Submitted:
11 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
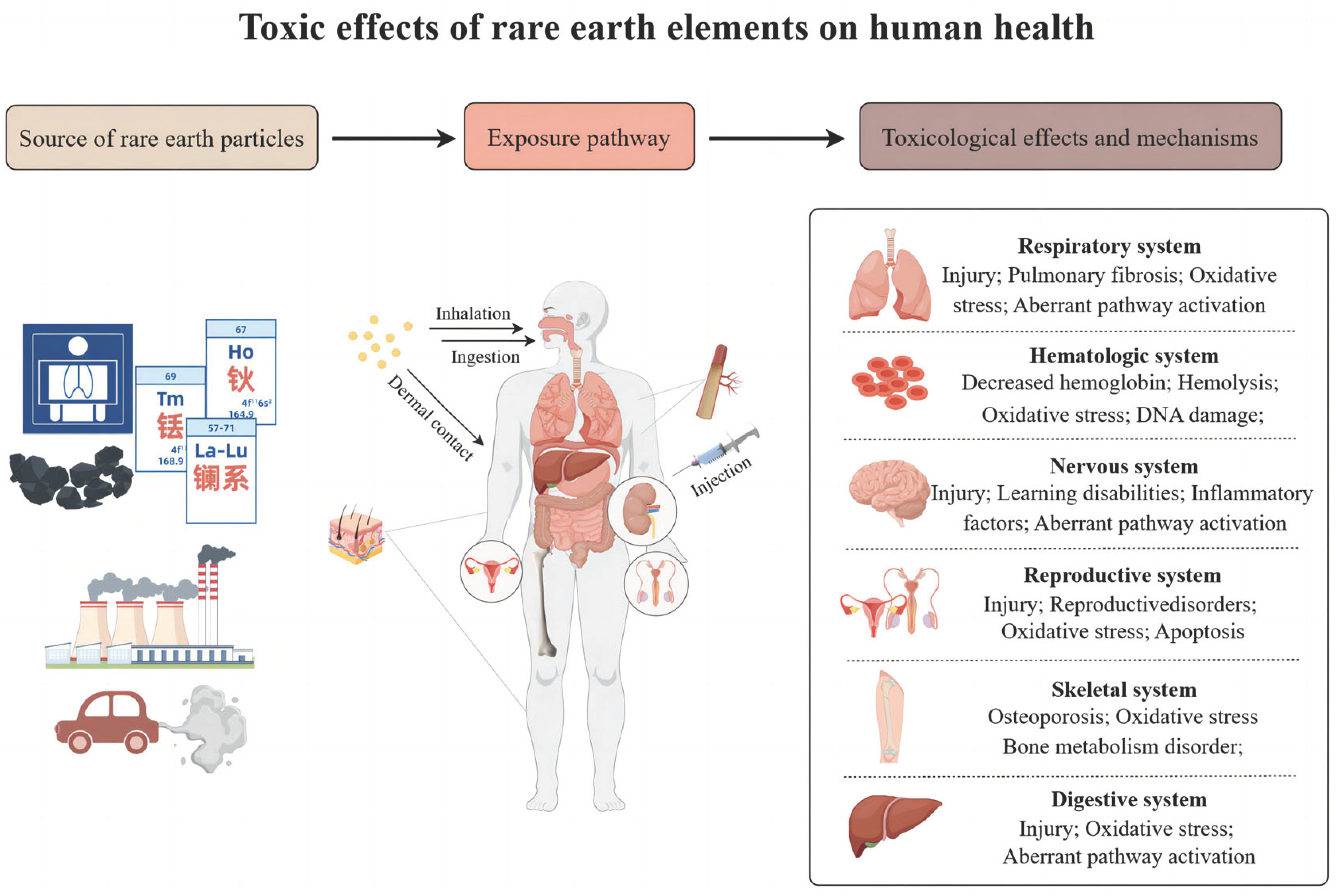
1. Introduction
2. Rare earth Exposure
3. Rare earth Toxicity
3.1. Respiratory System
| Element | Section Studied | Toxicity Outcome | Reference | |
|---|---|---|---|---|
| Respiratory system | Y | Endotracheal | Dyspnea and pulmonary edema, Pleural effusions | [14] |
| Ce | Environmental exposure, Skin contact | Extrapulmonary translocation, Interstitial lung disease, Pulmonary fibrosis, Pneumoconiosis, Cytotoxicity |
[29] [33] |
|
| Dy | Endotracheal instillation | Lung injury, Oxidative stress, Inflammatory response | [38] | |
| La | Environmental exposure | Phosphate deposition, Pulmonary fibrosis | [30] | |
| Nd | Occupational exposure, Environmental exposure | Cytotoxicity, Genotoxicity, Lung cancer | [35] | |
| Sm | Endotracheal | Lung injury, Inflammatory response, Pulmonary fibro | [57] | |
| Th | Environmental exposure, Skin contact | Dyspnea, Pneumoconiosis, Lung cancer | [62] | |
| Nervous system | La | Environmental exposure, Skin contact, Food chain | Learning and memory impairment, Decreased spatial discrimination, Cytotoxicity, Memory disorders | [47] [49] |
| Nd | Environmental exposure, Food chain | Fetal neural tube defects | [44] | |
| Gd | Iatrogenic exposure | Deposits in the brain, Brain damage | [42] | |
| Cardiovascular system | La | Occupational exposure Food chain |
Deposition in blood vessels | [57] |
| Nd | Environmental exposure, Skin contact, Food chain | Abnormal cardiovascular and cerebrovascular development, DNA damage, Cytotoxicity |
[58] | |
| Ce | Environmental exposure, Food chain |
The hemoglobin level is reduced, Anemia | [53] | |
| Gd | Endotracheal instillation | Cytotoxicity, Hematopoietic destruction | [56] | |
| Reproductive system | Ce | Environmental exposure, Oral administration | Oxidative stress, Placental dysfunction, Fetal abortion, Growth restriction |
[64] |
| Gd | Iatrogenic exposure | Inflammatory or invasive skin diseases, Stillbirth, Neonatal death |
[67] | |
| Skeleton | Nd | Occupational exposure, Environmental exposure | Disorders of bone metabolism, Decreased bone mineral density |
[72] |
| La | Environmental exposure, Food chain | Abnormal metabolism of calcium and phosphorus, Decreased bone mineral density |
[73] | |
| Gd | Iatrogenic exposure | Bone deposits, Osteoporosis | [70] | |
| Y | Iatrogenic exposure | Bone deposits | [71] |
3.2. Nervous System
3.3. Cardiovascular System
3.4. Reproductive System
3.5. Other Systems
4. Toxicity Mechanisms
5. Conclusions
| Type | Sample | REE Exposure | Toxicity | Reference | |
|---|---|---|---|---|---|
| Genetic | In vivo | C57-ras | 12.5, 25, and 50 mg/kg lanthanum nitrate for 180 d | The rare earth deposition causes direct damage | [39] |
| In vitro | SH-SY5Y | 10, 25, 50, and 100 µg/mL Gd2O3 for 24 and 48 h | Apoptosis is regulated by bcl-2/bax protein expression | [83] | |
| In vivo | Sprague-Dawley rat | 1.5 mg/kg body weight Indium chloride for 8 weeks | Oxidative stress, Chromatin DNA damage | [81] | |
| In vivo | Rat | 1mg / kg CeO2 for 6 d | Oxidative stress, Inflammation, DNA damage | [96] | |
