Submitted:
11 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
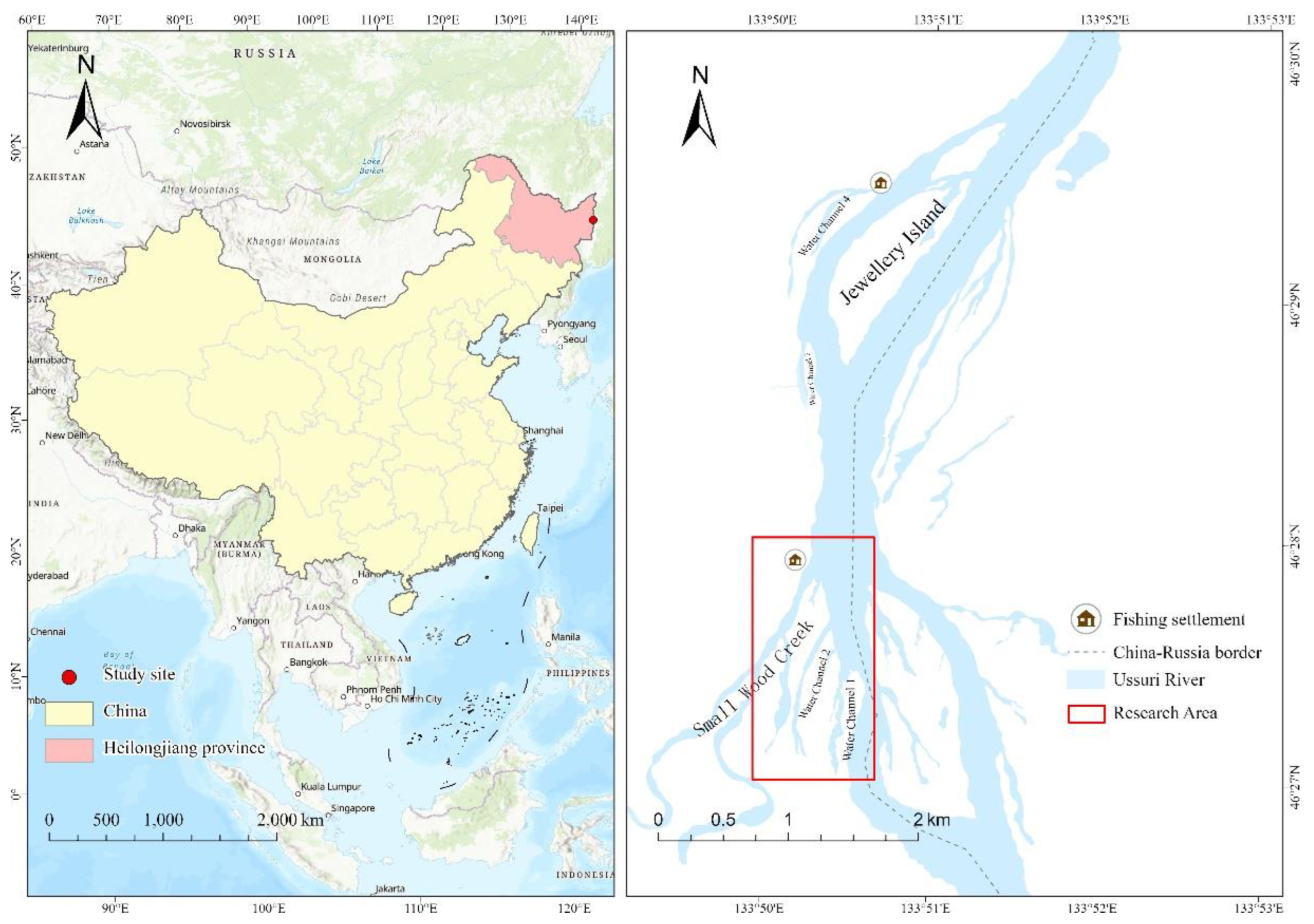
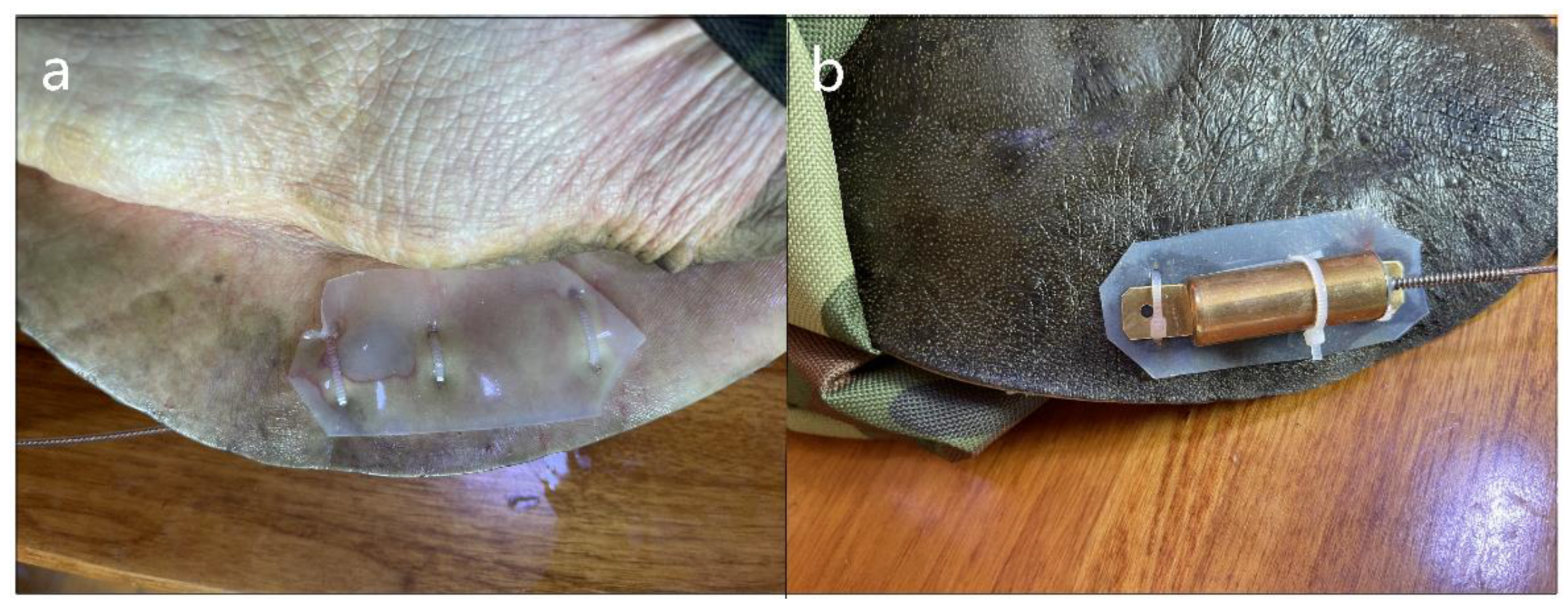
2.1. Study Site, Radio Telemetry, and Data Collection
2.2. Home Range Analysis
2.3. Movement Analysis
3. Results
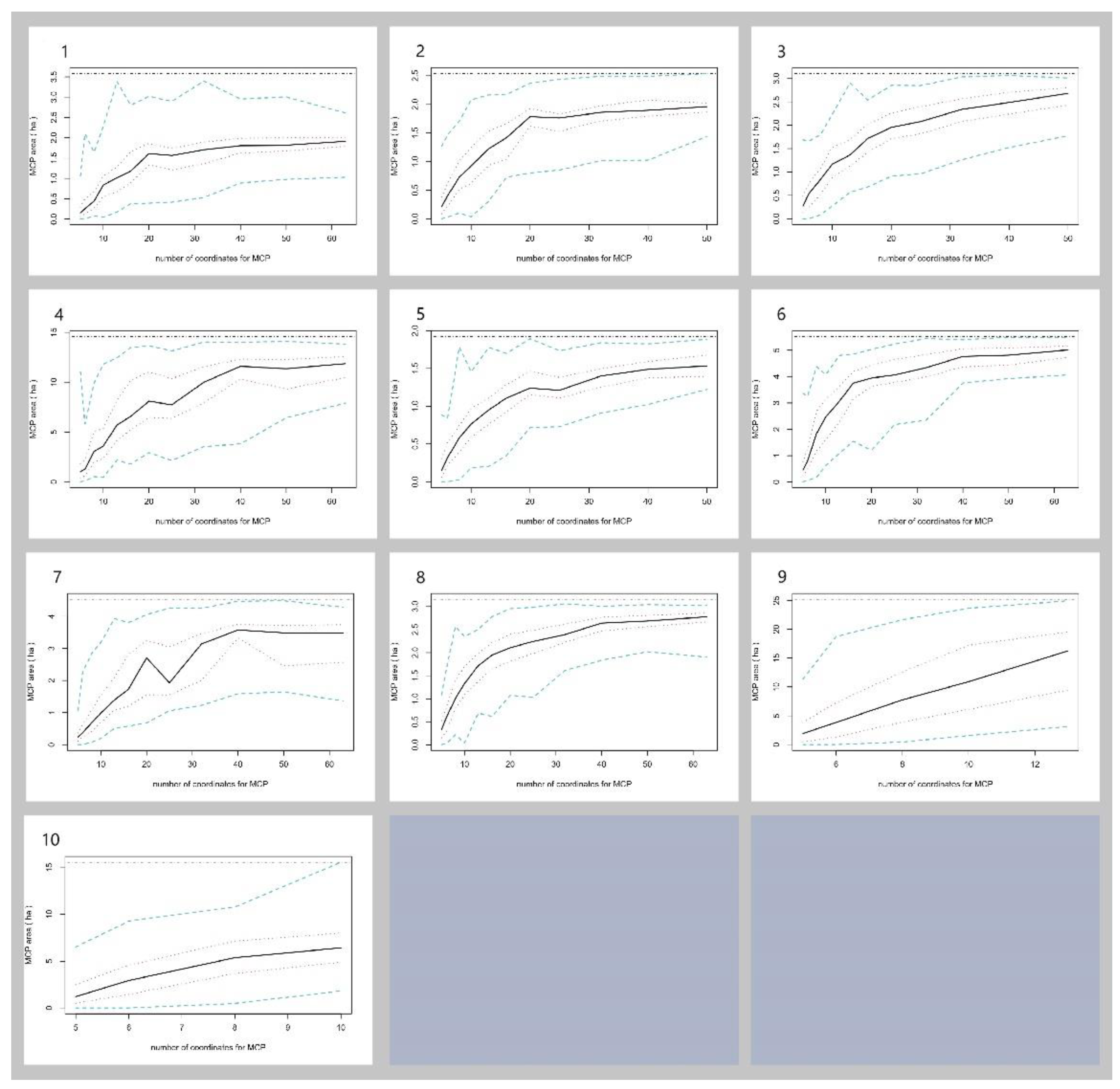
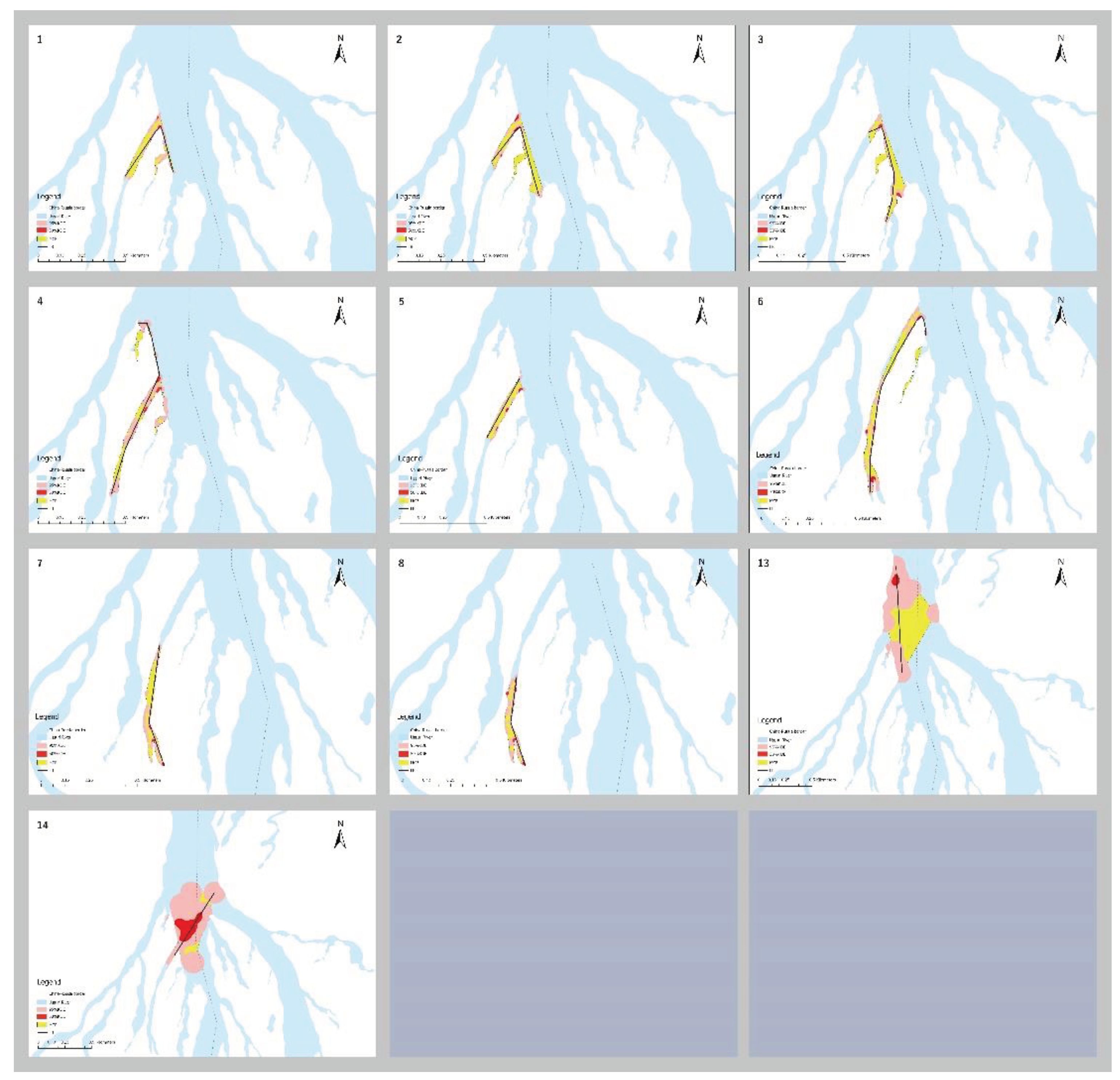
3.1. Home Range Analysis
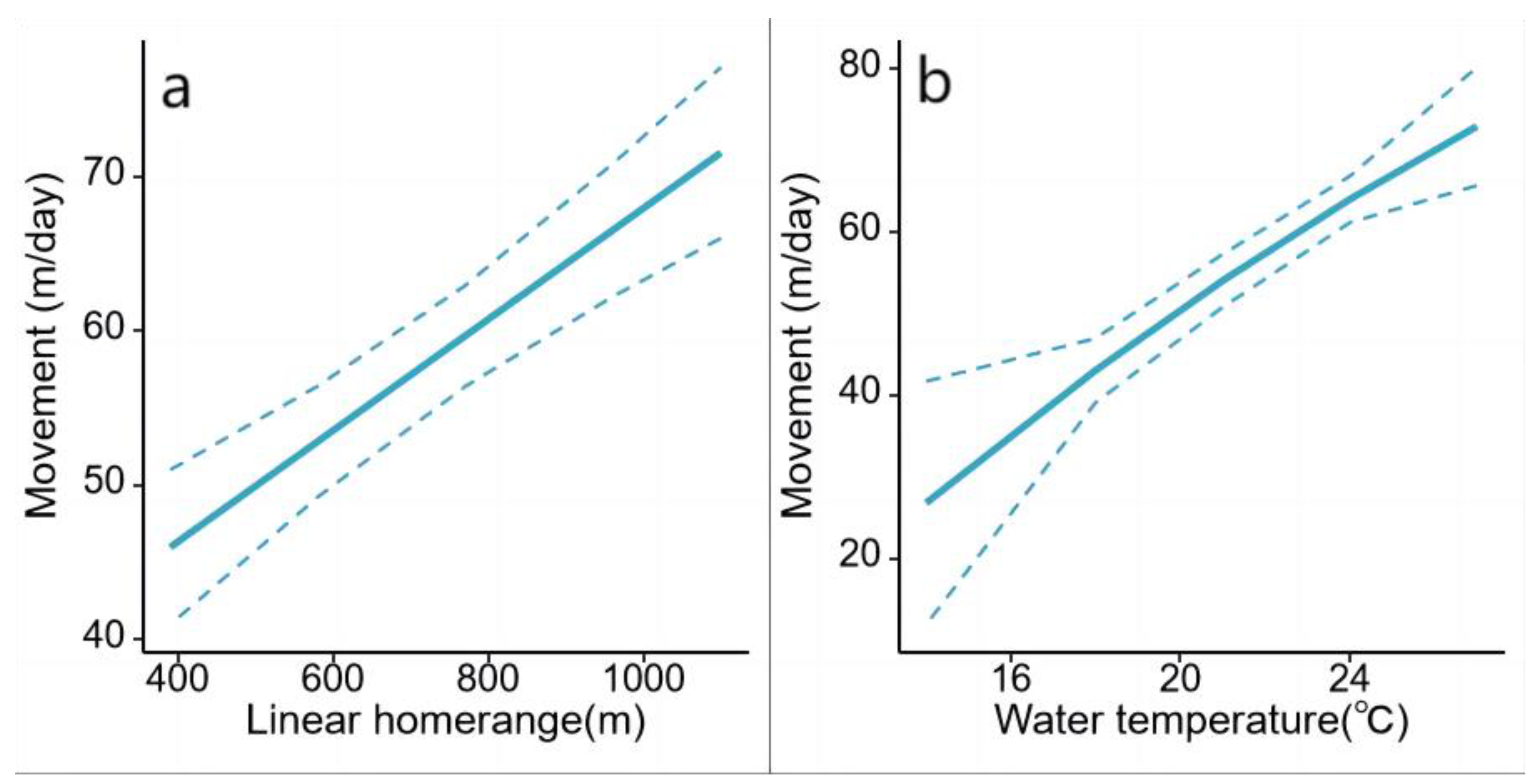
3.2. Movement Analysis
4. Discussion
4.1. Home Range
4.2. Movement
4.3. Conservation and Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Age group | Sex | PL | Body mass | Locations | Linear Range | Min Move | Max Move | Mean Move | Move SD | MCP | KDE 95% | KDE 50% |
Prop. Asy. |
End |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sub adult | Male | 13.8 | 560 | 68 | 615 | 14 | 137.4 | 60.25 | 28.77 | 1.79 | 0.86 | 0.09 | 1.01 | complete home range, released at the end |
