Submitted:
11 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
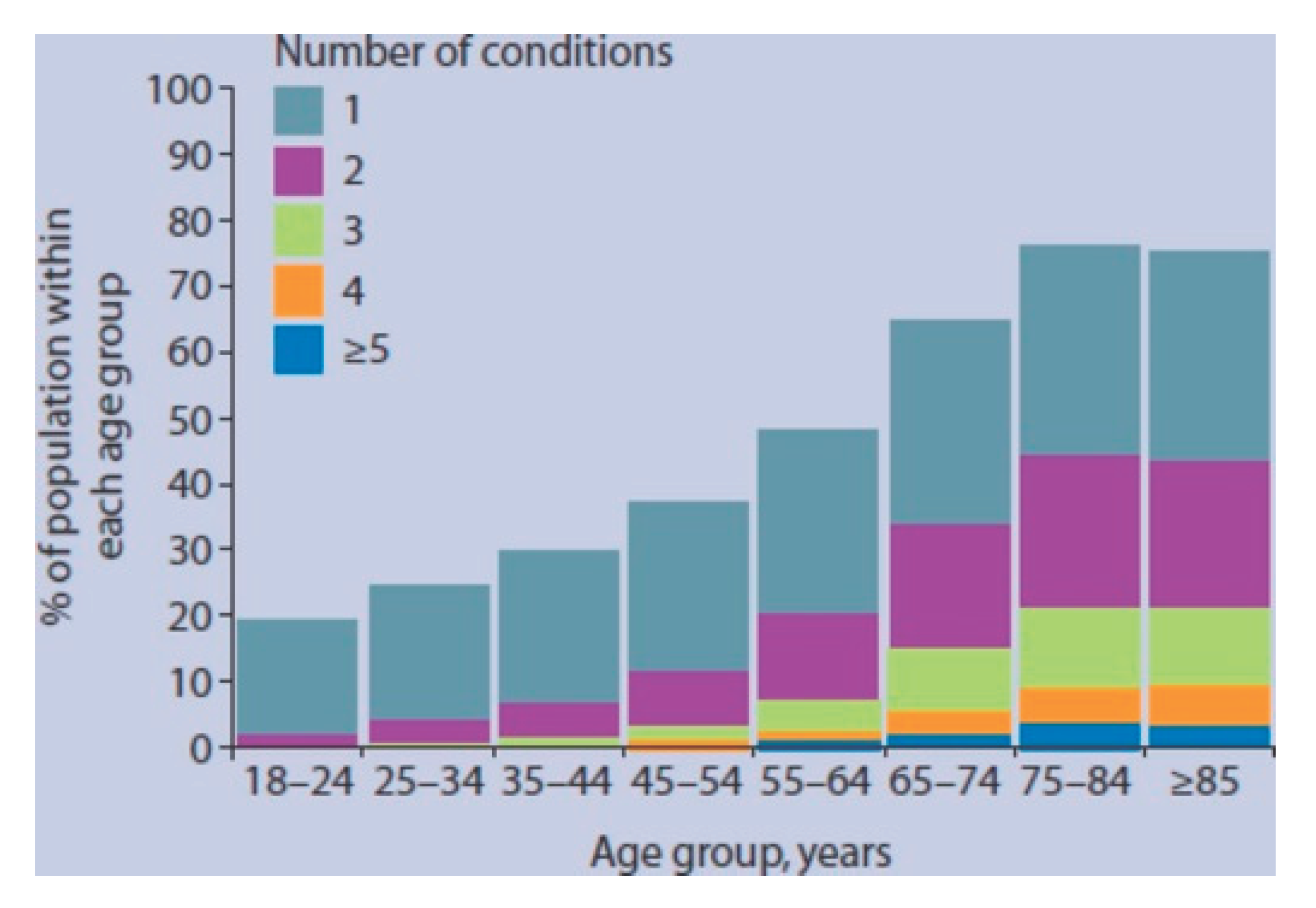
1. Introduction
| Age cohort | 2020 | 2035 | 2050 | Relative change between 2020 and 2050(%) |
| Population (million) | ||||
| Total adult population | 137 | 180 | 221 | 61% |
| 50–59 years | 48 | 56 | 69 | 42% |
| 60–79 years | 72 | 95 | 112 | 56% |
| 80 and older | 17 | 30 | 40 | 137% |
| ≥1 chronic condition (million) | ||||
| Adult population | 72 | 114 | 143 | 100% |
| 50–59 years | 16 | 18 | 22 | 40% |
| 60–79 years | 45 | 71 | 84 | 86% |
| 80 and older | 11 | 26 | 37 | 244% |
| Multimorbidity (million) | ||||
| Adult population | 8 | 12 | 15 | 91% |
| 50–59 years | 2 | 2 | 2 | 40% |
| 60–79 years | 5 | 7 | 9 | 79% |
| 80 and older | 1 | 3 | 4 | 203% |
| Prevalence of ≥1 chronic condition (%) | 22% | 35% | 48% | 120% |
| Prevalence of multimorbidity (%) | 2% | 4% | 5% | 111% |
2. Metabolism
2.1. Fatigue
3. Cognition
| Training Type | Trend (absent training) | System | Associated tests and biomarkers | Training | Adaptations |
|---|---|---|---|---|---|
| Strength Training | Sarcopenia, muscle loss, bone loss | Musculoskeltal | Grip Strength [65] | Weightlifting | Increase in muscle mass and bone density |
| Endurance training | Lower VO2 max | Metabolic, cardiopulmonary | Resting Metabolic Rate, Creatine phosphokinase [66] | Running, swimming, walking, cycling, cross-country skiing, hiking, etc. | Increased mitochondrial size, greater ability to metabolize fat, increased (heart) stroke volume |
| Balance training | Poorer coordination | Musculoskeletal, nervous | Self-selected gait velocity [67], Chair rise test (timed 5 chair rises), Tandem standing and walking, timed up and go test, clinical gait analysis with special focus on regularity, mechanography [68] | Yoga | Neuromuscular control [69] |
| Flexibility | Decrease in joint flexion [70,71] | Musculoskeletal, tendons, fascia | Flexibility tests: Flexindex [71] | Yoga, Pilates | Improved flexibility and stability |
| Preservation of genomic integrity | Accumulation of mutations [72], accumulation of methylation, higher cancer rates [73] | Genomic Integrity |
Telomere Length [74], Methylation level [75] | Low inflammation practices, avoiding carcinogenic exposures, possibly fasting [76] | Low inflammation practices, avoiding carcinogenic exposures, possibly fasting [76] |
| Cognition | Impairment on task switching [78], working and long-term memory [78] | Nervous | Cognitive tests [79]: Mini-Mental State Examination, Isaacs Set Test, Benton Visual Retention Test, Digit Symbol Substitution Test [80], Combined panel [81] |
Equivocal evidence for transfer effects of cognitive training [82], Combined program (exercise, brain training and lecture) [83], Reading [84], Hobbies [85], Multilingualism [86], Dance [87], Social Activity [88], meditation [89] |