| Epigenetic | In vitro | 16HBE | 10 μg/ml NPs-Nd2O3 for 48 h | Promote NF-κ B activation and promote cellular inflammation by negatively regulating adiponectin receptor 1 expression | [89] |
| In vitro | 16HBE | 0, 5, 10, 20, 40 and 80 μg/ml Nd2O3 for 6, 12, 24, 48 and 72 h | circ_009773 regulates DNA damage | [88] | |
| In vitro | Human fibroblast cell | 0.05 to 1.6 mg/mL of Tb-MOF for 48 h | Altered gene methylation, Induced genetic damage | [91] | |
| Signaling pathways | In vivo | Rat | 0, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL Nd2O3 for 24h | Activating the NF-κ B and caspase-3 signaling pathways, promoting the synthesis and release of inflammatory chemokine | [97] |
| In vivo | Rat | 0, 1mg / kg CeO2 nanoparticles for 6 d | Activation of oxidative stress, Nrf2 signaling pathways | [96] | |
| In vivo | C57BL/6J mice | Long-term exposure to cerium nanoparticles | Activation of NF-κ B signaling pathway can increase the cytotoxic activity of immune cells | [98] |
6. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner A, Scott JW, Green LA. Rare earth elements in plastics. Sci Total Environ. 2021 Jun 20;774:145405. [CrossRef]
- Grosjean N, Le Jean M, Armengaud J, Schikora A, Chalot M, Gross EM, Blaudez D. Combined omics approaches reveal distinct responses between light and heavy rare earth elements in Saccharomyces cerevisiae. J Hazard Mater. 2022 Mar 5;425:127830. [CrossRef]
- Han G, Liu M, Li X, Zhang Q. Sources and geochemical behaviors of rare earth elements in suspended particulate matter in a wet-dry tropical river. Environ Res. 2023 Feb 1;218:115044. [CrossRef]
- Gwenzi W, Mangori L, Danha C, Chaukura N, Dunjana N, Sanganyado E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci Total Environ. 2018 Sep 15;636:299-313. [CrossRef]
- Dai L, Ge J, Wang L, Wan X, Guo G, Liang T, Bolan N, Rennert T, Rinklebe J. Hair-biomonitoring assessment of rare-earth-element exposure in residents of the largest rare-earth mining and smelting area of China. Environ Int. 2023 Sep;179:108177. [CrossRef]
- Brouziotis AA, Giarra A, Libralato G, Pagano G, Guida M and Trifuoggi M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci.2022 Sep 10:948041. [CrossRef]
- Squadrone S, Brizio P, Stella C, Mantia M, Battuello M, Nurra N, Sartor RM, Orusa R, Robetto S, Brusa F, Mogliotti P, Garrone A, Abete MC. Rare earth elements in marine and terrestrial matrices of Northwestern Italy: Implications for food safety and human health. Sci Total Environ. 2019 Apr 10;660:1383-1391. [CrossRef]
- Li X, Chen Z, Chen Z, Zhang Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere. 2013 Oct;93(6):1240-6. [CrossRef]
- Liu C, Liu WS, van der Ent A, Morel JL, Zheng HX, Wang GB, Tang YT, Qiu RL. Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolacca americana in response to soil properties. Chemosphere. 2021 Nov;282:131096. [CrossRef]
- Mitsumori LM, Bhargava P, Essig M, Maki JH. Magnetic resonance imaging using gadolinium-based contrast agents. Top Magn Reson Imaging. 2014 Feb;23(1):51-69. [CrossRef]
- Cashion W, Weisbord SD. Radiographic Contrast Media and the Kidney. Clin J Am Soc Nephrol. 2022 Aug;17(8):1234-1242. [CrossRef]
