reaction is responsible for a rise in the amount of insoluble dietary fibre that occurs during cooking. It is possible that thermal processing may have caused production of maillard reaction products and thus increased its IDF value (33). An increase in total dietary fibre content might be occurred due to apparent increase of cellulose content (34). Boiling or microwave heating of instant mashed potatoes has been shown to increase TDF and IDF contents (35). Another study found that microwave cooking and frying reduced the in-vitro digestible starch and increased the resistant starch (RS) and water-insoluble dietary fibre (IDF) (36). Heat processing of rice increased the dietary fibre content (37,38). Hence, increase in cellulose content leads to increased amount of TDF and IDF. Compared to storage temperatures, low temperature might be the reason to increase the amount of resistant starch in T3, which results in higher levels of insoluble and total dietary fibre.
Resistant starch (RS) is defined as starch that is not digested by hydrolytic enzymes in the small intestine for at least 120 minutes after digestion and instead passes to the colon to be fermented by microbiota (39). It helps to control cholesterol, blood sugar levels and essentially acts as insoluble dietary fibre in the body (40). Boiling as a cooking method will increase or decrease the resistant starch content relying on the form of food (41). RS found high in boiling 1 method because of presence of heat and water. Starch granules absorb moisture, swell, and gelatinize when food is heated in the presence of water, whereas amylose degrades and washes out of solution and produces a larger degree of gelatinization with longer heating times (42,43). In boiling 2, the starch was leached out of rice and discarded along with the water. Hence, less amount of starch was available to convert into resistant starch probably due to low amount of amylose content. Fried rice found high amount of RS because Stir-frying reduces the starch hydrolysis rate and increases the RS content. According to a study, stir-fried food's RS concentration increased from 7 to 12% (44). As cooking time increased, so seemed the reductions in moisture content and amylose. Absence of water in the fried samples also inhibits the process of crystallization of amylose chains resulting in decreased RS content (22,45).
The pressure cooked basmati rice had a significantly lower level of resistant starch than any other cooking processes because of the bursting of starch cells at high temperature and pressure. The mechanism of high pressure gelatinisation of starch is different from heat induced gelatinisation. The amorphous region of starch granules swells when comes in contact with water and high temperature, leads to helix-coil transitions in amylose and amylopectin, loss of granular structure and crystalline order (46). Pressure cooking significantly reduced the RS content of fresh jasmine rice, compared to the rice cooker and oven baking (47). Among storage temperatures, RS content was found high in T3 than T2, T4 and T1. Because of the retrogradation, recrystallization of starch granules occurred while storing at 4° C. The rate and amount of retrogradation are determined by starch properties like molecular or crystalline structure and storage conditions such as time, temperature, and water content (10).
Since retrogradation at low temperatures produces RS, insoluble dietary fibre, a high amylose concentration, and a longer amylopectin chain, it was discovered that the In vitro starch digestion rate of Basmati Rice products was lowest in T3 products, followed by T2, T4, and T1. High amylose content reduced the starch digestibility, because of presence of large numbers of hydrogen bonds. It was also found low in fried rice. It might also be due to an interaction between amylose and fatty acids that results in complex formation on the surface of starch granules, and these components may act as physical barriers to digestion (48).
The starch digestion rate is calculated by taking the glucose measurements upto 180 minutes from the start of digestion as rapidly digestible starch are digested at 20 mins and slowly digestible starch are digested at 120 mins of digestion and complete starch digestion takes place by 180 minutes (49). Rapidly digestible starch (RDS) gets converted to glucose within 20 minutes of food intake while slowly digestible starch (SDS) takes 20 to 120 minutes to convert to glucose. Rapidly digestible starches are thought to cause an abrupt rise in blood glucose levels after consumption, whereas slowly digestible starches are completely digested in the small intestine, resulting in a slower rise in blood glucose than rapidly digestible starches. Slowly digestible starch are linked to satiety, stable glucose metabolism and diabetes management (50).
The RDS was significantly found high in pressure cooked basmati rice, followed by boiling 2, boiling 1 and fried rice. RDS was found high in T1, followed by T4, T2 and T3. SDS found high in boiling1, followed by frying, boiling 2 and pressure cooking. The low digestibility could be caused by variations in the morphological and physical characteristics of the starches in cereals. Cooking cereal starches causes the starch granules to gelatinize and undergo physical and chemical disruption. In turn, the amount of water present, cooking time, and temperature all affect the extent to which gelatinization occurs (51). Protein structures surrounding starch granules may limit starch gelatinization and granule swelling, which lessens the granules' vulnerability to enzymatic attack. This may be responsible for a certain amount of the low digestibility. The type of starch has a direct effect on the rate at which cereal starches can be digested. The digestibility of starch decreases with increasing amylose concentration. The greater surface area of amylopectin and the insoluble aggregates are considered to be the cause of the apparent variations in digestibility between amylose and amylopectin. These factors also probably reduce the accessibility of cleavage sites to enzyme attack (52). Therefore, the kind and supply of starch in cereals may affect how easily they digest.
Similarly, the amylose content in basmati rice was found high boiling 1 stored at T3, which could be because of the amylose aligns and associates with itself during cooking and cooling, a process known as retrogradation (53). Cooking process decreased amylose content , probably due to its leaching into the water, resulting in low amylose content in boiling 2 (54,55).
