Introduction
Modern scientific and therapeutic applications of arsenic trioxide (As
2O
3 or ATO) started in the 1990s with the treatment of acute promyelocytic leukemia (APL), demonstrating striking efficacy and a good safety profile [
1,
2]. Since early 2000, arsenic in its tri-oxidized form (As
2O
3), has been used as an anticancer agent for acute promyelocytic leukemia or APL [
3,
4] and numerous trials have been or are underway for various types of leukaemia and myelodysplastic syndromes. The transformation of APL from an often fatal to highly curable cancer with long-term survival exceeding 90% is one of the greatest and most inspiring proven successes in oncology [
5,
6]. GvHD is the most common complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT). It develops in around 40% of cases into acute GvHD (aGvHD) and in 20-50% of cases into the chronic form (cGvHD) [
7,
8].
Not all the mechanisms triggered by Arsenic are known yet. The mode of action of arsenic trioxide in APL is thought to be mediated via proteasome activation to lead to a rapid destruction of the fusion protein responsible for the abnormal lymphocyte cell differentiation [
9]. However, the exact mechanism of action of ATO in autoimmune diseases is different, since ATO has been shown to have a clear selective effect on activated immune cells through apoptosis induction coupled to down regulation of pro inflammatory cytokines [
10,
11]. ATO reduces B and T lymphocyte cytokines and chemokines production and decreases inflammation, modulates macrophage phenotype towards the non-inflammatory type, and decreases the number of activated dendritic cells. In addition, ATO is able to prevent the differentiation of cell types, such as fibroblasts into myofibroblasts, which produce collagen at the origin of fibrosis [
10,
14]. Thus, ATO combines immunomodulatory and antifibrotic activities, which are key properties for the treatment of cGvHD. From 2016 to 2020, we conducted a phase 2 clinical trial using ATO in chronic GvHD entitled ‘Treatment of Chronic Graft Versus Host Disease with Arsenic Trioxide (GvHD-ATO)’ (ClinicalTrials.gov ID NCT02966301 - GMED16-001) [
12]. The first results were originally published in July 2022 in a paper entitled “High Response Rate and Corticosteroid Sparing with ATO-Based First-Line Therapy in Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation” [
13]. These results demonstrated the convincing efficacy and safety of the medication (Trisenox
®/Arscimed
®) on top of CS (with or without cyclosporine A). We observed a high clinical response rate and correlated rapid CS sparing effect, in moderate to severe cGvHD following allo-hematopoietic stem cells transplantation (HSCT). We performed an additional analysis of the GMED16-001 Phase 2 clinical trial data aiming at determining the best schedule to treat cGvHD patients with ATO, taking into account the benefit-risk balance.
Method & Results
The prospective national multicenter single-arm open-label phase 2 study was conducted in 5 university hospital centers in France. ATO was started within 10 days of CS administration (1 mg/kg/day). Patients received 11 infusions of ATO per cycle of 4 weeks at a dose of 0.15 mg/kg per infusion. The patients received either 1 or 2 cycles, depending on the clinical response and assessment by the physician. Patients were evaluated at 6 weeks, 14 weeks, 6 months, 9 months, and 12 months after the start of ATO, using the cGvHD Activity Assessment Form [
14]. The primary endpoint was efficacy based on the overall response rate (ORR; complete response [CR] or partial response [PR]) at 6 months. Response rates are reported with 2-sided 95% exact confidence intervals (CIs). Twenty-one patients entered in this phase 2 study and received at least one ATO infusion (1 incomplete cycle for 1 patient, 1 complete cycle for 11 patients, and 2 complete cycles for 9 patients). ATO on top of prednisone and, for some patients, cyclosporine A (CsA), demonstrated high ORRs 75.0% [95% CI 50.9%; 91.3%] for patients with cGvHD following allo-HSCT (primary endpoint), with CR in 35% and PR in 40% of the patients in the FAS population (Full Analysis Set).
-
A.