- Pagano G, Thomas PJ, Di Nunzio A, Trifuoggi M. Human exposures to rare earth elements: Present knowledge and research prospects. Environ Res. 2019 Apr;171:493-500. [CrossRef]
- Rim KT, Koo KH, Park JS. Toxicological evaluations of rare earths and their health impacts to workers: a literature review. Saf Health Work. 2013 Mar;4(1):12-26. [CrossRef]
- Shin SH, Kim HO, Rim KT. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf Health Work. 2019 Dec;10(4):409-419. [CrossRef]
- Gerhardsson L, Wester PO, Nordberg GF, Brune D. Chromium, cobalt and lanthanum in lung, liver and kidney tissue from deceased smelter workers. Sci Total Environ. 1984 Aug 1;37(2-3):233-46. [CrossRef]
- Waring PM, Watling RJ. Rare earth deposits in a deceased movie projectionist. A new case of rare earth pneumoconiosis? Med J Aust. 1990 Dec 3-17;153(11-12):726-30. [CrossRef]
- Wang L, Liang T, Zhang Q, Li K. Rare earth element components in atmospheric particulates in the Bayan Obo mine region. Environ Res. 2014 May;131:64-70. [CrossRef]
- Wang L, Zhong B, Liang T, Xing B, Zhu Y. Atmospheric thorium pollution and inhalation exposure in the largest rare earth mining and smelting area in China. Sci Total Environ. 2016 Dec 1;572:1-8. [CrossRef]
- Li K, Liang T, Wang L, Tian S. Inhalation exposure and potential health risk estimation of lanthanides elements in PM2.5 associated with rare earth mining areas: a case of Baotou city, northern China. Environ Geochem Health. 2018 Dec;40(6):2795-2805. [CrossRef]
- Qiu F, Zhang H, Cui Y, Zhang L, Zhou W, Huang M, Xia W, Xu S, Li Y. Associations of maternal urinary rare earth elements individually and in mixtures with neonatal size at birth. Environ Pollut. 2024 Feb 15;343:123163. [CrossRef]
- Fang X, Peng B, Guo X, Wu S, Xie S, Wu J, Yang X, Chen H, Dai Y. Distribution, source and contamination of rare earth elements in sediments from lower reaches of the Xiangjiang River, China. Environ Pollut. 2023 Nov 1;336:122384. [CrossRef]
- Yan Y, Chi HF, Liu JR, Hu GR, Yu RL, Huang HB, Lin CQ. Provenance and bioaccessibility of rare earth elements in atmospheric particles in areas impacted by the optoelectronic industry. Environ Pollut. 2020 Aug;263(Pt A):114349. [CrossRef]
- Liu WS, Guo MN, Liu C, Yuan M, Chen XT, Huot H, Zhao CM, Tang YT, Morel JL, Qiu RL. Water, sediment and agricultural soil contamination from an ion-adsorption rare earth mining area. Chemosphere. 2019 Feb;216:75-83. [CrossRef]
- Bakhshalizadeh S, Liyafoyi AR, Mora-Medina R, Ayala-Soldado N. Bioaccumulation of rare earth elements and trace elements in different tissues of the golden grey mullet (Chelon auratus) in the southern Caspian Sea. Environ Geochem Health. 2023 Aug;45(8):6533-6542. [CrossRef]
- Mauro M, Crosera M, Monai M, Montini T, Fornasiero P, Bovenzi M, Adami G, Turco G, Filon FL. Cerium Oxide Nanoparticles Absorption through Intact and Damaged Human Skin. Molecules. 2019 Oct 18;24(20):3759. [CrossRef]
- Tong SL, Zhu WZ, Gao ZH, Meng YX, Peng RL, Lu GC. Distribution characteristics of rare earth elements in children's scalp hair from a rare earths mining area in southern China. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39(9):2517-32. [CrossRef]
