1. Introduction
Aquatic bodies are essential for sustaining aquatic and terrestrial life as well as they continuously are a major economical and touristic resource, therefore, they generate a special attention for a proper monitoring, protection and pollution issues mitigation. Most ecosystems are highly dynamic in terms of both their species composition and abundances and their functioning. The abiotic and biotic elements of rivers and streams watersheds have been described as having a continuous pronounced ecological unpredictability from the beginning of the industrial age up to now, especially by the Covid-19 pandemic development. The diversity and structure of microbial communities were inevitably impacted by an environmental stress linked to natural ecosystems pollution. The microbial resilience capacity has been under the impact of a selective pressure triggered by untreated chemicals from wastewater plants effluents.
The overexploitation of water during anthropic activities conducted to a water depletion and/or pollution which triggered a warning signal for a proper water resource management. Furthermore, wastewaters have need known sources of chemical pollutants which mainly originated from human activities and medical unsupervised consumption of pharmaceuticals which end up as parent or metabolized molecules into domestic wastewater [
1,
2].
Wastewater treatment plants (WWTP) are part of a water resource management plan to reduce chemical or microbiological pollution before reaching the environment [
3]. Water, besides being a strategic resource for society, is an important vector in spreading infectious diseases over large areas. Certainly, it was the case of Covid-19 pandemic when the water management was a major factor in SARS-CoV-2 virus spreading as well as its associated infections [
4].
Despite the primary mechanism of SARS-CoV-2 transmission, via respiratory droplets that people cough, sneeze or exhale, an increasing number of articles reported the detection of this virus in feces of Covid-19 patients. Most of viral particles remained viable, infectious and/or able to replicate in stool under certain conditions. In the same time, during the epidemic peak, a massive use of pharmaceuticals caused a sudden increase of these drugs concentrations in wastewaters. Unfortunately, the chemical compounds increase triggered a rise of microorganism’s resistance to pharmaceutical compounds [
1,
5]. Changes in the microbial structures during WWTP treatment steps were correlated to virus presence in faecal matter, which eventually ended up in sewage systems.
The origin point of Covid-19 pandemic was China, Wuhan province from where it had a very fast progression reaching European countries in just a few months, when by March 2020 all European countries confirmed the infection presence. The very fast virus worldwide spreading took by surprise the medical authorities which, in response, imposed long periods of harsh social measures, such as lockdowns, to minimize the spread of the virus.
In Romania, the Covid-19 pandemic steadily spread from second part of 2020, reaching an infection peak in the middle of 2021. Covid-19 spreading rate into population was mainly monitored by hospital units, but there were reports that it could be efficiently monitored by Covid-19 quantification in domestic wastewater [
6]. Furthermore, many studies have shown that wastewater-based epidemiology has become a practical approach for monitoring Covid-19 outbreaks. At the same time, microbiological analyses of wastewater could provide indirect data of Covid-19 progression among certain human communities. The concept of wastewater-based epidemiology indicated that wastewater has been one of the main ways of disseminating antimicrobials and resistant microorganisms into the environments [
3,
7].
Large amounts of wastewater, including those from hospital units, have been daily generated into the sewage system from where they reached as influents the municipal WWTPs [
6,
8]. The modern WWTPs could process large quantities of wastewater (up to 440 000 000m3/year) and subsequently help to maintain a safe urban development and a clean environment. Consequently, it is necessary for WWTPs to have efficient and stable physco-chemical and microbiological treatment steps. The microbiological treatment step relies on activated sludge, which is a unique microbial community with a high bacterial diversity and high concentration of bio-mass based on wastewater pollutants composition [
9]. Any impairment of wastewater treatment steps decreases the effluents quality thus posing high risk to downstream users and environment, especially to the aquatic systems [
10].
The Covid-19 presence and its spreading rate in wastewater was clearly linked to its indirect effect microbial populations due to an anti-Covid-19 overused medical treatment in hospitals. Unfortunately, pathogenic microorganisms and pharmaceutical compounds, abundantly present in hospital wastewaters, were partially bio-degraded by the microbial communities from the activated sludge during a WWTP biological treatment step. In addition, the activated sludge became a hot spot for selection and dissemination of bacterial resistance under the pressure of chemicals, most of them used during the Covid-19 treatment.