| 2 | Sub adult | Male | 14 | 590 | 55 | 672 | 10.8 | 118.9 | 61.58 | 26.08 | 1.77 | 0.68 | 0.08 | 1.01 | complete home range caught by fish net and released |
| 3 | Sub adult | Male | 13 | 520 | 59 | 624 | 16.1 | 113.8 | 57.86 | 23.83 | 1.65 | 0.97 | 0.11 | 1.01 | complete home range, released at the end |
| 4 | Sub adult | Female | 13.6 | 580 | 67 | 1145 | 18.6 | 275.6 | 72.45 | 29.36 | 3.65 | 2.07 | 0.18 | 0.96 | complete home range, released at the end |
| 5 | Sub adult | Female | 14.3 | 617 | 62 | 388 | 10.8 | 89.9 | 48.12 | 19.64 | 1.30 | 0.66 | 0.07 | 1.01 | complete home range, released at the end |
| 6 | Sub adult | Female | 13.5 | 550 | 70 | 995 | 15.2 | 158.5 | 62.25 | 26.41 | 1.80 | 0.78 | 0.08 | 1.03 | complete home range, released at the end |
| 7 | Sub adult | Male | 14.2 | 615 | 75 | 632 | 9.2 | 133.7 | 49.77 | 22.58 | 2.71 | 1.32 | 0.11 | 0.92 | complete home range, released at the end |