- He X, Zhang H, Ma Y, Bai W, Zhang Z, Lu K, Ding Y, Zhao Y, Chai Z. Lung deposition and extrapulmonary translocation of nano-ceria after intratracheal instillation. Nanotechnology. 2010 Jul 16;21(28):285103. [CrossRef]
- Nemery, B. Metal toxicity and the respiratory tract. Eur Respir J. 1990 Feb;3(2):202-19.
- Gong H, Jr. Uncommon causes of occupational interstitial lung diseases. Curr Opin Pulm Med. 1996 Sep;2(5):405-11. [CrossRef]
- Censi P, Tamburo E, Speziale S, Zuddas P, Randazzo LA, Punturo R, Cuttitta A, Aricò P. Yttrium and lanthanides in human lung fluids, probing the exposure to atmospheric fallout. J Hazard Mater. 2011 Feb 28;186(2-3):1103-10. [CrossRef]
- Snow SJ, McGee J, Miller DB, Bass V, Schladweiler MC, Thomas RF, Krantz T, King C, Ledbetter AD, Richards J, Weinstein JP, Conner T, Willis R, Linak WP, Nash D, Wood CE, Elmore SA, Morrison JP, Johnson CL, Gilmour MI, Kodavanti UP. Inhaled diesel emissions generated with cerium oxide nanoparticle fuel additive induce adverse pulmonary and systemic effects. Toxicol Sci. 2014 Dec;142(2):403-17. [CrossRef]
- Keller J, Wohlleben W, Ma-Hock L, Strauss V, Gröters S, Küttler K, Wiench K, Herden C, Oberdörster G, van Ravenzwaay B, Landsiedel R. Time course of lung retention and toxicity of inhaled particles: short-term exposure to nano-Ceria. Arch Toxicol. 2014 Nov;88(11):2033-59. [CrossRef]
- Ma J, Bishoff B, Mercer RR, Barger M, Schwegler-Berry D, Castranova V. Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol Appl Pharmacol. 2017 May 15;323:16-25. [CrossRef]
- Zhao AN, Yin HJ, Fan MG, Zhang Z, Li N, Ma T. [Study on lung injury induced by rare earth samarium oxide particles in rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021 Dec 20;39(12):881-886. Chinese. [CrossRef]
- Ahmad J, Wahab R, Siddiqui MA, Farshori NN, Saquib Q, Ahmad N, Al-Khedhairy AA. Neodymium oxide nanostructures and their cytotoxic evaluation in human cancer cells. J Trace Elem Med Biol. 2022 Sep;73:127029. [CrossRef]
- Chen XA, Cheng YE, Rong Z. Recent results from a study of thorium lung burdens and health effects among miners in China. J Radiol Prot. 2005 Dec;25(4):451-60. [CrossRef]
- Gosens I, Mathijssen LE, Bokkers BG, Muijser H, Cassee FR. Comparative hazard identification of nano- and micro-sized cerium oxide particles based on 28-day inhalation studies in rats. Nanotoxicology. 2014 Sep;8(6):643-53. [CrossRef]
- Han Y, Lee DK, Kim SH, Lee S, Jeon S, Cho WS. High inflammogenic potential of rare earth oxide nanoparticles: the New Hazardous Entity. Nanotoxicology. 2018 Sep;12(7):712-728. [CrossRef]
- Han L, Jiang C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm Sin B. 2021 Aug;11(8):2306-2325. [CrossRef]
- Zhu W, Xu S, Shao P, Zhang H, Wu D, Yang W, Feng J. Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol Trace Elem Res. 1997 Apr;57(1):71-7. [CrossRef]
- Zheng L, Zhang J, Yu S, Ding Z, Song H, Wang Y, Li Y. Lanthanum Chloride Causes Neurotoxicity in Rats by Upregulating miR-124 Expression and Targeting PIK3CA to Regulate the PI3K/Akt Signaling Pathway. Biomed Res Int. 2020 ;2020:5205142. 5 May.