World Health Organization acknowledged the bacterial multidrug resistance as one of humanity greater challenge, especially for those bacterial strains linked to the ESKAPE group, composed by Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and species of the genus Enterobacter spp. 50% of patients with severe SARS-CoV-2 infections were reported to develop bacterial coinfections, especially with a bacterial strain from ESKAPE, isolated from several extra-hospital reservoirs such as various water sources, including wastewater [
11,
12]. The ESKAPE group may be a relevant component of drug resistant dissemination pattern [
8].
Aquatic environments under anthropic pressure have been hot spots for antibiotics and antibiotic resistance genes. In addition to them, municipal WWTPs have been also major incubators for stimulating bacterial resistance mechanisms due to the fact that most antibiotics used in medical systems were excreted, via urine and feces, into sewage systems [
13].
The COVID-19 pandemic offered a unique opportunity to study the modulation of environmental antibiotic resistance that could be associated with changes in disinfectant and/or antibiotic usage patterns, coinfections, or other behaviors. COVID-19 cases positively correlated with the disinfectants/antiseptics group of ARGs and negatively correlated with the sulfonamide and aminoglycoside resistance classes. Antibiotic residues were present in hospital wastewater and subsequently they can adversely affect the natural biota at multiple trophic levels by exacerbating antibiotic resistance genes contamination of aquatic systems. Unfortunately, antibiotics released from clinical units reached the municipal sewage system and from there, they could reach the aquatic environment where they were further in contact with opportunistic pathogens, enhancing the bacterial resistance mechanism [
14,
15]. Municipal WWTPs reduce the abiotic and biotic pollution by three main treatment steps such as primary treatment to reduce the solid matter, secondary biological treatment to break down the organic waste matter and tertiary treatment to remove pathogenic organisms through chlorine disinfection. Recent studies showed the presence of the same Klebsiella pneumonia clone in both hospital and WWTP influent and effluent after chlorination, suggesting a high adaptive potential of this clone [
16]. The majority of fecal bacteria strains, such as Klebsiella pneumonia, isolated from effluent, influent and clinical samples were multidrug resistant and they especially have been resistant to antibiotics (beta-lactams) [
13]. Unfortunately, the microbiological and pharmaceutical compounds presence in sewage systems through excreted feces from infected people have not been significantly reduced by the WWTP therefore, they could reach and contaminate the environment, including the human communities nearby [
17]. Putative waterborne pathogens with ARG were identified in community coinfections with Covid-19 [
14].
In spite of progress in water treatment systems, the occurrence of waterborne infections remained a worldwide hot issue [
18,
19]. The enteric bacteria are the most commonly used bacterial indicator groups of fecal pollution, these being used as indicators to evaluate the quality of wastewater influents, effluents and rivers. The evaluation of faecal bacteria dynamics in sewage systems, including the wastewater treatment facilities, and their resistance and resilience to pharmaceutical compounds is essential for establishing a potential impact of con-trolled or uncontrolled wastewater discharges on the aquatic environment. Pathogenic or potentially pathogenic faecal bacteria in aquatic ecosystems could be used as warning bioindicators of a major pollution with a public health harmful potential, especially when they are resistant to pharmaceutical compounds widely used in the Covid-19 pandemic. [
6].
This work analyzed the microbiological modifications induced in influent and effluent wastewaters from three WWTPs in Romania during ante-Covid-19 and the peak period of the Covid-19 pandemic.
The centralized sewage systems that were studied are implemented in regions with moderate to high socio-economic capacity that have experienced a large number of cases of COVID-19. The aim of this study was to highlight the conditions that indicate an urgent need for monitoring programs and adapted risk assessments of SARS-Cov-2 in wastewater, which can help in the early detection and limitation of future outbreaks of viral diseases.
3. Results and Discussion
The sampling points were analyzed from West to East of Romania base on the Covid-19 progression.