Benefit/risk of a second ATO cycle
-
1-
Overall clinical responses
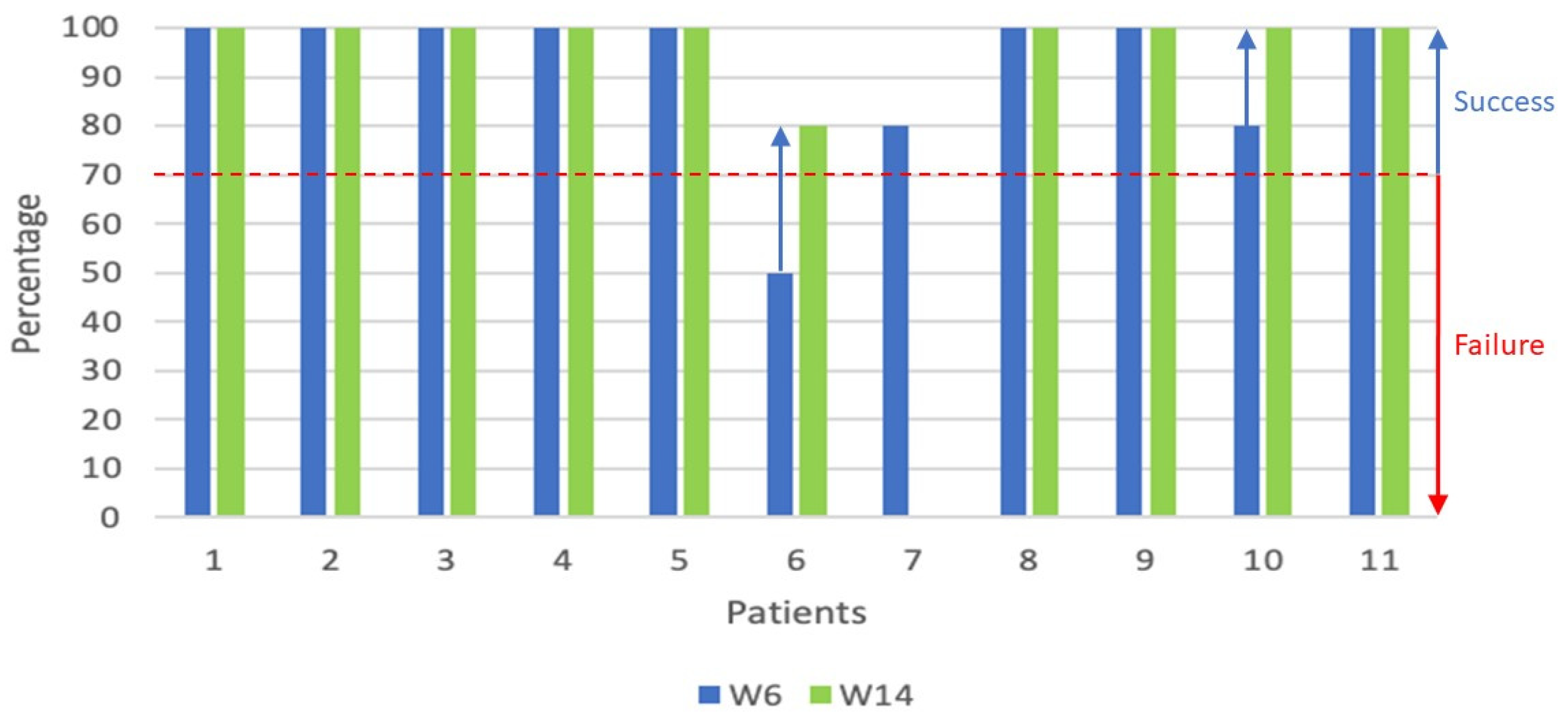
We focused on the full set of clinical data collected during the phase 2 trial on the evolution of the overall response between W6 and W14 for the 2 populations of patients who received either 1 or 2 cycles of ATO.
A graphical representation was used (
Figure 1 and
Figure 2) to visualize the evolution of the overall clinical responses as reported by the physicians [
14]. For visual/graphical representation purposes, the following transposition was done: Complete Response (CR) = 100%, Partial Response (PR) = 80%, Mixed Response = 50%, Progressive Disease (PD) = 10%, no assessment = 0%.
For all 20 patients treated with at least 1 complete cycle of ATO, the overall response rate at 6 months was 75.0% with a CR of 35% and a PR of 40% in the Full Analysis Set (FAS) population. For the population of 11 patients who received a single cycle of ATO (
Figure 1), the overall response rates (including partial – 80% and complete responses - 100%) at respectively W6 and W14 were 91% (10/11 patients) and 100% (10/10 patients – one patient not evaluated).