| 8 | Sub adult | Female | 13.9 | 600 | 65 | 473 | 8.5 | 94.6 | 39.18 | 20.04 | 1.58 | 0.78 | 0.07 | 1.01 | complete home range, released at the end |
| 9 | Sub adult | Female | 14.6 | 680 | 29 | * | * | * | * | * | * | * | * | 0.58 | incomplete home range radio transmitter peeled off by fish net and released |
| 10 | Sub adult | Female | 14 | 600 | 18 | * | * | * | * | * | * | * | * | 0.68 | incomplete home range radio transmitter peeled off by fish net and released |
| 11 | Sub adult | Female | 13.7 | 578 | 30 | * | * | * | * | * | * | * | * | 0.70 | incomplete home range radio transmitter peeled off by fish net and escaped |
| 12 | Sub adult | Male | 14.5 | 620 | 32 | * | * | * | * | * | * | * | * | 0.69 | incomplete home range radio transmitter peeled off by unknown reason |
| 13 | Adult | Female | 16.8 | 1000 | 14 | * | 98.2 | 345.3 | 178.2 | * | * | * | 0.45 | incomplete home range signal stayed on Russia side | |
| 14 | Adult | Male | 17 | 1230 | 11 | * | 89.5 | 420.2 | 159.6 | * | * | * | 0.50 | incomplete home range signal disappeared after 11 locations | |
| 15 | Adult | Female | 22 | 2000 | 1 | * | * | * | * | * | * | * | * | * | signal disappeared the second day |
| 16 | Adult | Female | 26 | 2720 | 1 | * | * | * | * | * | * | * | * | * | signal disappeared the second day |
| 17 | Adult | Male | 19.5 | 1880 | 3 | * | * | * | * | * | * | * | * | * | signal disappeared the fourth day |
| 18 | Adult | Male | 24.5 | 2450 | 1 | * | * | * | * | * | * | * | * | * | signal disappeared the second day |
| 19 | Adult | Female | 29 | 2950 | 1 | * | * | * | * | * | * | * | * | * | signal disappeared the second day |
Appendix B
| Model Name | Structure |
|---|---|
| Global | 1+W+W2+LR+Sex+PL+W*LR+W*PL+W*Sex+1|id |
| Temp. and linear home range interaction | 1+W+W2+LR+W*LR+1|id |
| Temp., linear home range, and sex interaction | 1+W+W2+LR+Sex+W*LR+W*Sex+1|id |