The rate at which a particular food raises blood sugar is determined by its glycemic index. Selecting low-glycemic index foods has been shown to improve a healthy person's post-prandial glucose and lipid metabolism (56). Glycemic Load (GL) of an average portion of food is made up by the amount of available carbohydrate in that serving and the GI of the food. The higher the GL, the greater the expected elevation in blood glucose and in the insulinogenic effect of the food. The consumption of high GL diet for longer period of time leads to increased risk of type 2 diabetes and coronary heart disease (57).
Glycemic index and glycemic load of the human subjects fed with basmati rice prepared by boiling 1 was found low in storage temperature T3 followed by T2 which can be due to the higher amount of RS and dietary fibre found in these products. The results of the present study is supported by a randomized, single-blind crossover study where 15 healthy participants were given white rice cooked and stored at different temperatures. The findings showed that the white rice cooked and stored at 4°C for 24 hours had highest RS content and also led to a reduced glycemic response compared to control rice(58). Following the administration of the treatment diet, the blood glucose levels of the pre-treatment groups (G3–G8) in the rat research dramatically restored to the near normal range. G7 showed a significant fall in blood glucose levels—22.5%. Products made from rice that were kept at T3 were rich in resistant starch, which causes the sugar to be released into the bloodstream gradually and decreases the absorption of sugar. The control of glucose output is significantly impacted by the slow digestion of resistant starch (RS) (59). The starch is nearly freshly digested, but the metabolism of resistant starch can occur anywhere from 5 to 7 hours after a meal. Digestion takes 5-7 hours, gradually raises blood sugar levels, reduces blood sugar and insulinemia, and leads to provide satiety for longer time (60). Because insoluble dietary fibre absorbs sugar molecules, it inhibits the passage of glucose in the small intestine (61). In the human digestive tract, fibre slows the rise in blood glucose levels and decreases the absorption of glucose (62,63). also, when fibre is hydrated it acts more effectively to reduce blood glucose level (64).
Similar results were reported in a study where rats fed on resistant starch from the rice group (12.9±3.2 mmoles/L) showed decreasing tendency in blood glucose concentrations as compared to resistant starch of corn (15.6±8.1 mmoles/L( (65). The relative body weight in treatment group and diabetic group (G2) was significantly lower (<0.001) than that of the normal control (G1) group, due to the protein breakdown during diabetes. In diabetic control rats, plasma insulin levels were found lower (G2), as compared to normal control group (G1). However, the level of plasma insulin was found to be elevated in the treatment groups (G6-G8) compared to diabetic control group (G2) after the treatment diet was given. STZ (Streptozotocin) induces diabetes in rats by preferential accumulation of the chemical in β-cells after entry through the GLUT2 (glucose transporter) receptor (chemical structural similarly with glucose) that allows STZ to bind to this receptor. Under this condition, the destruction of β- cells and induction of the hyperglycemic state is associated with inflammatory infiltrates including lymphocytes in the pancreatic islets (66). The destruction of β-cells of the islets of Langerhans results in massive reduction of insulin release. But in treatment group plasma insulin levels increased significantly. This could happen because of release of insulin in bound form from beta-cells of islets of Langerhans (67).
After the treatment of 28 days, the cholesterol level reduced significantly in all treatment groups except diabetic control (G2). Insulin inhibits the lipolysis process because it is important to synthesise fatty acids and triglycerides in fat tissues (68). In diabetic group, due to reduction in insulin level, body of the rat started to remove fat for the production of energy through the lipolysis mechanism (6) hence resulted in increased production of Acetyl-coA, which further increased the level of ketone bodies and cholesterol levels (69).
The diabetic group exhibited lower levels of high density lipoprotein (HDL) and higher levels of total cholesterol, triglycerides, and LDL in comparison to the normal control and treatment groups. The maximum reduction in triglyceride found in two groups G7 compared to other groups. These results may be related to soluble fiber consumption, which is considered as an important dietary factor in the prevention of cardiovascular diseases in many epidemiological studies (70).
Interestingly, the results indicate that at the end of the treatment, all groups of diabetic rats treated with basmati rice products had lower cholesterol levels, lower triglyceride and LDL levels, and higher HDL levels. The amount of rice resistant starch in each diet has an impact on this outcome. The highest concentrations of resistant starch demonstrated their ability to lower LDL, triglyceride, and total cholesterol levels. Diabetes is abnormally linked to increased amounts of endogenous glucose and fat synthesis. Through regulation of glycolysis and gluconeogenesis, the liver plays a critical role in preserving glucose utilisation and storage equilibrium (71).
Microscopic observations of liver cells of the rats showed that group that consumed basmati rice T3 shad less degeneration compared to group T1 group which could be due to the presence of more amount of resistant starch in basmati rice T3, which showed better insulin sensitivity and improved lipolysis mechanism. Retrograded starch has also been suggested to reduce blood LDL and cholesterol concentrations by a number of pathways, such as an increase in bile acid excretion from the faeces (72). In addition to short chain fatty acids in the liver and gut of rats (73), soluble dietary fibres regulate cholesterol levels by inhibiting hepatic cholesterol production through large intestine fermentations (74).