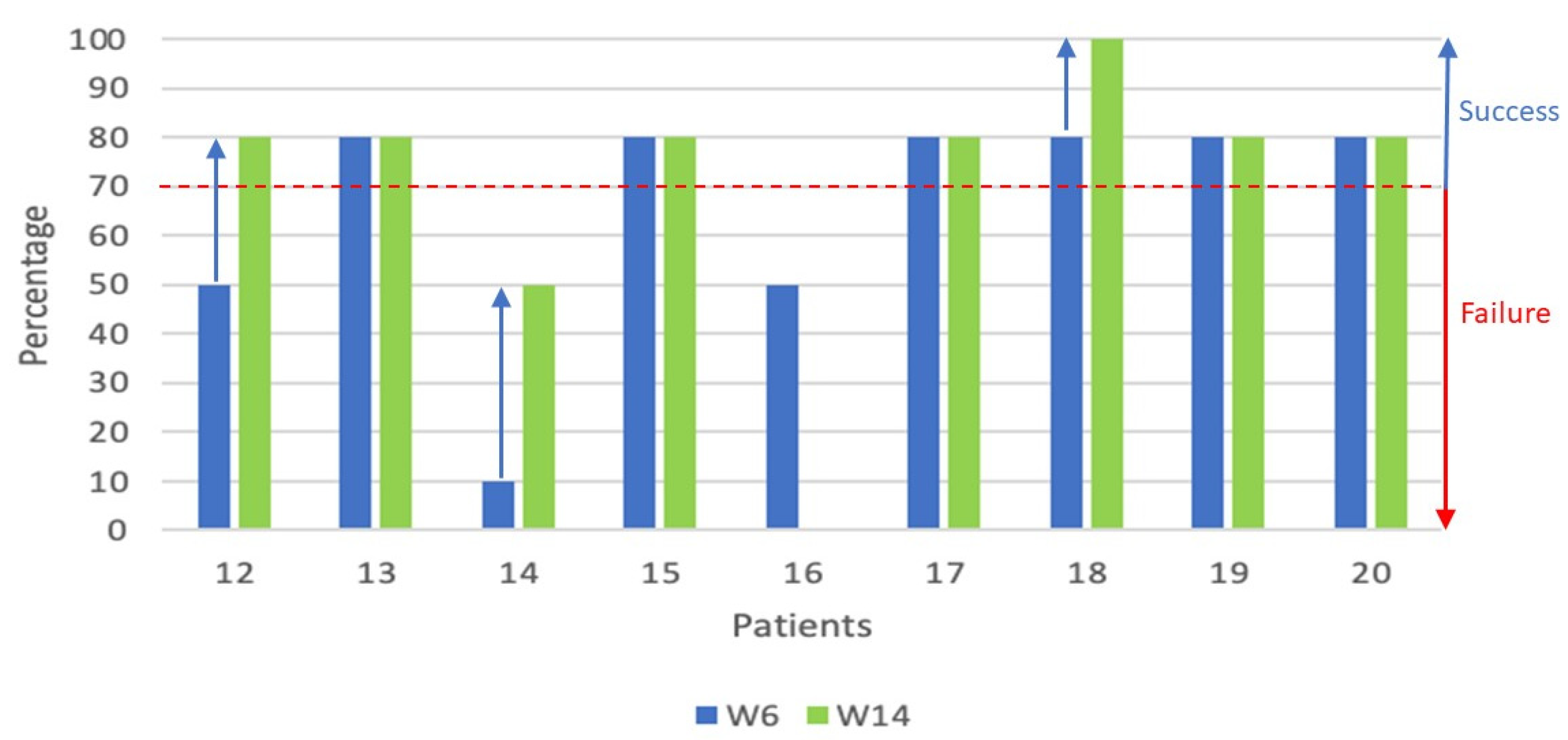
For the population of 9 patients who received 2 cycles of ATO (
Figure 2), the overall response rate at W6 and W14 was 66% (6/9 patients) and 75% (6/8 patients - one patient not assessed), respectively, as assessed by the physicians. We found that in the '2 cycles' population, 3 patients had an improvement in the clinical signs of their cGvHD after the second cycle of ATO, representing one third of patients in this group. In fact, one patient in the 'mixed response' group achieved a 'partial response' (efficacy success), one patient with disease progression achieved a mixed response and one patient in the partial response group no longer had clinical signs of cGvHD (complete response). The physicians reported an overall positive evolution of cGvHD over the first 14 weeks, in particular for three additional patients in ‘2 cycles’ population.