| Temp., linear home range, and body size interaction | 1+W+W2+LR+PL+W*LR+W*PL+1|id |
| Temp., sex, and body size interaction | 1+W+W2+Sex+PL+W*Sex+W*PL+1|id |
| Temp. and linear home range | 1+W+W2+LR+1|id |
| Temp. and linear home range linear effect and interaction | 1+W+LR+W*LR+1|id |
| Temp. | 1+W+W2+1|id |
| Temp., sex, and body size | 1+W+W2+Sex+PL+1|id |
| Main linear effect | 1+W+LR+Sex+PL+1|id |
| Temp. and sex interaction | 1+W+W2+Sex+W*Sex+1|id |
| Temp. and body size interaction | 1+W+W2+PL+W*PL+1|id |
| Temp. and linear home range linear effect | 1+W+LR+1|id |
| Temp., sex, and body size linear effect | 1+W+Sex+PL+1|id |
| Temp. and sex | 1+W+W2+Sex+1|id |
| Temp. and body size | 1+W+W2+PL+1|id |
| Null | 1+1|id |
References
- Brandt, J.F. Observationes quaedam ad generis trionychum species duas novas spectantes. Bull. Acad. Imper. Sci. St. Petersbourg Cl. Phys.-Mathemat 1857, 16: 110-111.