Histopathological study of pancreas of Group 4 showed improved architecture of pancreas with increment in the formation of beta cells and regeneration of islets of langerhans compared to group 3 leading to improved blood glucose level. High levels of RS, which function as an insoluble fibre and are fermented by the intestinal microbiota, are responsible for this. They also release carbon dioxide, methane, hydrogen, and metabolically active short chain fatty acids, which have an impact on insulin secretion (76), hepatic gluconeogenesis (75). According to a recent study, RS from a high-fat diet can control the expression of genes related to lipid and hepatic glucose metabolism pathways, as well as hyperglycemia and hyperlipidemia in diabetic rats (84). Resistant starch and insoluble dietary fibre also helps to reduce lipolysis and increase GLP-1, peptide YY and insulin secretion (77). GLP-1 (Glucagon-like- peptide 1) stimulates insulin secretion and reduces glucagon secretion. Pancreatic peptide (YY) helps to reduce appetite and increased the feeling of fullness which helps to control blood glucose and lipid levels.
6. Conclusions
The quantity of resistant starch in basmati rice increased when cooking methods such boiling 1, boiling 2, and frying were used, while the amount dropped when pressure cooking was used. While freshly prepared food (T1) and reheating food (T4) reduced the amount of resistant starch, products held at 4°C (T3) and at room temperature for 24 h (T2) increased the amount of resistant starch. Products kept at 4°C (T3) exhibited high levels of amylose, slowly digesting starch, and insoluble dietary fibre. The trial rats' blood glucose and cholesterol levels dropped as a result of eating this rice, and the human participants' glycemic index and glycemic load also fell. A slower rise in blood glucose and cholesterol levels was also caused by the food product's greater RS, which stimulated the regeneration of beta-cells in the pancreas and hepato cells in the liver. Given the wide range of starchy preparations that Indians eat, modifying the method of cooking and storage temperature of starchy foods may have multiple positive health effects. In order to manage blood glucose levels, awareness about the nutritional and health benefits of consuming resistant starch should be raised. In order to boost the amount of resistant starch in food at the home level, people must also be educated on proper food preparation and storage techniques.
Acknowledgments
The authors thank all the study participants for their valuable contributions. This paper was derived from a thesis for fulfillment of obtaining a PhD degree.
Scope Statement
This research was designed to boost the knowledge among people about the resistant starch present in commonly consumed Indian basmati rice products. People recognition of the connection between a nutritious food regimen and good health is one of the important reasons for the popularity of functional foods that provide health advantages and lessen the chance of persistent sicknesses further to fundamental vitamins. Resistant starch (RS) which is recognized as a type of dietary fibre is one of the most widely used ingredients in functional foods. It has long been noted as an outstanding feature among fibers for its several nutritional. Consuming it lowers postprandial glycemia, enhances insulin sensitivity, and decreases fat accumulation. In considering the previously mentioned data, the goal of the current study was to ascertain how various cooking techniques and storage temperatures affected the production of resistant starch in Indian basmati rice products and how this affected the stability of blood glucose and cholesterol levels. The production of consumer-friendly foods with adequate resistant starch and raising public awareness of the dietary and health benefits of resistant starch consumption are the major challenges facing the food industry. Additionally, to enhance the amount of resistant starch in food at the domestic level by educating people on proper cooking techniques.
Conflicts of Interest
The research was conducted without any commercial or financial relationships that could be seen as a conflict of interest, as declared by the authors.
References
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M. J. , Sanchez-Zapata, E.; Perez-Alvarez, J. A. Resistant Starch as functional ingredient—A review. Food Res. Int. 2010, 43, 921–42. [Google Scholar] [CrossRef]
- Higgins, J. A.; Higbee, D. R.; Donahoo, W. T.; Brown, I. L.; Bell, M. L.; Bessesen, D. H. Resistant starch consumption promotes lipid oxidation. Nutr. Meta. 2004, 1, 1–8. [Google Scholar]
- Rahim, M. S.; Kumar, V.; Mishra, A.; Fandade, V.; Kumar, V.; Kondepudi, K. K.; Bishnoi, M.; Roy, J. High resistant starch mutant wheat ‘TAC 35’ reduced glycemia and ameliorated high fat diet induced metabolic dysregulation in mice. J. Cereal Sci. 2022, 105. [Google Scholar] [CrossRef]
- Englyst, H. N.; Kingman, S. M.; Cummings, J. H. Classification and measurement of nutritionally important starch fractions. Eur. J. of Clin. Nutr. 1992, 46:1, 33–50. [Google Scholar]
- Asp, N. G. Resistant starch. Proceedings of the 2nd plenary meeting of EURESTA: European Flair Concerted Action No. 11 on physiological implications of the consumption of resistant starch in man. Eur. J. Clin. Nutr. 1992, 46: 1 -148.
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–a review. Compr. Rev. Food Sci. Food Saf 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hernández, O.; Emaldi, U.; Tovar, J. In vitro digestibility of edible films from various starch sources. Carboh. Polym 2008, 71, 648–55. [Google Scholar] [CrossRef]
- Sanz-Penella, J. M.; Tamayo-Ramos, J. A.; Sanz, J.; Haros, M. Phytate reduction in bran-enriched bread by phytase producing bifidobacteria. J. Agric. Food Chem. 2009, 57, 10239–244. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J. A. Resistant starch as prebiotic: A review. Starch - Stärke. 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, A.; Shevkani, K. Maize: grain structure, composition, milling, and starch characteristics. Nutrition dynamics and novel uses Springer India. 2013, 65-76. [Google Scholar] [CrossRef]
- Reader, D.; Johnson, M. L.; Hollander, P.; Franz, M. Response of resistant starch in a food bar vs. two commercially available bars in persons with type II diabetes mellitus. Diabetes 1997, 1997, 254. [Google Scholar]
- Cummings, J. H.; Englyst, H. N. Measurement of starch fermentation in the human large intestine. Can. J. Physiol. Pharmacol. 1991, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bullock, N. R.; Norton, G. Techniques to assess the fermentation of resistant starch in the mammalian gastrointestinal tract. Carbohydrate Polymers. 1999, 38, 225–230. [Google Scholar] [CrossRef]
- Lin, H. V.; Frassetto, A.; Kowalik, E. J. J.; Nawrocki, A. R. Butyrate and propionate protect against diet induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Haralampu, S. G. Resistant starch – a review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–92. [Google Scholar] [CrossRef]
- Baghurst, K.; Baghurst, P. A.; Record, S. J. CRC handbook of dietary fiber in human health, CRC Press, Boca Raton, Florida. 2001, 583–91.