The Covid-19 incidence average, daily measured in hospitals, was around 5 (five Covid-19 cases per thousand inhabitants) for the city of Timisoara (West side of Romania) and 3 for Rm. Valcea (central part of Romania) and Iasi (Eastern part of Romania) (
Figure 1).
The Covid-19 pandemic was also associated with digestive issues in human infected with SARS-CoV-2 during infection. The binding of the virus to some proteases of the intestinal tract led to the production of inflammatory factors secondary to the viral infection that were potentially harmful to the intestinal barrier. The imbalances of the intestinal microbiota from secondary Covid-19 infections [
23] have caused an increase in the number of hospitalized patients with both SARS-CoV-2 infections and secondary bacterial infections. A clinical research study from Romania demonstrated that both the number of hospitalized patients and the number of bacterial isolates was lower before the emergence and installation of the Covid-19 pandemic. This phenomenon also led to the excretion and dissemination of bacteria from secondary infections in the wastewater and then in the receiving aquatic environments. [
24]. In the same time, Amin and co. [
25] showed that microbiological analysis on Covid and non-Covid hospitals from Bangladesh indicated 97% from wastewater samples positive for E. coli were positive for Sars-CoV-2 too. There results indicated that high concentration of fecal bacteria and viral particles were discharged through the hospital wastewater into WWTP.
In addition, the Covid-19 treatment procedures heavily relied on antibiotic and biocides chemical which in fact disturbed the balance of intestinal bacterial communities, including generation of antibiotic resistant bacteria. Even before the establishment of SARS-CoV-2 infections the bacterial resistance was a global problem, after this period the resistance rate of pathogenic microorganisms isolated from both clinical cases and the environment increased. The pathogenic microorganism from patients were released into the sewage system and therefore could spread their resistance during the steps of WWTP. The influence of disinfection processes and specific infection treatments with antibiotics caused the dissemination of antibiotic resistance. The antibiotics presence in wastewater can exert a selective pressure and promote a development of antibiotic resistance genes which was an essential factor of changing the microbial community structure [
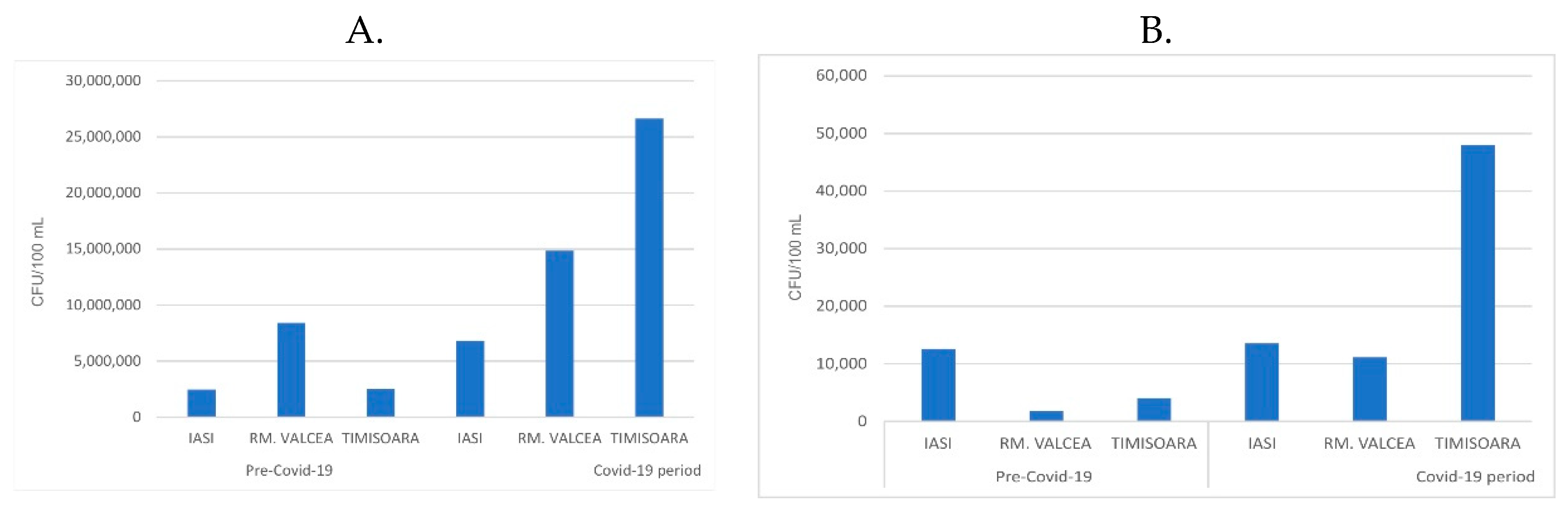
26]. The fecal bacteria from the sewage systems, especially in the WWTP influents, increased during Covid-19 progression compared to the period before Covid-19 pandemic (
Figure 2A.). The ratio between fecal from Covid-19 vs ante-Covid-19 was similar in Iasi (2.8 ration Covid-19/ante-Covid-19) and Rm. Valcea (2 ration Covid-19/ante-Covid-19). Interestingly, in Timisoara, a city with higher Covid-19 incidence, compared to Iasi and Rm. Valcea, the fecal ration between Covid-19 and ante-Covid-19 increased to 10.8.