- Bodie, J.R.; Semlitsch, R.D. Spatial and temporal use of floodplain habitats by lentic and lotic species of aquatic turtles. Oecologia 2000, 122: 138-146. [CrossRef]
- Blundell, G.M.; Maier, J.A.K.; Debevec, E.M. Linear home ranges: effects of smoothing, sample size, and autocorrelation on kernel estimates. Ecological monographs 2001, 71(3): 469-489.
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information—Theoretic Approach, 2nd ed.; Springer-Verlag: New York, NY, USA, 2002; pp. 1–488.
- Börger, L.; Dalziel, B.D.; Fryxell, J.M. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecology letters 2008, 11(6): 637-650. [CrossRef]
- Bates, M.M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 2015, 67(1): 1-48.
- Barton, B., 2019. K. MuMIn: Multi-Model Inference. R Package Version 1.42.1. Available online: https://CRAN.R-project.org/package=MuMIn.
- Baek, H.; Kim, P.; Kim, Y.C.; et al. The complete mitochondrial genome of the Amur soft-shelled turtle (Pelodiscus maackii Brandt, 1858), from South Korea. Mitochondrial DNA Part B 2022, 7(3): 498-500.
- Calenge, C. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model 2006, 197, 516–519. [CrossRef]
- Carrière, M.A. Movement patterns and habitat selection of common map turtles (Graptemys geographica) in St.Lawrence Islands National Park, Ontario, Canada. MSc Thesis, University of Ottawa, Ottawa, Canada,., 2007.
- Chang, M.H.; Song J.Y.; Koo, K.S. The Status of Distribution for Native Freshwater Turtles in Korea, with Remarks on Taxonomic Position. Korean J. Environ. Biol 2012, 30, 151-155.
- Dormann, C.F.; Elith, J.; Bacher, S.; et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36(1): 27-46.
- Duncan, M.B. Distributions of small nongame fishes in the lower Yellowstone River. The American Midland Naturalist 2016, 175(1): 1-23. [CrossRef]
- Ernst, C.H. Environmental temperatures and activities in wild spotted turtles, Clemmys guttata. Journal of Herpetology 1982: 112-120. [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada, 2nd ed., The Johns Hopkins University Pres:Baltimore, MD, USA, 2009; pp. 1–827.
- ESRI, 2022. ArcGis Pro 3.0.1. Environmental Systems Research Institute, Redlands, CA.
- Gibbons, J.W. Movement patterns among turtle populations: applicability to management of the desert tortoise. Herpetologica 1986: 104-113.
- Geffen, E.; Mendelssohn, H. Home range use and seasonal movements of the Egyptian tortoise (Testudo kleinmanni) in the northwestern Negev, Israel. Herpetologica 1988: 354-359.
- Gibbons, J.W. The slider turtle. In Life History and Ecology of the Slider Turtle; Gibbons, J.W., Ed., Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 3–18.
- Harris, S.; Cresswell, W. J.; Forde, P.G.; et al. Home-range analysis using radio-tracking data–a review of problems and techniques particularly as applied to the study of mammals. Mammal review 1990, 20(2-3): 97-123.
- Horne, J.S.; Garton, E.O. Likelihood cross-validation versus least squares cross-validation for choosing the smoothing parameter in kernel home-range analysis. The Journal of Wildlife Management 2006, 70(3): 641-648. [CrossRef]
- Hodder, K.H.; Masters, J.E.G.; Beaumont, W.R.C.; Gozlan, R.E.; Pinder, A.C.; Knight, C.M.; Kenward, R.E. Techniques for evaluating the spatial behaviour of river fish. Hydrobiologia 2007, 582, 257–269. [CrossRef]
- Horne, J.S.; Garton, E.O. 2015. Animal Space Use 1.3. Available online: http://www.webpages.uidaho.edu/population_ecology/animal_space_use.htm (accessed on 13 February 2015).
- Jennrich, R.I.; Turner, F.B. Measurement of non-circular home range. Journal of theoretical Biology 1969, 22(2): 227-237. [CrossRef]
- Jones, M.C.; Marron, J.S.; Sheather, S.J. A brief survey of bandwidth selection for density estimation. Journal of the American statistical association 1996, 91(433): 401-407.
- Kenward, R.E. A Manual for Wildlife Radio Tagging. London: Academic Press, 2001; pp. 1-311.
- .Kernohan, B.J.; Gitzen, R.A.; Millspaugh, J.J. Analysis of animal space use and movements. In Radio Tracking and Animal Populations; Millspaugh, J.J., Marzluff, J.M., Eds., Academic Press: San Diego, CA, USA, 2001; pp. 125–166.