- Alsaffar, A.A. Effect of food processing on the resistant starch content of cereals and cereal products: a review. Int. J. Food Sci. Tech. 2011, 46, 455–62. [Google Scholar] [CrossRef]
- Kale, C. K.; Kotecha, P. M.; Chavan, J. K.; Kadam, S. Effect of processing conditions of bakery products on formation of resistant starch. J. of Food Sci. Technol. 2002, 39, 520. [Google Scholar]
- AOAC. Official Methods of Analysis. 16th ed. Washington, DC: Association of Official Analytical Chemists (2000).
- AOAC. Official Methods of Analysis. 16th ed. Washington, DC: Association of Official Analytical Chemists (2002). A: DC.
- Sopade, P. A.; Gidley, M. J. A rapid in-vitro digestibility assay based on glucometry for. investigating the kinetics of starch digestion. Starch-Stärke 2009, 61, 245-55. [Google Scholar] [CrossRef]
- Goni, I.; Garia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427-37. [Google Scholar] [CrossRef]
- Ndaong, N. A. Efek Pemaparan Deltamethrin Pada Broiler Terhadap Aktivitas Enzim Alanine Amino Transferase, Asparat Aminotransferase, Gambaran Histopatologi Hepar Dan Feed Convertion Ratio, Tesis. Fakultas Kedokteran Hewan. Universitas Gadjah Mada, Yogyakarta, 2013.
- Selan, Y. N. Morfologi Dan Momfometri Saluran Pencernaan Kalong Kapauk (Pteropus vampyrus) Beserta Distribusi Sarafnya. Tesis. Fakultas Kedokteran Hewan. Universitas Gadjah Mada, Yogyakarta, 2013.
- Ebuehi, O.; Oyewole, C. A. Effect of cooking and soaking on physical, nutrient composition and sensory evaluation of indigenous and foreign rice varieties in Nigeria. Nutr. Food Sci. 2008, 38, 15–21. [Google Scholar] [CrossRef]
- Tarade, K. M.; Singhal, R. S.; Jayram, R. V.; andit, A. B. Kinetics of degradation of ODAP in Lathyrus sativus L. flour during food processing. Food Chem. 2007, 104, 643–49. [Google Scholar] [CrossRef]
- Siregar, K.; Sofyan, H.; Nasution, I. S.; Ichwana, R.; Syafriandi, S.; Sholihati Sofiah, I.; Miharza, T. Study of life cycle assessment in biodiesel production from crude palm oil and its benefits for the sustainability of oil palm industry in Aceh province Indonesia. IOP Conference Series: Earth and Environ. Sci. 2021, 644, 012-17. [Google Scholar] [CrossRef]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Polonor. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef]
- Kate, F. Everything You Need to Know about Soluble Fiber. 2020. Retrieved 24 July, from. http://www.katefarms.com.
- Cheng, L.; Zhang, X.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. Characterisation of physicochemical and functional properties of soluble dietary fibre from potato pulp obtained by enzyme-assisted extraction. Int. J. Bio. Macromol. 2017, 101, 1004–11. [Google Scholar] [CrossRef]
- Akinola, O. O.; Raji, T. J.; Olawoye, B. Lignocellulose, dietary fibre, inulin and their potential application in food. Heliyon. 2022, 29, 104–59. [Google Scholar]
- Siregar, K.; Sofyan, H.; Nasution, I. S.; Ichwana, R.; Syafriandi, S.; Sholihati, S.I.; Miharza, T. Study of life cycle assessment in biodiesel production from crude palm oil and its benefits for the sustainability of oil palm industry in Aceh province Indonesia. IOP Conference Series: Earth and Environ. Sci. 2021, 644, 012. [Google Scholar] [CrossRef]
- Chang, M. C.; Morris, W. C. Effect of heat treatments on chemical analysis of dietary fiber. J. of Food Sci. 1990, 55, 1647–50. [Google Scholar] [CrossRef]
- Herranz, J. Vidal-Valverde, C. Rojas-Hidalgo, E. Cellulose, Hemicellulose and Lignin Content of Raw and Cooked Spanish Vegetables. J. Food Sci. 1981, 46, 1927–33. [Google Scholar] [CrossRef]
- Thed, S. T.; Philips, R. D. Changes of dietary fibre and starch composition of processed potato products during domestic cooking. Food Chem. 1995, 52, 301–04. [Google Scholar] [CrossRef]
- Devi, K.; Geervani, P. Rice processing effect on dietary fiber components and in vitro starch digestibility. J. Food Sci. Technol. 2000, 37, 315–18. [Google Scholar]
- Ramulu, P.; Udayasekhararao, P. Effect of processing on dietary fibre content of cereals and pulses. Plant Foods Hum. Nutr. 1997, 50, 249–57. [Google Scholar] [CrossRef]
- Chung, H.; Hoover, R.; Liu, Q. The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch. Int. J. Biol. Macromol. 2009, 44, 203−10. [Google Scholar] [CrossRef] [PubMed]
- Birt, D. F.; Boylston, T.; Hendrich, S.; Jane, J. L.; Hollis J, Li. L. Resistant starch: promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D. W.; Tyler, R. T.; Toews, T.; Gawalko, E. J. Effect of cooking on the consumption of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L). Food Res. Intl. 2010, 43, 589–94. [Google Scholar] [CrossRef]
- Ek, K. L.; Brand-Miller, J.; Copelan, L. Glycemic effect of potatoes. Food Chem. 1230, 133, 1230-40. [Google Scholar] [CrossRef]