- Gaman L, RaDOI MP, Delia CE, Luzardo OP, Zumbado M, Rodríguez-Hernández Á, Stoian I, Gilca M, Boada LD, Henríquez-Hernández LA. Concentration of heavy metals and rare earth elements in patients with brain tumours: Analysis in tumour tissue, non-tumour tissue, and blood. Int J Environ Health Res. 2021 Nov;31(7):741-754. [CrossRef]
- Fan G, Yuan Z, Zheng H, Liu Z. [Study on the effects of exposure to rare earth elements and health-responses in children aged 7-10 years]. Wei Sheng Yan Jiu. 2004 Jan;33(1):23-8. Chinese.
- Wei J, Wang C, Yin S, Pi X, Jin L, Li Z, Liu J, Wang L, Yin C, Ren A. Concentrations of rare earth elements in maternal serum during pregnancy and risk for fetal neural tube defects. Environ Int. 2020 Apr;137:105542. [CrossRef]
- Jin C, Gao L, Li Y, Wu S, Lu X, Yang J, Cai Y. Lanthanum damages learning and memory and suppresses astrocyte-neuron lactate shuttle in rat hippocampus. Exp Brain Res. 2017 Dec;235(12):3817-3832. [CrossRef]
- Xiao X, Yong L, Jiao B, Yang H, Liang C, Jia X, Liu Z, Sang Y, Song Y. Postweaning exposure to lanthanum alters neurological behavior during early adulthood in rats. Neurotoxicology. 2021 Mar;83:40-50. [CrossRef]
- Lin CH, Liu GF, Chen J, Chen Y, Lin RH, He HX, Chen JP. Rare-earth Nanoparticle-induced Cytotoxicity on Spatial Cognition Memory of Mouse Brain. Chin Med J (Engl). 2017 Nov 20;130(22):2720-2725. [CrossRef]
- Xu T, Zhang M, Hu J, Li Z, Wu T, Bao J, Wu S, Lei L, He D. Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere. 2017 Aug;181:55-62. [CrossRef]
- Lin C, Liu G, Huang Y, Liu S, Tang B. Rare-earth nanoparticles induce depression, anxiety-like behavior, and memory impairment in mice. Food Chem Toxicol. 2021 Oct;156:112442. [CrossRef]
- Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent. Invest Radiol. 2019 Aug;54(8):453-463. [CrossRef]
- Bai Y, Long C, Hu G, Zhou D, Gao X, Chen Z, Wang T, Yu S, Han Y, Yan L. Association of blood chromium and rare earth elements with the risk of DNA damage in chromate exposed population. Environ Toxicol Pharmacol. 2019 Nov;72:103237. [CrossRef]
- Yu L, Dai Y, Yuan Z, Li J. Effects of rare earth elements on telomerase activity and apoptosis of human peripheral blood mononuclear cells. Biol Trace Elem Res. 2007 Apr;116(1):53-9. [CrossRef]
- Gaman L, Delia CE, Luzardo OP, Zumbado M, Badea M, Stoian I, Gilca M, Boada LD, Henríquez-Hernández LA. Serum concentration of toxic metals and rare earth elements in children and adolescent. Int J Environ Health Res. 2020 Dec;30(6):696-712. [CrossRef]
- Wingard CJ, Walters DM, Cathey BL, Hilderbrand SC, Katwa P, Lin S, Ke PC, Podila R, Rao A, Lust RM, Brown JM. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011 Dec;5(4):531-45. [CrossRef]
- Cheng J, Cheng Z, Hu R, Cui Y, Cai J, Li N, Gui S, Sang X, Sun Q, Wang L, Hong F. Immune dysfunction and liver damage of mice following exposure to lanthanoids. Environ Toxicol. 2014 Jan;29(1):64-73. [CrossRef]
- Gao J, Wang S, Tang G, Wang Z, Wang Y, Wu Q, Yang X, Liu Y, Hu L, He B, Qu G, Jiang G. Inflammation and accompanied disrupted hematopoiesis in adult mouse induced by rare earth element nanoparticles. Sci Total Environ. 2022 Jul 20;831:155416. [CrossRef]