- Koya, P.R.; Goshu, A.T. Solutions of rate-state equation describing biological growths. American Journal of Mathematics and Statistics 2013, 3(6): 305-311.
- Kranstauber, B.; Smolla, M.; Scharf, A. move: Visualizing and Analyzing Animal Track Data_. R package version 4.2.2, 2023.
- Lovich, J.E. Geographic variation in the seasonal activity cycle of spotted turtles, Clemmys guttata. Journal of Herpetology 1988, 22(4): 482-485. [CrossRef]
- Moll, D.; Moll, E.O. The Ecology, Exploitation and Conservation of River Turtles; Oxford University Pres:New York, NY, USA, 2004; pp. 1–393.
- Mueller, T.; Fagan, W.F. Search and navigation in dynamic environments–from individual behaviors to population distributions. Oikos 2008, 117(5): 654-664. [CrossRef]
- Mazerolle, M.J. Model selection and multimodel inference using the AICcmodavg package. R Vignette 2020, 2020: 22.
- Nilsen, E.B.; Pedersen, S.; Linnell, J.D.C. Can minimum convex polygon home ranges be used to draw biologically meaningful conclusions?. Ecological research 2008, 23: 635-639. [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in ecology and evolution 2013, 4(2): 133-142.
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; et al. Where is positional uncertainty a problem for species distribution modelling?. Ecography 2014, 37(2): 191-203. [CrossRef]
- Plummer, M.V.; Shirer, H.W. Movement Patterns in A River Population of the Softshell Turtle, Trionyx Muticus; Occasional Papers of the Museum of Natural History 1975.; The University of Kansas: Lawrence, KS, USA, 43, pp. 1–26.
- Plummer, M.V. Activity, habitat and population structure in the turtle, Trionyx muticus. Copeia 1977: 431-440. [CrossRef]
- Pluto, T.G.; Bellis, E.D. Seasonal and annual movements of riverine map turtles, Graptemys geographica. Journal of Herpetology 1988: 152-158. [CrossRef]
- Park, B.U.; Marron, J.S. Comparison of data-driven bandwidth selectors. Journal of the American Statistical Association 1990, 85(409): 66-72.
- Plummer, M.V.; Mills, N.E.; Allen, S.L. Activity, habitat, and movement patterns of softshell turtles (Trionyx spiniferus) in a small stream. Chelonian Conservation and Biology 1997, 2: 514-520.
- Powell, R.A. Animal home ranges and territories and home range estimators. Research techniques in animal ecology: controversies and consequences, 2000; 442: 65-110.
- Row, J.R.; Blouin-Demers, G. Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 2006, 2006(4): 797-802. [CrossRef]
- Ross, J.P.; Bluett, R.D.; Dreslik, M.J. Movement and home range of the smooth softshell turtle (Apalone mutica): Spatial ecology of a river specialist. Diversity 2019, 11(8): 124. [CrossRef]
- R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Sexton, O.J. Spatial and temporal movements of a population of the painted turtle, Chrysemys picta marginata (Agassiz). Ecological Monographs 1959, 29(2): 113-140. [CrossRef]
- Stejneger, L. Herpetology of Japan and adjacent territory. Government Printing Office, Washington DC, 1907. [CrossRef]
- Sutcliffe, A.G.; Poole, T.B. An experimental analysis of social interaction in the common marmoset (Callithrix jacchus jacchus). International Journal of Primatology 1984, 5: 591-607. [CrossRef]
- Swihart, R.K.; Slade, N.A. Influence of sampling interval on estimates of home-range size. The Journal of Wildlife Management 1985: 1019-1025. [CrossRef]
- Silverman, B.W. Density estimation for statistics and data analysis. Chapman & Hall, London, United Kingdom, 1986.
- Sain, S.R.; Baggerly, K.A.; Scott, D.W. Cross-validation of multivariate densities. Journal of the American Statistical Association 1994, 89(427): 807-817.
- Svanbäck R.; Bolnick, D.I. Intraspecific competition affects the strength of individual specialization: an optimal diet theory method. Evolutionary Ecology Research 2005, 7(7): 993-1012.