- Kolaric, L.; Minarovicova, L.; Lauková, M.; Karovicovaand, J.; Kohajdova, Z. Pasta enriched with sweet potato starch: Impact on quality parameters and resistant starch content. J. Text Stud. 2020, 51, 464–74. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Katawal, S. B.; Shrestha, A. K. Formation of resistant starch during processing and storage of instant noodles. Int. J. Food Prop. 2010, 13, 454-63. [Google Scholar] [CrossRef]
- Mangala, S. L.; Vidyasankar, K.; Tharanathan, R. N. Resistant starch from processed cereals. The influence of amylopectin and non-carbohydrate constituents on its formation. Food Chem. 1999, 64, 391-96. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. High pressure phase transition kinetics of maize starch. J. Food Eng. 2007, 81, 469-75. [Google Scholar] [CrossRef]
- Chiu, Y. T.; Stewart, M. L. Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac. J. Clin. Nutr. 2013, 22, 372–79. [Google Scholar]
- Svihus, B.; Uhlen, A. K.; Harstad, O. M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: a review. Anim. Feed Sci. Technol. 2005, 122, 303–20. [Google Scholar] [CrossRef]
- Englyst, K. N.; Liu, S.; Englyst, H. N. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2006, 61, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Robin, F. Slowly digestible starch – its structure and health implications: A review. Trends in Food Sci. Technol. 2007, 18, 346–55. [Google Scholar] [CrossRef]
- Williams, M. R.; Bowler, P. Starch gelatinization – a morphological study of Triticeae and other starches. Starch/Stärke 1982, 34, 221-23. [Google Scholar] [CrossRef]
- Goddard, M. S.; Young, G.; Marcus, R. The effect of amylase content on insulin and glucose responses to ingested rice. Am. J. Clin. Nutr 1984, 39, 388–92. [Google Scholar]
- Sagum, R.; Arcot, J. Effect of domestic processing methods on the starch, non starch polysaccharides and in vitro starch and protein digestibility of three varieties of rice with varying levels of amylose. Food Chem. 2000, 70, 107–111. [Google Scholar] [CrossRef]
- Ahmed, N.; Tetlow, I. J.; Nawaz, S.; Iqbal, A.; Mubin, M.; Nawaz, U.; Rehman, M. S.; Butt, A.; Lightfoot, D. A.; Maekawa, M. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice: Effect of high temperature in basmati rice. J. Sci. Food Agric. 2015, 95, 2237–43. [Google Scholar] [CrossRef]
- Chung, H.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2011, 75, 436–47. [Google Scholar] [CrossRef]
- Rizkalla, S. W.; Bellisle, F.; Slama, G. Health benefits of low glycemic index foods, such as pulses, in diabetic patients and health individuals. BrJ. of Nutr. 2002, 88, 255–62. [Google Scholar]
- Liu, Q.; Zhang, Y.; Laskowski, J. S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–45. [Google Scholar] [CrossRef]
- Sonia, S.; Witjaksono, F.; Ridwan, R. Effect of cooling of cooked white rice on resistant starch content and glycemic response. Asia Pacific J. Cln. Nutr. 2015, 24, 620-25. [Google Scholar]
- Kim, W. K.; Chung, M. K.; Kang, N. E.; Kim, M. H.; Park, O. J. Effect of resistant starch from corn or rice on glucose control, colonic events, and blood lipid concentrations in streptozotocin-induced diabetic rats. J. of Nutr. Biochem. 2003, 14, 166–72. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A. P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Raben, A.; Tagliabue, A.; Christensen, N.; Madsen, J.; Holst, J.; Astrup, A. Resistant starch: The effect on postprandial glycemia, hormonal response, and satiety. Am. J. Clin. Nutr. 1994, 60, 544–51. [Google Scholar] [CrossRef]
- Lopez, H. W.; Levrat-Verny, M. A.; Coudray, C.; Besson, C.; Krespine, V.; Messager, A. Class 2 resistant starches lower plasma and liver lipids and improve mineral retention in rats. J. of Nutr. 2001, 131, 1283–89–89. [Google Scholar] [CrossRef]
- Leclere, C.; Champ, M.; Boillot, J.; Guille, G.; Lecannu, G.; Molis, C. Role of viscous guar gums in lowering the glycemic response after a solid meal. Am. J. Clin. Nutr 1994, 59, 914–21. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K. C.; Li, Y.; Fu, L. In Vitro Study of Possible Role of Dietary Fiber in Lowering Postprandial Serum Glucose. J. Agric. Food Chem. 2001, 49, 1026–29. [Google Scholar] [CrossRef]
- Wood, L.; Wilbourne, J.; Kyne-Grzebalski, D. Administration of insulin by injection. Practical Diabetes International. 2002, 19, S1–S4. [Google Scholar] [CrossRef]
- Graham, K. L.; Krishnamurthy, B.; Fynch, S.; Mollah, Z. U.; Slattery, R.; Santamaria, P.; Kay, T. W.; Thomas, H. E. Autoreactive cytotoxic T lymphocytes acquire higher expression of cytotoxic effector markers in the islets of nod mice after priming in pancreatic lymph nodes. Am. J. Pathol. 2011, 178, 2716–25. [Google Scholar] [CrossRef]