- Zhao Y, Liang J, Meng H, Yin Y, Zhen H, Zheng X, Shi H, Wu X, Zu Y, Wang B, Fan L, Zhang K. Rare Earth Elements Lanthanum and Praseodymium Adversely Affect Neural and Cardiovascular Development in Zebrafish (Danio rerio). Environ Sci Technol. 2021 Jan 19;55(2):1155-1166. [CrossRef]
- Chen Y, Zhu W, Shu F, Fan Y, Yang N, Wu T, Ji L, Xie W, Bade R, Jiang S, Liu X, Shao G, Wu G, Jia X. Nd2O3 Nanoparticles Induce Toxicity and Cardiac/Cerebrovascular Abnormality in Zebrafish Embryos via the Apoptosis Pathway. Int J Nanomedicine. 2020 Jan 22;15:387-400. [CrossRef]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007 Mar;115(3):403-9. [CrossRef]
- Kennedy IM, Wilson D, Barakat AI; HEI Health Review Committee. Uptake and inflammatory effects of nanoparticles in a human vascular endothelial cell line. Res Rep Health Eff Inst. 2009 Jan;(136):3-32.
- Miller HA, Schake MA, Bony BA, Curtis ET, Gee CC, McCue IS, Ripperda TJ Jr, Chatzizisis YS, Kievit FM, Pedrigi RM. Smooth muscle cells affect differential nanoparticle accumulation in disturbed blood flow-induced murine atherosclerosis. PLoS One. 2021 Dec 9;16(12):e0260606. [CrossRef]
- Chen J, Xiao HJ, Qi T, Chen DL, Long HM, Liu SH. Rare earths exposure and male infertility: the injury mechanism study of rare earths on male mice and human sperm. Environ Sci Pollut Res Int. 2015 Feb;22(3):2076-86. [CrossRef]
- Lee WY, Park HJ. Toxicity of cerium oxide nanoparticles on neonatal testicular development in mouse organ culture. Reprod Toxicol. 2022 Aug;111:120-128. [CrossRef]
- Qin F, Shen T, Li J, Qian J, Zhang J, Zhou G, Tong J. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J Nanobiotechnology. 2019 Mar 21;17(1):41. [CrossRef]
- Li M, Zhuang L, Zhang G, Lan C, Yan L, Liang R, Hao C, Li Z, Zhang J, Lu Q, Wang B. Association between exposure of light rare earth elements and outcomes of in vitro fertilization-embryo transfer in North China. Sci Total Environ. 2021 Mar 25;762:143106. [CrossRef]
- Zhong H, Geng Y, Chen J, Gao R, Yu C, Yang Z, Chen X, Mu X, Liu X, He J. Maternal exposure to CeO2NPs during early pregnancy impairs pregnancy by inducing placental abnormalities. J Hazard Mater. 2020 May 5;389:121830. [CrossRef]
- Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA. 2016 Sep 6;316(9):952-61. [CrossRef]
- Liu L, Wang L, Ni W, Pan Y, Chen Y, Xie Q, Liu Y, Ren A. Rare earth elements in umbilical cord and risk for orofacial clefts. Ecotoxicol Environ Saf. 2021 Jan 1;207:111284. [CrossRef]
- Nemati A, Beyranvand F, Assadollahi V, Salahshoor MR, Alasvand M, Gholami MR. The effect of different concentrations of cerium oxide during pregnancy on ovarian follicle development in neonatal mice. Birth Defects Res. 2021 Mar;113(4):349-358. [CrossRef]
- Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009 Nov;1(6):479-88. [CrossRef]
- Galusha AL, Kruger PC, Howard LJ, Parsons PJ. An assessment of exposure to rare earth elements among patients receiving long-term parenteral nutrition. J Trace Elem Med Biol. 2018 May;47:156-163. [CrossRef]