-
2-
Maintained response over time / introduction second line systemic therapy
In our study, a second line therapy is defined as “secondary systemic therapy that includes any intervention intended to control chronic GvHD through an immunosuppressive effect by oral or parenteral administration of any systemic medication not originally given under auspices of this protocol”. Only 3 types of second line systemic therapies were started during the year of patient follow-up: extracorporeal photopheresis, Rituximab and Ruxolitinib.
To continue the comparison between patients who received 1 or 2 cycles, it is important to follow the response to treatment over time and the introduction of second-line therapy. In the ‘1 cycle’ population, 5 in 11 patients received second line therapies (
Table 1); one patient 5 months after the first infusion of ATO (Rituximab), 2 patients at 6 months (photopheresis) and 2 other patients at 9 months (Rituximab) after baseline. In all 3 Rituximab treated patients, the start dose was 5 mg, then increased to 10 mg or 15 mg. These second line therapies, implemented at different time points, were ongoing at the end of patient follow-up. Apart from the first of the five patients (Patient 6), with disease progression and the start of a second-line treatment before month 6, the response at month 6 was still partial or complete in the other 4 patients (Patients: 1, 2, 4 and 5). The introduction of a second treatment line in these 4 patients was done because the treatment response was not maintained till the end of the follow-up (12 months) (
Table 1).
It is interesting to note that patient 1, for whom photopheresis started from M6 onwards, is a patient who received an incomplete cycle of ATO due to an ATO-related SAE (
Table 3). The administration schedule was: 4 infusions at 0.15 mg/kg/d, a temporary discontinuation during 10 days and 6 infusions at a lower dose of 0.075 mg/kg/d with a total of 10 infusions (
Table 3).
In the 2 ‘cycles’ population, only 1 in 9 patients received a second line therapy (
Table 2). In this one patient photopheresis was started at 4 months after the first infusion of ATO, Rituximab was added at 5 months and given for 1 month and then finally Ruxolitinib (5mg) was added to the ongoing photopheresis at 11 months. The two second line therapies, photopheresis and Ruxolitinib, were ongoing at the end of the study follow-up (1 year after the first ATO infusion). This patient had been treated with cyclosporine since the graft and throughout the cGvHD study. The corticotherapy, started at inclusion in the study, was gradually reduced, then increased again at 6 months and continued, with some additional changes, till study-end. This patient responded very poorly to the various treatments.
-
3-
Safety (SAE/AE ATO-related)
During the entire follow-up of 12 months, a total of 197 Adverse Events including 22 Serious Adverse Events (AEs / SAEs) were reported in 21 patients having received at least one infusion of ATO (safety population). Thirteen of these AEs, reported in 7 patients were ATO-related including 2 SAEs in 2 patients. To assess the tolerability and safety of a second treatment cycle of ATO, a descriptive analysis was performed to compare the events reported during the 2 treatment cycles separately: 2 ATO-related SAEs and 10 ATO-related AEs were reported during the first cycle of any of the 21 patients having received one cycle (
Table 3 and
Table 4), only 1 ATO-related AE occurred during the second cycle.
During the first ATO cycle, 3 ATO related events were reported in 3 patients (2 SAEs - 1 AE) and resulted in a change in the treatment of the study product (
Table 3). Patient 21; hepatotoxicity detected after 2 infusions, resolved after 7 days without sequelae; the event led to the patient's premature withdrawal from the study. Patient 1: hepatotoxicity detected after 4 infusions, resolved after 10 days without sequelae; after normalization of liver values, this patient received 6 infusions of ATO at 0.075 mg/kg/d (i.e. a dose reduced by 50%). Patient 8; QTc prolongation (moderate grade) reported after 2 infusions; the treatment cycle was only discontinued for 2 days, and the patient received all 9 infusions at 0.15 mg/kg/d without other discontinuation or modification. The latter 2 patients (1 and 8) received only 1 cycle of ATO, because they had a complete response at W6.
Table 3.
ATO-related SAEs/AEs reported in the safety population (22 patients having received at least one ATO dose) during the first cycle of treatment, leading to a temporary or definitive discontinuation of the study treatment.
Table 3.
ATO-related SAEs/AEs reported in the safety population (22 patients having received at least one ATO dose) during the first cycle of treatment, leading to a temporary or definitive discontinuation of the study treatment.