- Steury, T.D.; McCarthy, J.E.; Roth, T.C.; et al. Evaluation of Root-n Bandwidth Selectors for Kernel Density Estimation. The Journal of Wildlife Management 2010, 74(3): 539-548. [CrossRef]
- Suzuki D; Hikida T. Taxonomic status of the soft-shell turtle populations in Japan: a molecular approach. Current herpetology 2014, 33(2): 171-179. [CrossRef]
- Worton, B.J. A review of models of home range for animal movement. Ecological modelling 1987, 38(3-4): 277-298. [CrossRef]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70(1): 164-168. [CrossRef]
- Worton, B.J. Using Monte Carlo simulation to evaluate kernel-based home range estimators. The Journal of wildlife management 1995: 794-800. [CrossRef]
- Woolnough, D.A.; Downing, J.A.; Newton, T.J. Fish movement and habitat use depends on water body size and shape. Ecology of Freshwater Fish 2009, 18(1): 83-91. [CrossRef]

| MCP | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 | 88.89 | 55.84 | 20.53 | 84.04 | 38.5 | 0 | 0 | |
| 2 | 63.01 | 80.14 | 17.38 | 73.08 | 30.81 | 0 | 0 | |
| 3 | 48.42 | 31.97 | 15.96 | 17.4 | 15.67 | 0 | 0 | |
| 4 | 83.63 | 70.84 | 37.42 | 100 | 64.68 | 41.49 | 8.55 | |
| 5 | 38.12 | 45.89 | 10.77 | 13.18 | 29.86 | 0 | 0 | |
| 6 | 0 | 35.77 | 12.7 | 76.91 | 82.82 | 81.05 | 80.52 | |
| 7 | 0 | 0 | 0 | 6.67 | 0 | 56.49 | 90.47 | |
| 8 | 0 | 0 | 0 | 1.85 | 0 | 43.03 | 69.31 | |
| 95% KDE | ||||||||
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 | 64.75 | 54.78 | 36.92 | 68.86 | 29.85 | 0 | 0 | |
| 2 | 0.73 | 49.79 | 31.49 | 76.58 | 31.14 | 0 | 0 | |
| 3 | 0.56 | 44.96 | 20.07 | 30.41 | 22.45 | 0 | 0 | |
| 4 | 0.78 | 86.75 | 61.26 | 97 | 43.09 | 24.1 | 19.83 | |
| 5 | 0.65 | 79.9 | 31.87 | 34.4 | 22.8 | 0 | 0 | |
| 6 | 0.35 | 42.38 | 0 | 40.31 | 45.56 | 82.19 | 72.53 | |
| 7 | 0 | 0 | 0 | 9.21 | 0 | 54.55 | 86.94 | |
| 8 | 0 | 0 | 0 | 7.46 | 0 | 47.11 | 69.66 | |
| 50% KDE | ||||||||
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 | 51.3 | 43.31 | 28.63 | 35.97 | 24.32 | 0 | 0 | |
| 2 | 88.54 | 42.42 | 43.39 | 63.79 | 23.89 | 0 | 0 | |
| 3 | 80.45 | 47.29 | 23.3 | 28.81 | 22.76 | 0 | 0 | |
| 4 | 100 | 92.16 | 42.06 | 96.5 | 28.43 | 0 | 0 | |
| 5 | 77.09 | 76.88 | 41.43 | 39.47 | 23.45 | 0 | 0 | |
| 6 | 0 | 30.38 | 34.43 | 27.71 | 34.33 | 74.64 | 80.79 | |
| 7 | 0 | 0 | 0 | 0 | 0 | 33.17 | 45.04 | |
| 8 | 0 | 0 | 0 | 0 | 0 | 52.96 | 53.93 | |
| Rank | Model Name | K | -2LL | AICc | ∆AICc | wi | R2(m) | R2(c) |
|---|---|---|---|---|---|---|---|---|
| 1 | Global | 11 | -2284.17 | 4590.87 | 0 | 0.83 | 0.26 | 0.26 |
| 2 | Temp., linear home range, and sex interaction | 9 | -2288.68 | 4595.73 | 4.86 | 0.07 | 0.26 | 0.26 |
| 3 | Main linear effect | 7 | -2291.24 | 4596.71 | 5.84 | 0.04 | 0.25 | 0.26 |
| 4 | Temp. and linear home range interaction | 7 | -2292.5 | 4599.23 | 8.36 | 0.01 | 0.26 | 0.26 |
| 5 | Temp., linear home range, and body size interaction | 9 | -2290.54 | 4599.45 | 8.57 | 0.01 | 0.26 | 0.26 |
| 17 | Null | 3 | -2345.27 | 4696.58 | 105.71 | 0 | 0 | 0.15 |
| Effect | Estimate | SE | LCI | UCI |
|---|---|---|---|---|
| Intercept | 54.83 | 2.49 | 49.94 | 59.72 |
| Water Temp. | 12.6 | 1.92 | 8.84 | 16.37 |
| (Water Temp.)2 | -0.49 | 1.19 | -2.81 | 1.84 |
| Linear home range (LR) | 8.71 | 1.45 | 5.87 | 11.55 |
| Sex | 5.5 | 4.51 | -3.35 | 14.35 |
| Plastron Length (PL) | -2.35 | 1.97 | -6.22 | 1.51 |
| Water Temp. * LR | -1.73 | 1.28 | -4.24 | 0.78 |
| Water Temp. * PL | 2.8 | 2.05 | -1.21 | 6.82 |
| Water Temp. * Sex | -7.1 | 4.74 | -16.39 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