- Sheela, C. G.; Augusti, K. T. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J. Exp. Biol. 1992, 30, 523-26. [Google Scholar]
- Wilson, E. A. Studies on the surgical induction of endometriosis in the rat. Fertility and Sterility 1985, 4, 684–694. [Google Scholar]
- Kaplan, L.; Lee, C.; Schaeffer, A. J. Effect of castration on experimental bacterial prostatitis in rats. The Prostate. 1983, 4, 4:625–30. [Google Scholar] [CrossRef]
- Rimm, E. B. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease Among Men. JAMA: J. Am. Med.Assoc. 1996, 275, 447. [Google Scholar] [CrossRef]
- Chandalia, M.; Garg, A.; Lutjohann, D. ; von, Bergmann. K.; Grundy, S. M.; Brinkley, L. J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. New Engl. J. Med. 2000, 342. [Google Scholar]
- Levrat, M.A. , Moundras, C., Younes, H. Effectiveness of resistant starch, compared to guar gum, in depressing plasma cholesterol and enhancing fecal steroid excretion. Lipids. 1996, 31, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen,W. J. L.; Anderson, J.W.; Jennings, D. Propionate May Mediate the Hypocholesterolemic Effects of Certain Soluble Plant Fibers in Cholesterol-Fed Rats. Proceedings of the Society for Experimental Biology and Medicine. 1984, 175(2):215-218. [CrossRef]
- Hara, H.; S, Haga.; Y, Aoyama.; S, Kiriyama. J. of Nutr. 1999, academic.oup.com.
- Anderson, L.; Dinesen, B.; Jorgensen, P. N.; Poulsen, F.; Roder, M. F. Enzyme immunoassay for intact human insulin in serum or plasma. Clin. Chem. 1993, 578. [Google Scholar] [CrossRef]
- Sasaik, Y.; Weekes, T. E. C.; Bruice, J. B. Effects of glucose and butyrate on insulin release from perifused fragments of sheep pancreas. J. Endocr. 1977, 72, 415–416. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, X. Role of Gut Microbiota in High-Fat-Diet-Induced Diabetes: Lessons from Animal Models and Humans. Nutrients 2023, 15(4), 922. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary Resistant Starch Upregulates Total GLP-1 and PYY in a Sustained Day-Long Manner through Fermentation in Rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1160–166. [Google Scholar] [CrossRef]
Figure 1.
Methodology of the study.
Figure 1.
Methodology of the study.
Figure 2.
Effect of different cooking methods and storage temperature on resistant starch content of basmati rice products (per 100g).
Figure 2.
Effect of different cooking methods and storage temperature on resistant starch content of basmati rice products (per 100g).
Figure 3.
Effect of different storage temperature on In vitro starch digestion rate of Basmati rice boiled by absorption.
Figure 3.
Effect of different storage temperature on In vitro starch digestion rate of Basmati rice boiled by absorption.
Figure 4.
Effect of different storage temperature on In vitro starch digestion rate of Basmati rice boiled in extra amount of water.
Figure 4.
Effect of different storage temperature on In vitro starch digestion rate of Basmati rice boiled in extra amount of water.
Figure 5.
Effect of different storage temperature on In vitro starch digestion rate of Pressure cooked Basmati rice.
Figure 5.
Effect of different storage temperature on In vitro starch digestion rate of Pressure cooked Basmati rice.
Figure 6.
Effect of different storage temperature on In vitro starch digestion rate of Fried Basmati rice.
Figure 6.
Effect of different storage temperature on In vitro starch digestion rate of Fried Basmati rice.
Figure 7.
Effect of different cooking methods and storage temperature on Rapidly Digestible Starch in Basmati rice products.
Figure 7.
Effect of different cooking methods and storage temperature on Rapidly Digestible Starch in Basmati rice products.
Figure 8.
Effect of different cooking methods and storage temperature on Slowly Digestible Starch in Basmati rice products.
Figure 8.
Effect of different cooking methods and storage temperature on Slowly Digestible Starch in Basmati rice products.
Figure 9.
Effect of different cooking methods and storage temperature on Glycemic Index of basmati rice products.
Figure 9.
Effect of different cooking methods and storage temperature on Glycemic Index of basmati rice products.
Figure 10.
Effect of different cooking methods and storage temperature on Glycemic Load of basmati rice products.
Figure 10.
Effect of different cooking methods and storage temperature on Glycemic Load of basmati rice products.
Figure 11.
Hiatopathology study of Liver of Rats. (a) Control (b) Diabetic control (c) Freshly prepared basmati rice fed diet group (d) Basmati riceT3 fed diet group. Black arrow shows hepatocytes, yellow arrow shows sinusoidal layer.