The WWTP efficiency in removing the bacterial load, especially faecal coliforms, from influent (around 1.5 x 10
7 CFU/100 mL) to effluent (around 1.5 x 10
4 CFU/100 mL) was very high by decreasing the bacterial magnitude range load with 1 x 10
3 CFU/100 mL. In spite of this significant reduction of the bacterial load, the fecal presence in effluent was still substantial and their fecal ratio between Covid-19 and ante-Covid-19 was up to 6 for Rm. Valcea and up to 13 for Timisoara, but decreased almost to 1 for Iasi. (
Figure 2B.). The overall tendency of fecal presence was to be increased during Covid-19 pandemic compared to ante-Covid-19 progression.
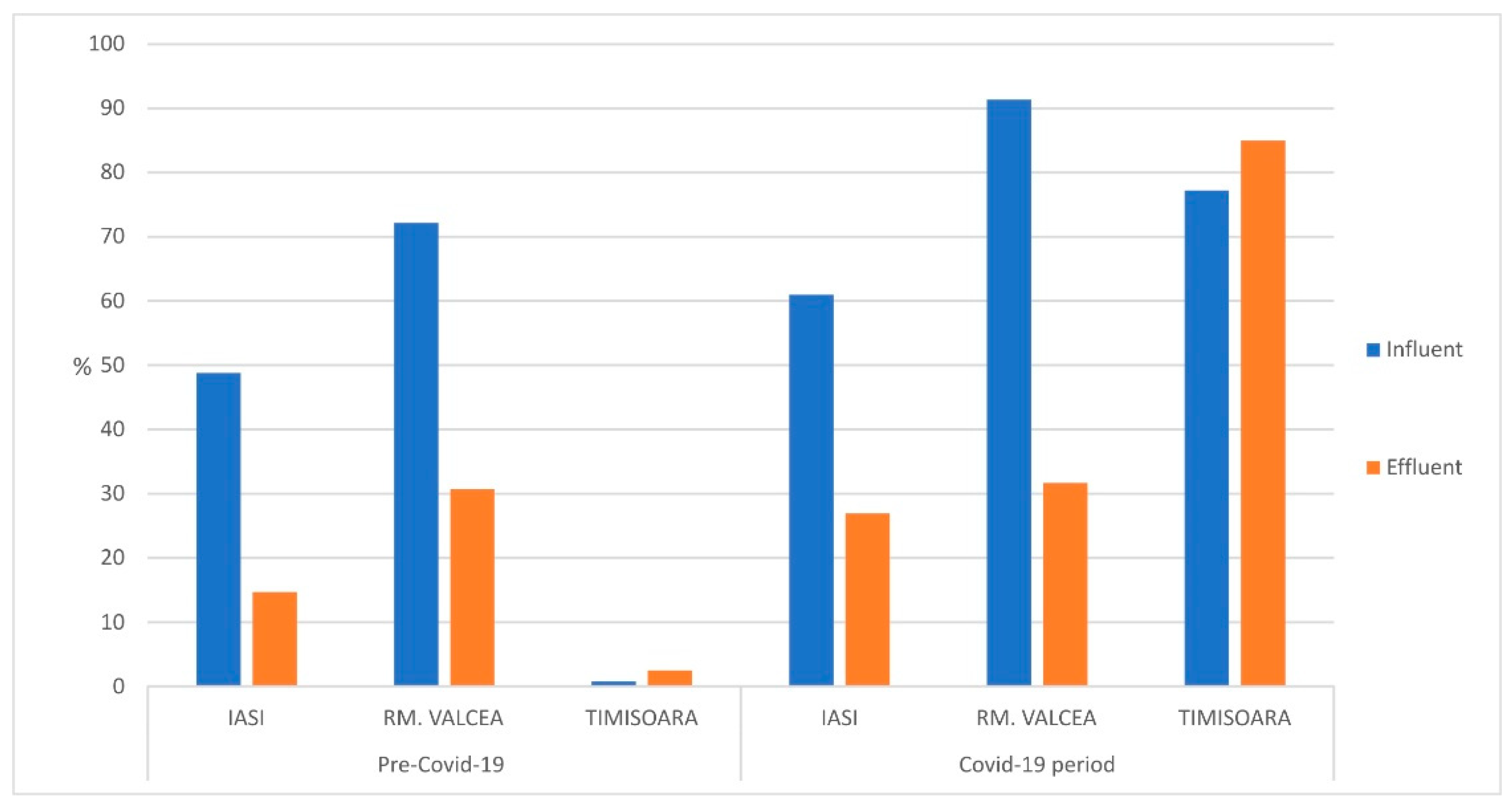
The fraction of fecal bacteria detected in total coliforms from WWTP’s influents and effluents was increased during Covid-19 pandemic compared to ante-Covid-19 (
Figure 3). The statistical analysis of these results indicated a p value <0.03 in all cases. In the city of Iasi (Eastern part of Romania) the percentage raise, during Covid-19 pandemic, in influent was around 10% compared to ante-Covid-19 time. A more significant fecal percentage raise (around 15%) was observed in effluent and this could be explained by an acquired resistance of fecal coliforms to WWTP treatment steps and chemical inputs, such as pharmaceutical compounds used in hospital units and released in the sewage system.
In Rm. Valcea (central part of Romania) the fecal coliforms percentage raised on total coliforms was observed only for WWTP influent, which was up to 20% in Covid-19 compared to ante-Covid-19. No significant changes in the fecal percentage from total coliforms in effluent. In Timisoara (Western part of Romania) it was observed a significant raised of fecal percentage from total coliforms during Covid-19, regardless of influent or effluent. This could be explained by the fact that Timisoara was the SARS-CoV-2 entrance gate in Romania and therefore the Covid-19 incidence was higher than central and eastern side of Romania as well as the medical treatment was implemented earlier which could induce an early acquired resistance of fecal bacteria to pharmaceutical compounds.
Some studies showed that Gram-negative bacteria antibiotic resistance, especially faecal such as E. coli, increased during the COVID-19 progression compared to Gram-positive bacteria [
24].
In this respect, during SARS-CoV-2 infection, Gram-negative bacteria such as total coliforms and E. coli were in contact with a large amount of pharmaceutical compounds, compared to ante-COVID-19 pandemic, increasing the resistance mechanism and subsequently the resistant Gram-negative bacterial population. This case scenario was analysed for Romanian WWTP during before Covid-19 pandemic and infection progression.