- Liu H, Liu H, Yang Z, Wang K. Bone Mineral Density in Population Long-Term Exposed to Rare Earth Elements from a Mining Area of China. Biol Trace Elem Res. 2021 Feb;199(2):453-464. [CrossRef]
- Shankar VS, Alam AS, Bax CM, Bax BE, Pazianas M, Huang CL, Zaidi M. Activation and inactivation of the osteoclast Ca2+ receptor by the trivalent cation, La3+. Biochem Biophys Res Commun. 1992 Sep 16;187(2):907-12. [CrossRef]
- Cui X, Jiang S, Liu L, Tang X, Chen Y. Effect of low-dose lanthanum carbonate on calcium and phosphorus metabolism in Asian Patients with end-stage renal disease, maintenance hemodialysis and hyperphosphatemia. Afr Health Sci. 2022 Jun;22(2):362-368. [CrossRef]
- Zhu W, Xu S, Shao P, Zhang H, Wu D, Yang W, Feng J, Feng L. Investigation on liver function among population in high background of rare earth area in South China. Biol Trace Elem Res. 2005 Apr;104(1):1-8. [CrossRef]
- Chen F, Deng Q, Wu Y, Wu Y, Chen J, Chen Y, Lin L, Qiu Y, Pan L, Zheng X, Wei L, Liu F, He B, Wang J. U-Shaped Relationship of Rare Earth Element Lanthanum and Oral Cancer Risk: A Propensity Score-Based Study in the Southeast of China. Front Public Health. 2022 May 12;10:905690. [CrossRef]
- Hao Z, Li Y, Li H, Wei B, Liao X, Liang T, Yu J. Levels of rare earth elements, heavy metals and uranium in a population living in Baiyun Obo, Inner Mongolia, China: a pilot study. Chemosphere. 2015 Jun;128:161-70. [CrossRef]
- Guo C, Wei Y, Yan L, Li Z, Qian Y, Liu H, Li Z, Li X, Wang Z, Wang J. Rare earth elements exposure and the alteration of the hormones in the hypothalamic-pituitary-thyroid (HPT) axis of the residents in an e-waste site: A cross-sectional study. Chemosphere. 2020 Aug;252:126488. [CrossRef]
- Adebayo OA, Akinloye O, Adaramoye OA. Cerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of balb/c mice. Andrologia. 2018 Apr;50(3). [CrossRef]
- Martín-Aguilar L, Presas-Rodriguez S, Rovira À, Capellades J, Massuet-Vilamajó A, Ramió-Torrentà L, Tintoré M, Brieva-Ruiz L, Moral E, Cano-Orgaz A, Blanco Y, Batlle-Nadal J, Carmona O, Gea M, Hervás-García JV, Ramo-Tello C. Gadolinium-enhanced brain lesions in multiple sclerosis relapse. Neurologia (Engl Ed). 2022 Sep;37(7):557-563. [CrossRef]
- Lee KH, Chen HP, Leung CM, Chen HL, Tsai SS, Hsu PC. Effects of indium chloride exposure on sperm morphology and DNA integrity in rats. J Food Drug Anal. 2015 Mar;23(1):152-160. [CrossRef]
- Yang J, Liu Q, Wu S, Xi Q, Cai Y. Effects of lanthanum chloride on glutamate level, intracellular calcium concentration and caspases expression in the rat hippocampus. Biometals. 2013 Feb;26(1):43-59. [CrossRef]
- Alarifi S, Ali H, Alkahtani S, Alessia MS. Regulation of apoptosis through bcl-2/bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int J Nanomedicine. 2017 Jun 21;12:4541-4551. [CrossRef]
- Han G, Tan Z, Jing H, Ning J, Li Z, Gao S, Li G. Comet Assay Evaluation of Lanthanum Nitrate DNA Damage in C57-ras Transgenic Mice. Biol Trace Elem Res. 2021 Oct;199(10):3728-3736. [CrossRef]
- Porosnicu I, Butnaru CM, Tiseanu I, Stancu E, Munteanu CVA, Bita BI, Duliu OG, Sima F. Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules. 2021 Jun 4;26(11):3403. [CrossRef]