Increased BDNF and neurogenesis [90] preservation of white matter |
4. Cardiovascular and Pulmonary Health
5. Musculoskeletal (Strength and Stability)
6. Emotional Health for Aging
| Factor | Interventions |
|---|---|
| Regret | Reflect and change behaviour going forward [131] Create positive life experiences |
| Gratitude | Express, journal [132] |
| Forgiveness | Reflecting on events, moving from resentment to compassion [133] |
| Openness | Explore, try new things [134] |
| Meaning/Purpose Intrinsic Motivation | Life crafting [135] |
| Conscientiousness | Adhere to a program [136] |
| Belongingness | Connect [137] |
| Self-transcendence | Experience flow [138] |
7. Resiliency in Aging
- (1)
- Financial
- (2)
- Living
- (3)
- Material
- (4)
- Knowledge
- (5)
- Emotional and Spiritual
- (6)
- Social
- (7)
- Cultural
- (8)
- Time.
| Type of Capital | Explanation | Relationship with healthy aging | Interventions |
| Financial | One’s financial resources | Financial health associated with lower rates of dementia, and better outcomes in case of dementia [152] | Saving, Investing, Increasing Earning Potential |
| Living | One’s natural surroundings | Surrounding green space associated with lower dementia risk [154,155] | Gardening, planting trees, regenerative agriculture/silviculture |
| Knowledge | One’s knowledge base and skillset | Lifelong learning associated with lower risk of dementia [156] | Learning, Hobbies |
| Emotional and Spiritual | One’s personal faith | Religious attendance associated with lower dementia risk [157,158] | Religious and spiritual practice, prayer, meditation |
| Social | One’s connections with other people | Loneliness associated with dementia [159] | Social activity |
| Cultural | Values and traditions | Frequent family visits associated with decrease in dementia symptoms [160] | Story Telling |
| Time Capital | One’s time remaining | Age associated with dementia [161] | Maximize health span, leave unfulfilling time obligations, optimize practices, delegate |
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ansah, J.P.; Chiu, C.-T. Projecting the Chronic Disease Burden among the Adult Population in the United States Using a Multi-State Population Model. Front Public Health 2023, 10, 1082183. [Google Scholar] [CrossRef]
- Lau, S.-H.P.; Tsui, A.K. ECONOMIC-DEMOGRAPHIC DEPENDENCY RATIO IN A LIFE-CYCLE MODEL. Macroeconomic Dynamics 2020, 24, 1635–1673. [Google Scholar] [CrossRef]
- Mason, C.N.; Miller, T. International Projections of Age Specific Healthcare Consumption: 2015–2060. The Journal of the Economics of Ageing 2018, 12, 202–217. [Google Scholar] [CrossRef]
- Braendle, T.; Colombier, C. Healthcare Expenditure Projections up to 2045. Available online: https://mpra.ub.uni-muenchen.de/104737/ (accessed on 7 March 2024).