Figure 11.
Hiatopathology study of Liver of Rats. (a) Control (b) Diabetic control (c) Freshly prepared basmati rice fed diet group (d) Basmati riceT3 fed diet group. Black arrow shows hepatocytes, yellow arrow shows sinusoidal layer.
Figure 12.
Hiatopathology study of Pancreas of Rats. (a) Control; (b) Diabetic Control; (c)T1 Basmati Rice fed diet group (d) T3 Basmati Rice fed diet group. Black arrows show beta cell, yellow arrows show Langerhans islet.
Figure 12.
Hiatopathology study of Pancreas of Rats. (a) Control; (b) Diabetic Control; (c)T1 Basmati Rice fed diet group (d) T3 Basmati Rice fed diet group. Black arrows show beta cell, yellow arrows show Langerhans islet.
Table 1.
Preparation of commonly consumed Basmati rice products in India.
Table 1.
Preparation of commonly consumed Basmati rice products in India.
| Cereal product |
Ratio of ingredients |
Method |
| Boiling 1 (boiling by absorption) |
Basmati rice to water (2:4w/v) |
Basmati rice was boiled in water for 15 mins. |
| Boiling 2 (after boiling, extra water was drained out) |
Basmati rice to water (2:7-8 w/v) |
Basmati rice was boiled in an extra amount of water separately for 15 mins, and then the extra water was drained out after cooking. |
| Frying (fried rice) |
Basmati rice to water (2:4w/v) |
Wok was heated on a high flame and 10ml of oil was used to fry rice. Then rice were cooked for about 20-25 mins. |
| Pressure cooking (pressure cooked rice) |
Basmati rice to water (2:4w/v) |
Basmati rice were pressure cooked separately in a pressure cook (15 psi) for 10 min. |
Table 2.
Effect of different cooking methods and storage temperature on crude protein, crude fat and ash content of Basmati rice products (per 100g).
Table 2.
Effect of different cooking methods and storage temperature on crude protein, crude fat and ash content of Basmati rice products (per 100g).
| Cooking methods |
Treatments |
| T1 |
T2 |
T3 |
T4 |
Treatment Mean |
| Crude protein |
|
| Boiling 1 |
8.68±0.22b
|
9.32±0.57b
|
10.44±0.19b
|
9.74±0.52a
|
9.54A
|
| Boiling 2 |
9.51±0.36b
|
7.62±0.07c
|
9.90±0.10b
|
6.87±0.42cd
|
8.22B
|
| Frying |
7.59±0.10c
|
8.80±0.10b
|
9.34±0.14c
|
7.40±0.46b
|
8.28B
|
| Pressure cooking |
6.34±0.44de
|
7.54±0.43a
|
8.21±0.26cd
|
5.57±0.20e
|
7.49C
|
| Storage Mean |
8.03B
|
8.32B
|
9.47A
|
7.39C
|
|
| Crude fat |
|
| Boiling 1 |
2.01±0.04b
|
2.37±0.15b
|
2.47±0.07b
|
2.38±0.83b
|
2.18B
|
| Boiling 2 |
1.91±0.08b
|
1.84±0.07bc
|
1.93±0.06b
|
1.95±0.05b
|
1.90B
|
| Frying |
10.60±0.40a
|
10.98±0.49a
|
11.31±0.30a
|
10.57±0.64a
|
10.86A
|
| Pressure cooking |
0.62±0.05d
|
0.83±0.14d
|
0.90±0.10cd
|
0.80±0.10d
|
0.78C
|
| Storage Mean |
3.78A
|
4.0A
|
4.03A
|
3.92A
|
|
| Ash |
|
| Boiling 1 |
1.79±0.09ab
|
1.82±0.04a
|
1.90±0.03a
|
1.95±0.05a
|
1.86A
|
| Boiling 2 |
1.72±0.14ab
|
1.71±0.05abc
|
1.87±0.03a
|
1.71±0.11abc
|
1.75B
|
| Frying |
1.89±0.01a
|
1.92±0.07a
|
1.85±0.06a
|
1.74±0.21ab
|
1.85AB
|
| Pressure cooking |
1.43±0.10cd
|
1.53±0.13bc
|
1.22±0.12d
|
1.69±0.08abc
|
1.46C
|
| Storage Mean |
1.70A
|
1.74A
|
1.70A
|
1.77A
|
|
Table 3.
Effect of different cooking methods and storage temperature on Dietary Fibre (Soluble, Insoluble and Total) content of basmati rice products (per 100g).
Table 3.