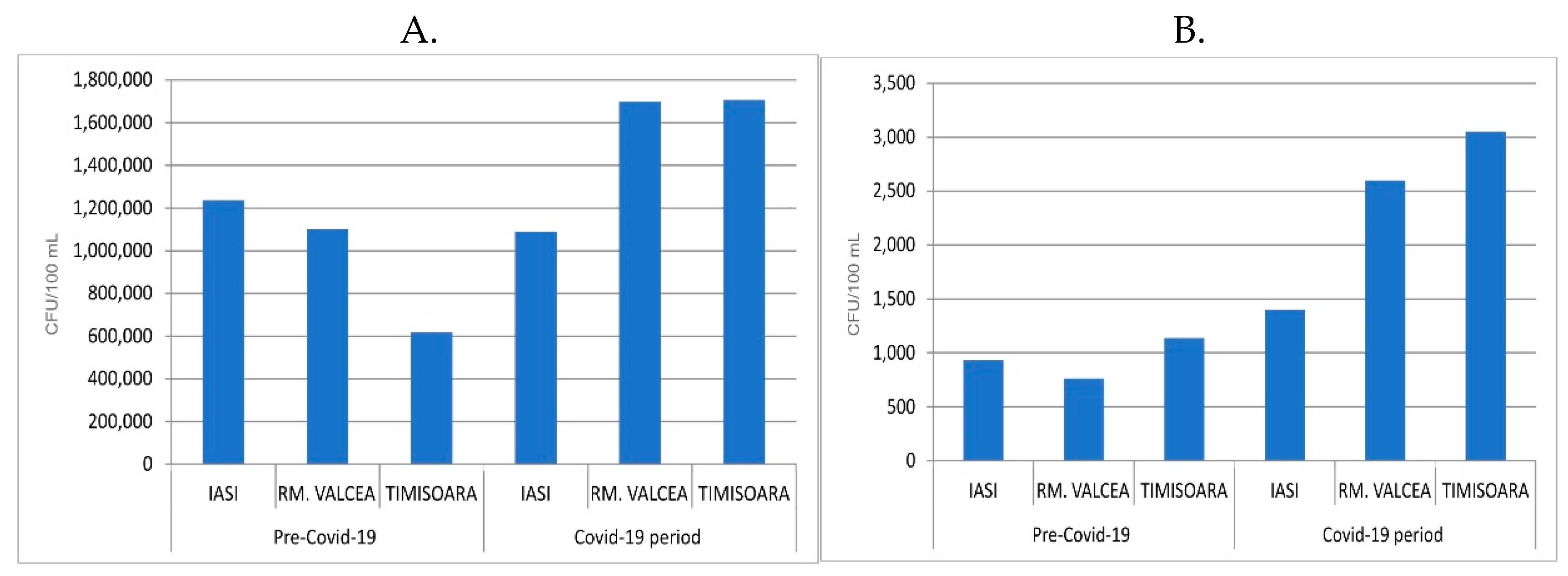
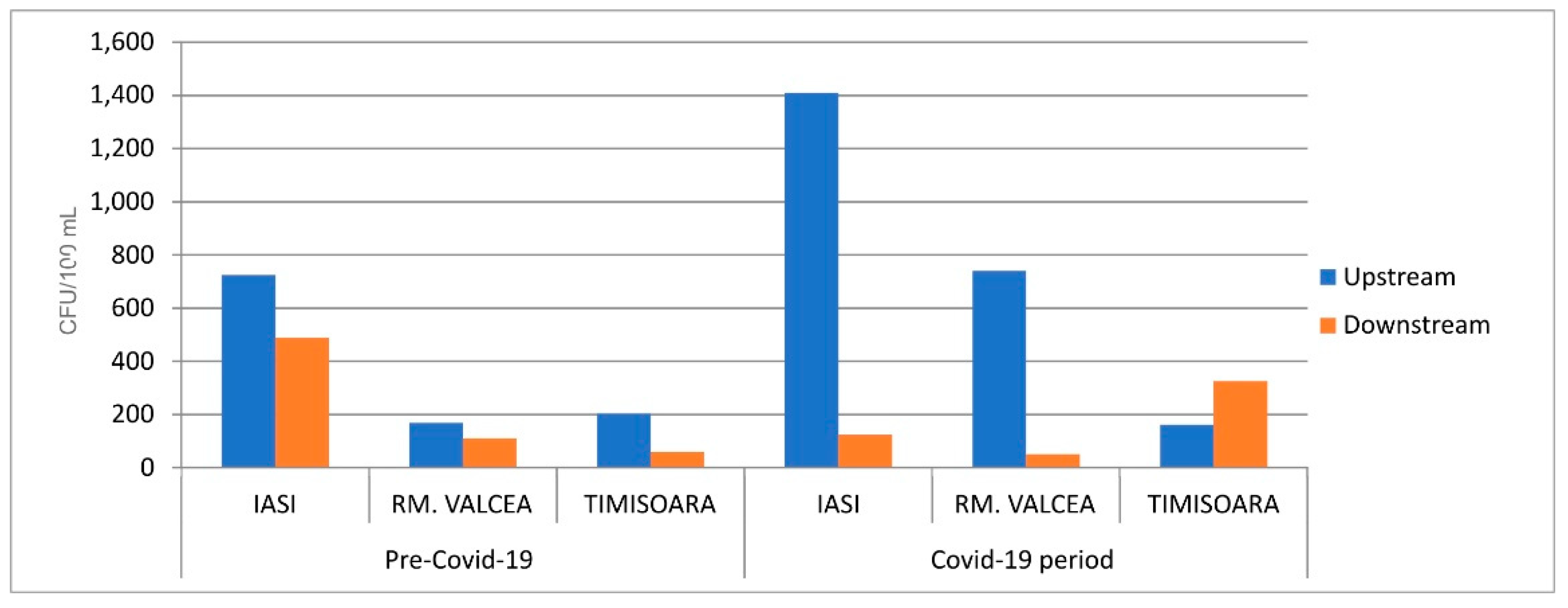
The fecal bacterial load from the streams, upstream the WWTP discharge site, was in the same rage in Covid-19 and ante-Covid-19 pandemic (
Figure 4) with the exception of Iasi where the fecal bacterial load was massively increased during SARS-CoV-2 infection. In this situation too, the statistical results recorded a p value around 0.03. Overall, the fecal bacterial load discharged into the environment had an effect of increasing the fecal contamination, downstream of WWTP discharge site. Downstream fecal coliforms load from Iasi and Rm. Valcea had a comparable increase in the bacterial contamination between ante-Covid-19 and Covid-19 (
Figure 4). Timisoara, with a much higher SARS-CoV-2 incidence, showed a huge fecal contamination of WWTP downstream during Covid-19 compared to ante-Covid-19, in spite of having a comparable fecal load into the environment, upstream WWTP discharge site.
At Iasi, Salmonella spp., a pathogenic bacteria belonging to fecal group, was detected in influent, effluent, downstream and upstream sites regardless of the sampling campaigned period, before Covid-19 infection or Covid-19 pandemic (data not shown). The same pattern of Salmonella spp. presence regardless or the sampling campaign was identified for WWTP’ influent from Rm. Valcea. A very interesting case was observed for Timisoara when Salmonella spp. was detected only in upstream during Covid-19 compared to ante-Covid-19 when Salmonella spp was detected in influent, effluent and upstream (data not shown). This could be explained by Salmonella spp sensitivity for pharmaceutical compounds present in the sewage system and discharged into the environment by the WWTP. The Covid-19 pandemic in Timisoara was characterized only by Salmonella spp presence in upstream which could be correlated with a high SARS-CoV-2 incidence, and subsequently with a high rate of usage of pharmaceutical compounds used during medical treatment for a large population. As an observation, Iasi (800k people) and Timisoara (500k people) have a comparable population, but a high Covid-19 incidence in Timisoara indirectly decreased Salmonella spp load due to an increased pharmaceutical treatment of Covid-19.
The enterococcal load pattern presence matched the fecal quantification in influent and effluent during ante-Covid-19 and after pandemic started. In Covid-19 progression, the enterococcal load increased, regardless of influent of effluent, compared to the period before pandemic (
Figure 5). In addition, the maximum increased was in Timisoara, which correlated with a high SARS-CoV-2 incidence compared to Rm. Valcea and Iasi.