Patient study number |
Adverse Event (AE) |
Severity |
Duration of AE |
Number of ATO infusions before AE/SAE |
Action Taken with
Study Treatment |
| 21 |
Hepatotoxicity |
Severe |
7 days |
2 |
Definitive discontinuation |
| 1 |
Hepatotoxicity |
Severe |
10 days |
4 |
Temporary discontinuation (10 days) |
| 8 |
QTc prolongation |
Moderate |
2 days |
2 |
Temporary discontinuation (1day) |
Nine non-serious ATO-related AEs were reported in 3 patients during the first cycle (
Table 4). Patient 13: nausea, vomiting, diarrhea, abdominal pain and anaemia. Patient 6: hypokalaemia. Patient 4: thrombopenia and muscular pain. The AEs resolved without sequelae and without requiring temporary or permanent cessation of ATO treatment. Patient 13 received a second cycle of ATO, without ATO-related AE. Patients 6 and 4 received only one cycle of ATO, because at W6 they had a mixed response and a complete response respectively.
Table 4.
ATO-related AEs reported in the safety population (22 patients having received at least one ATO dose) during the first cycle of treatment, none of these events led to a change of the study treatment. The AEs are listed in order of appearance based on the number of ATO infusions administered before the start of the event.
Table 4.
ATO-related AEs reported in the safety population (22 patients having received at least one ATO dose) during the first cycle of treatment, none of these events led to a change of the study treatment. The AEs are listed in order of appearance based on the number of ATO infusions administered before the start of the event.
Patient study number |
Adverse Event (AE) |
Severity |
Number of ATO infusions before AE |
13
|
Vomiting |
Moderate |
1 |
| Diarrhea |
Moderate |
2 |
| Abdominal pain |
Moderate |
3 |
| Anemia |
Moderate |
3 |
| Diarrhea |
Moderate |
10 |
| Nausea |
Moderate |
10 |
| 6 |
Hypokalemia |
Mild |
8 |
4
|
Thrombopenia |
Moderate |
8 |
| Muscular pain |
Mild |
9 |
During the second cycle of ATO treatment, only one ATO-related AE was reported after two infusions (hand oedema), which resolved without sequelae after one day and did not require a change in study treatment. The three ATO-related SAEs/AEs that affected the course of ATO treatment were all observed during the first cycle (22 patients). This allows us to conclude that the implementation of a second cycle in cGvHD patients is well tolerated and safe. Noteworthy is that all ATO-related SAEs/AEs are described in the Summary of Product characteristics (SmPC) of Trisenox®.
-
B.
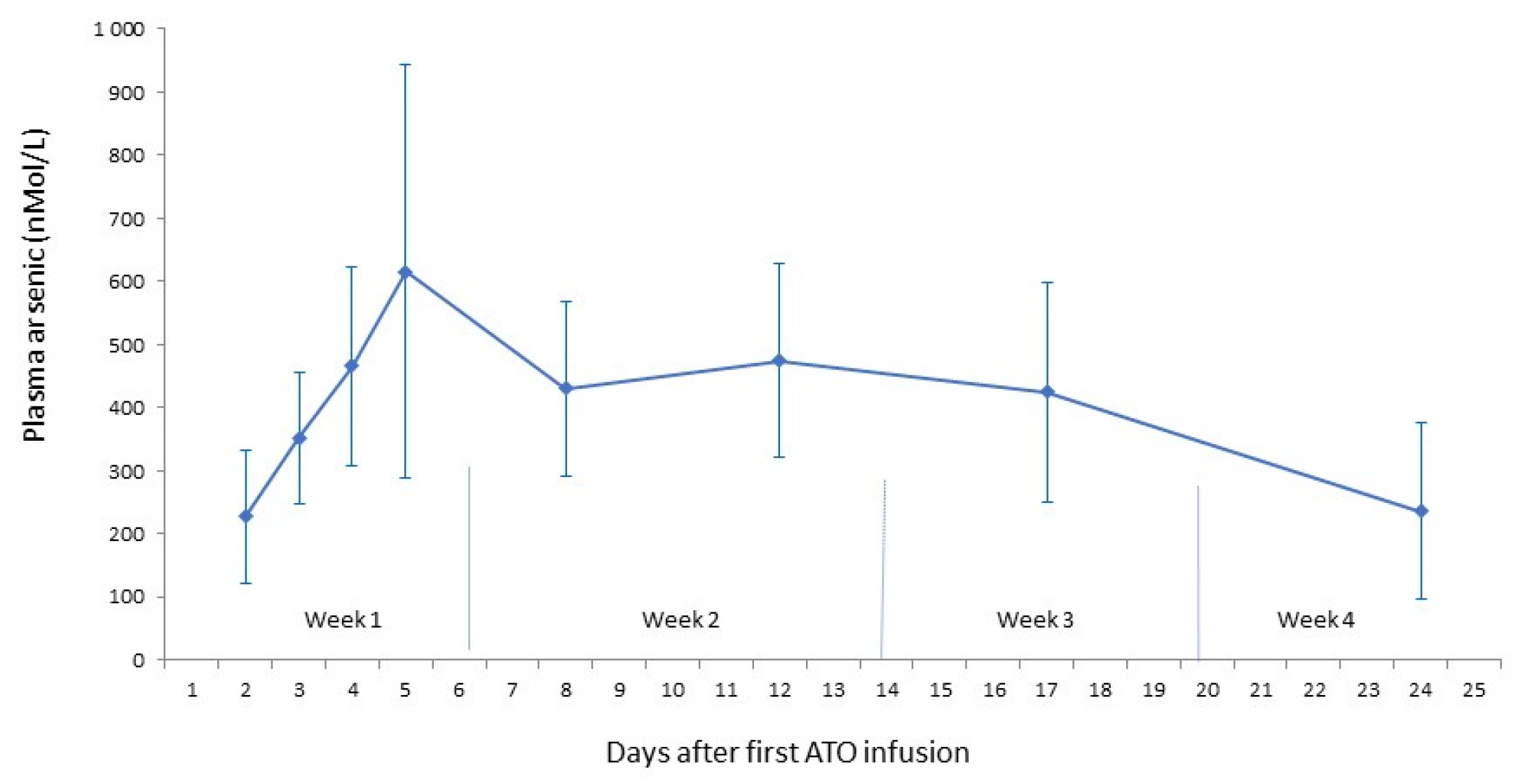
Residual arsenic plasma concentrations