Effect of different cooking methods and storage temperature on Dietary Fibre (Soluble, Insoluble and Total) content of basmati rice products (per 100g).
| Cooking methods |
Treatments |
| T1 |
T2 |
T3 |
T4 |
Treatment Mean |
| Soluble dietary fibre |
|
| Boiling 1 |
1.10±0.10ab
|
0.89±0.09bcde
|
0.59±0.02fg
|
0.93±0.06bcd
|
0.87A
|
| Boiling 2 |
1.16±0.06a
|
0.80±0.00cdef
|
0.63±0.06fg
|
0.97±0.06abcd
|
0.89A
|
| Frying |
0.89±0.10bcde
|
0.77±0.03def
|
0.55±0.05g
|
0.90±0.00bcd
|
0.78B
|
| Pressure cooking |
1.17±0.15a
|
0.87±0.06cde
|
0.68±0.09efg
|
0.99±0.01abc
|
0.92A
|
| Storage Mean |
1.08A
|
0.83C
|
0.61D
|
0.94B
|
|
| Insoluble dietary fibre |
|
| Boiling 1 |
2.43±0.06defg
|
3.10±0.10ab
|
3.33±0.12a
|
2.33±0.15defgh
|
2.80A
|
| Boiling 2 |
2.23±0.06fghi
|
2.50±0.00cdef
|
2.87±0.06bc
|
2.30±0.10efgh
|
2.47B
|
| Frying |
1.92±0.07i
|
2.50±0.24def
|
2.62±0.26cde
|
1.99±0.01hi
|
2.25C
|
| Pressure cooking |
2.00±0.10hi
|
2.36±0.14defgh
|
2.70±0.10cd
|
2.07±0.06ghi
|
2.28C
|
| Storage Mean |
2.14C
|
2.61B
|
2.88A
|
2.17C
|
|
| Total dietary fibre |
|
| Boiling 1 |
3.53±0.15bc
|
3.99±0.01a
|
3.92±0.14ab
|
3.27±0.15cde
|
3.67A
|
| Boiling 2 |
3.40±0.10cd
|
3.30±0.00cd
|
3.50±0.10c
|
3.27±0.06cde
|
3.36B
|
| Frying |
2.82±0.16f
|
3.27±0.21cde
|
3.17±0.21cdef
|
2.89±0.01ef
|
3.03D
|
| Pressure cooking |
3.17±0.21cdef
|
3.23±0.13cde
|
3.38±0.16cd
|
3.06±0.05def
|
3.20C
|
| Storage Mean |
3.22B
|
3.44A
|
3.49A
|
3.12B
|
|
Table 4.
Effectiveness of resistant starch on blood glucose and Plasma insulin levels in rats.
Table 4.
Effectiveness of resistant starch on blood glucose and Plasma insulin levels in rats.
| |
Blood glucose level (mg/dl) |
Plasma Insulin (µ/ml) |
| Diet Group |
Pre treatment |
During treatment |
Post treatment |
Treatment Mean |
Initial (pre) |
Final (post) |
P value |
| G1 |
114.3±6.86g
|
115±5.4g
|
112.6±4.32g
|
114.0E
|
24.33± 0.82 |
24.833± 1.17a
|
0.36NS
|
| G2 |
286.5±16.5a
|
285.6±13.8a
|
283.3±8.35a
|
285.1A
|
12.67± 1.63 |
10.00± 0.89d
|
≤0.001* |
| G3 |
286.3±16.4a
|
268±15.9abcd
|
244.2±11.0bcde
|
266.1B
|
12± 1.26 |
16.833± 1.72c
|
.005* |
| G4 |
282.1±18.4ab
|
238.17±19.4cde
|
198.3±18.1f
|
239.5D
|
13± 1.79 |
21.500± 0.54b
|
≤0.001* |
| G5 |
276.6±30.1abc
|
257.7±28.4abcde
|
232.5±25.8def
|
255.6BC
|
12.33± 1.03 |
17.167± 0.75c
|
≤0.001* |
Table 5.
Effect of resistant starch on lipid profile of rats.
Table 5.
Effect of resistant starch on lipid profile of rats.
| Total cholesterol (mg/dl) |
Triglyceride (mg/dl) |
HDL (High Density Lipoprotein) (mg/dl) |
LDL (Low Density Lipoprotein)(mg/dl) |
| Initial (pre) |
Final (post) |
P value |
Initial (pre) |
Final (post) |
P value |
Initial (pre) |
Final (post) |
P value |
Initial (pre) |
Final (post) |
P value |
| 85.17± 3.97 |
85.500± 2.58cd
|
0.805NS
|
72.33± 5.96 |
73.833±6.145d
|
0.122NS
|
54.16± 4.57 |
56.167±1.16a
|
0.356NS
|
23.5± 1.048 |
24.333±1.032f
|
0.201NS
|
| 137.3± 11.05 |
165.50± 05.57a
|
0.003* |
126.83±1.47 |
124.50±02.66a
|
0.084NS
|
24.66± 1.36 |
23.333±1.21e
|
0.034* |
74.33± 1.632 |
77.500±3.78a
|
0.141NS
|
| 134.83± 7.22 |
91.000± 0.89b
|
≤0.001* |
129.66±3.66 |
103.167±1.47b
|
≤0.001* |
23.5± 2.58 |
46.000±0.89c
|
≤0.001* |
70.5± 2.88 |
62.500±0.54b
|
0.019* |
| 138.33± 10.74 |
83.000± 0.89d
|
≤0.001* |
128.16±1.72 |
88.000±1.89c
|
≤0.001* |
24.66± 1.86 |
54.500±1.64a
|
≤0.001* |
68.5± 2.25 |
47.667±2.06e
|
≤0.001* |
| 134± 8.31 |
89.000± 0.89bc
|
≤0.001* |
125.83±3.76 |
99.333±1.50b
|
≤0.001* |
23.66± 1.21 |
47.500±0.54c
|
≤0.001* |
68.66± 2.65 |
56.500±1.76c
|
≤0.001* |
| |
180-200 |
|
<150 |
|
>50 |
|
<100 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
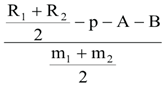 ×100
×100