- Mittal S, Pandey AK. Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int. 2014;2014:891934. [CrossRef]
- Wang S, Bu N, Yun Y, Shi X, Wang S, Gao Y. RNA-Seq Analysis of Testes from Mice Exposed to Neodymium Oxide. Toxics. 2023 Nov 22;11(12):952. [CrossRef]
- Liu L, Jia Y, Zhang X, Chen S, Wang S, Zhu J, Zheng L, Chen Z, Huang L. Identification of the function and regulatory network of circ_009773 in DNA damage induced by nanoparticles of neodymium oxide. Toxicol In Vitro. 2022 Feb;78:105271. [CrossRef]
- Yu F, Zhang X, Gao L, Xue H, Liu L, Wang S, Chen S, Huang L. LncRNA loc105377478 promotes NPs-Nd2O3-induced inflammation in human bronchial epithelial cells through the ADIPOR1/NF-κB axis. Ecotoxicol Environ Saf. 2021 Jan 15;208:111609. [CrossRef]
- Yu L, Xiong J, Guo L, Miao L, Liu S, Guo F. The effects of lanthanum chloride on proliferation and apoptosis of cervical cancer cells: involvement of let-7a and miR-34a microRNAs. Biometals. 2015 Oct;28(5):879-90. [CrossRef]
- Carrillo-Cocom LM, Juárez-Méndez L, Rincón S, Rivera-Villanueva JM, Nic-Can GI, Zepeda A. Induction of cytotoxic effects and changes in DNA methylation-related gene expression in a human fibroblast cell line by the metal-organic framework [H2NMe2]3 [Tb(III)(2,6 pyridinedicarboxylate)3] (Tb-MOF). Environ Sci Pollut Res Int. 2023 Apr;30(16):46685-46696. [CrossRef]
- Choi JH, Lee H, Lee H, Lee H. Dopant-Dependent Toxicity of CeO2 Nanoparticles Is Associated with Dynamic Changes in H3K4me3 and H3K27me3 and Transcriptional Activation of NRF2 Gene in HaCaT Human Keratinocytes. Int J Mol Sci. 2021 Mar 17;22(6):3087. [CrossRef]
- Li N, Cheng J, Cheng Z, Hu R, Cai J, Gao G, Cui Y, Wang L, Hong F. Molecular mechanism of inflammatory response in mouse liver caused by exposure to CeCl₃. Environ Toxicol. 2013 Jun;28(6):349-58. [CrossRef]
- Rice KM, Nalabotu SK, Manne ND, Kolli MB, Nandyala G, Arvapalli R, Ma JY, Blough ER. Exposure to Cerium Oxide Nanoparticles Is Associated With Activation of Mitogen-activated Protein Kinases Signaling and Apoptosis in Rat Lungs. J Prev Med Public Health. 2015 May;48(3):132-41. [CrossRef]
- Bu N, Wang SR, Gao YR, Zhao YH, Shi XM, Wang SH. [The role of Keap1/Nrf2/HO-1 signal pathway in liver injury induced by rare earth neodymium oxide in mice]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023 Mar 20;41(3):161-167. Chinese. [CrossRef]
- Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. Aortic Oxidative Stress, Inflammation and DNA Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury. Biomolecules. 2019 Aug 17;9(8):376. [CrossRef]
- Huang LH, Yang H, Su X, Gao YR, Xue HN, Wang SH. Neodymium Oxide Induces Cytotoxicity and Activates NF-κB and Caspase-3 in NR8383 Cells. Biomed Environ Sci. 2017 Jan;30(1):75-78. [CrossRef]
- Tang S, Zhou L, Liu Z, Zou L, Xiao M, Huang C, Xie Z, He H, Guo Y, Cao Y, Huang H, Wu X, Meng D, Ye L, Wu Y, Yang X, Zhou X. Ceria nanoparticles promoted the cytotoxic activity of CD8+ T cells by activating NF-κB signaling. Biomater Sci. 2019 May 28;7(6):2533‐2544. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