In this phase 2 study, the administration schedule was 5 infusions in week 1, 3 in week 2, 2 in week 3 and 1 in week 4. Although the results were encouraging with respect to effectiveness while observing an increase in arsenic plasma concentrations until the fifth ATO infusion, the treatment schedule did not lead to a plateau phase. (
Figure 3) [
15].
This observation encourages us to introduce a treatment schedule that will expectedly lead to a plateau phase of the arsenic plasma concentrations, by introducing 5 administrations per week during each of the 4 weeks in the treatment cycle, similar to the treatment schedule that is used for the consolidation cycle in patients with Acute Promyelocytic Leukemia.
Discussion
ATO has been approved by FDA in 2000 and EMA in 2002 in the indication of Acute Promyelocytic Leukemia. ATO (without ATRA and/or chemotherapy) has made APL the most curable blood cancer. The tolerability and efficacy in these patients has been well documented [
1,
5,
6].
Induction treatment schedule consists of intravenous ATO at a dose of 0.15 mg/kg/day, given daily until complete remission is achieved. If complete remission has not occurred by day 60, dosing must be discontinued. This is followed by a consolidation schedule, at an unchanged daily dose, 5 days per week for 4 weeks on and 4 weeks off, for a total of 4 cycles, with a therapy-free interval of 3 – 6 weeks. APL patients treated by ATO showed persistent, event free survival (EFS) at a rate of 96.6% in the ATO group versus 77.4% in the standard of care group, results at 72 months [
16,
17]. In these APL therapy schedules, the total number of ATO doses per patient ranges from 60 to 140 ATO administrations (at 0.15 mg/kg/d). These two APL treatment schedules led to a plateau of the residual arsenic plasma concentrations after two weeks ATO treatment [
18].
In the currently discussed Phase II study, with 5:3:2:1 infusions over the 4-weeks in each cycle, one to two cycles per patient, we demonstrated promising results with respect to safety and efficacy (75% overall response rate), even though patients only received 11 (one cycle) or 22 (two cycles) ATO administrations (at 0.15 mg/kg/d). This treatment schedule did not lead to a plateau phase in the residual arsenic plasma concentrations and at the same time, we observed a loss of efficacy over the course of the 12-month follow-up period in certain patients. Therefore, we hypothesize that a treatment schedule of 5 administrations/week for 4 weeks leading to a plateau phase, with an induction and consolidation cycle, as in APL, should be applied to optimize and consolidate ATO treatment results in cGvHD. With these two four-week cycles, separated by an equally long therapy-free interval, and a total of 40 doses, we will remain well below the 60 to 140 ATO administrations in APL treatment.