The enterococcus loads ration between upstream and downstream increased during Covid-19 pointing out to a possible increased sensitivity of upstream bacterial loads to pharmaceutical WWTP discharges for all cities (
Figure 6). An interesting situation arose for Timisoara when upstream vs downstream enterococcus ration was inversed, perhaps due to an antibiotic resistance of enterococcus discharged from Timisoara WWTP. Previous results showed an increased antibiotic resistance due to a high pharmaceutical compounds usage [
27,
28]. Timisoara had a higher Covid-19 incidence than Iasi and Rm. Valcea and therefore Timisoara had a higher usage of pharmaceutical compounds which triggered the presence of more antibiotic resistant bacteria.
In Romanian clinics, the Covid-19 pandemic marked an increase number of Gram-negative pathogenic bacilli compared to the previous period when Gram-positive cocci were more common [
28]. This observation corroborated with a higher Covid-19 incidence in Timisoara, where the bacterial load had more interaction with larger amount of pharmaceutical which generated resistant bacteria ended up in natural streams (downstream) compared to the bacterial load from upstream.
According to recent studies [
4,
6,
30,
31,
32], the degree of pollution of the aquatic systems in Romania decreased with the decrease in the incidence of Covid-19 pandemic. Post-pandemic effects can be seen in the horizontal transmission of acquired antibiotic resistance and the perpetuation of resistant bacteria in aquatic environments with impacts on population health. Although the pandemic was significantly reduced, bacterial infections with resistant microorganisms continued to be present, especially in hospital units. Thus, various pharmaceutical compounds, including antimicrobial substances, used in various clinical treatments end up in WWTP where they spread the ARG to environment and human communities, subsequently affecting the medical treatments. Recently, many solutions to improve the WWTP efficiency were proposed, focussing on the microbial treatment step, from increasing the energy efficiency by using bacterial-algae granules [
33,
34,
35] to resilient chemical removal by specific bacterial strains. Also, phosphate recovery by struvite crystallization and co2 degasification reactor can significantly reduce the level of contamination of wastewater subsequently discharged into natural ecosystems [
36,
37,
38].
Given that ecosystem stability is about temporal continuity, bacteria can continuously respond and adapt to secondary perturbations by changing patterns of assembly and function. Antimicrobial resistance remains a serious and emerging health system problem after Covid-19 that is exacerbated and widespread worldwide. This continues to generate unprecedented stimuli for doctors and researchers looking for the solution, prevention and management of this problem both at the medical level and at the environmental impact level.
4. Conclusions
Covid-19 had an indirect impact on microbial populations due the medical treatment against Covid-19 by using large amounts of pharmaceutical compounds, especially antibiotics. The Covid-19 progression in Romania could be monitored by the rise fecal bacterial loads from WWTP influent wastewaters. The same fecal pattern could be observed on enterococcus enteric bacteria. Enterococcus was linked to most of gastrointestinal infection and inflammatory bowel diseases, reported during Covid-19 peak period of time. Salmonella, another pathogenic bacteria, had no significant changes (monitored only by presence or absence) in influents and effluent, regardless of Covid-19 pandemic.
Unfortunately, it could be observed a clear fecal contamination of the natural aquatic bodies (downstream) by the WWTP effluent and this contamination seemed to increase in areas with a high Covid-19 incidence, Timisoara (5 – incidence number) city compared to Iasi and Valcea (3 – incidence number). The Enterococcus, Gram positive bacteria, got a more resistant rate when in contact with larger amount of pharmaceutical compounds and it could be observed in receptor river from Timisoara. In Valcea and Iasi with lower Covid-19 incidence rate the Enterococcus presence in downstream compared to upstream was similar, regardless of Covid-19 period of time. It was clear that a higher Covid-19 incidence increased the usage of pharmaceutical compounds which, at their turn, increased the number of resistant bacteria reaching the environment via WWTP effluents.
Fecal coliform bacteria in the final effluents play the role of quality indicators and indicate the presence of pathogens in wastewater with a high risk of transfer to natural water/environmental resources. In addition to information about the microbiological quality of wastewater or surface water, in the present case, coliforms also provide valuable subsequent information, such as the progression of Covid-19 and the increase in pharmaceutical use - phenomena with which they are correlated.