- Salisbury, C.; Johnson, L.; Purdy, S.; Valderas, J.M.; Montgomery, A.A. Epidemiology and Impact of Multimorbidity in Primary Care: A Retrospective Cohort Study. Br J Gen Pract 2011, 61, e12–e21. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.; Martin, A.B.; Whittle, L.; Catlin, A. The National Health Expenditure Accounts Team National Health Care Spending In 2022: Growth Similar To Prepandemic Rates: National Health Care Spending Growth in 2022 Similar to Prepandemic Rates. Health Affairs 2024, 43, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-Associated Chronic Diseases Require Age-Old Medicine: Role of Chronic Inflammation. Prev Med 2012, 54, S29–S37. [Google Scholar] [CrossRef]
- Davies, L.; Wolska, B.; Hilbich, C.; Multhaup, G.; Martins, R.; Simms, G.; Beyreuther, K.; Masters, C.L. A4 Amyloid Protein Deposition and the Diagnosis of Alzheimer’s Disease: Prevalence in Aged Brains Determined by Immunocytochemistry Compared with Conventional Neuropathologic Techniques. Neurology 1988, 38, 1688. [Google Scholar] [CrossRef] [PubMed]
- Poulain, M.; Herm, A.; Pes, G. The Blue Zones: Areas of Exceptional Longevity around the World. Vienna Yearbook of Population Research 2013, 11, 87–108. [Google Scholar] [CrossRef]
- Pes, G.M.; Dore, M.P.; Tsofliou, F.; Poulain, M. Diet and Longevity in the Blue Zones: A Set-and-Forget Issue? Maturitas 2022, 164, 31–37. [Google Scholar] [CrossRef]
- Poulain, M.; Pes, G.M.; Grasland, C.; Carru, C.; Ferrucci, L.; Baggio, G.; Franceschi, C.; Deiana, L. Identification of a Geographic Area Characterized by Extreme Longevity in the Sardinia Island: The AKEA Study. Experimental Gerontology 2004, 39, 1423–1429. [Google Scholar] [CrossRef]
- Fastame, M.C.; Hitchcott, P.K.; Penna, M.P. The Impact of Leisure on Mental Health of Sardinian Elderly from the ‘Blue Zone’: Evidence for Ageing Well. Aging Clin Exp Res 2018, 30, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, P.; Lee, J.T.; Oldenburg, B.; van Heusden, A.; Haregu, T.N.; Wang, H. The Prevalence of Metabolic Disease Multimorbidity and Its Associations With Spending and Health Outcomes in Middle-Aged and Elderly Chinese Adults. Frontiers in Public Health 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, J.A.; Reddy, P.; Davis-Lameloise, N.; Philpot, B.; Laatikainen, T.; Kilkkinen, A.; Bunker, S.J.; Best, J.D.; Vartiainen, E.; Kai Lo, S.; et al. Depression: An Important Comorbidity With Metabolic Syndrome in a General Population. Diabetes Care 2008, 31, 2368–2373. [Google Scholar] [CrossRef]
- Yang, Y.; Mauldin, P.D.; Ebeling, M.; Hulsey, T.C.; Liu, B.; Thomas, M.B.; Camp, E.R.; Esnaola, N.F. Effect of Metabolic Syndrome and Its Components on Recurrence and Survival in Colon Cancer Patients. Cancer 2013, 119, 1512–1520. [Google Scholar] [CrossRef]
- Braun, S.; Bitton-Worms, K.; LeRoith, D. The Link between the Metabolic Syndrome and Cancer. International journal of biological sciences 2011, 7, 1003. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild Cognitive Impairment and Progression to Dementia in People with Diabetes, Prediabetes and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Soc Psychiatry Psychiatr Epidemiol 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- Marseglia, A.; Darin-Mattsson, A.; Skoog, J.; Rydén, L.; Hadarsson-Bodin, T.; Kern, S.; Rydberg Sterner, T.; Shang, Y.; Zettergren, A.; Westman, E. Metabolic Syndrome Is Associated with Poor Cognition: A Population-Based Study of 70-Year-Old Adults without Dementia. The Journals of Gerontology: Series A 2021, 76, 2275–2283. [Google Scholar] [CrossRef]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef]
- Schnabel, L.; Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R.; et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results From the French NutriNet-Santé Cohort. Official journal of the American College of Gastroenterology | ACG 2018, 113, 1217. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of Ultra-Processed Foods and Health Outcomes: A Systematic Review of Epidemiological Studies. Nutrition Journal 2020, 19, 86. [Google Scholar] [CrossRef]
- Magalhães, V.; Severo, M.; Correia, D.; Torres, D.; de Miranda, R.C.; Rauber, F.; Levy, R.; Rodrigues, S.; Lopes, C. Associated Factors to the Consumption of Ultra-Processed Foods and Its Relation with Dietary Sources in Portugal. Journal of Nutritional Science 2021, 10, e89. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.A.; Baker, P.I. Ultra-Processed Food and Adverse Health Outcomes. BMJ 2019, 365, l2289. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T.; Arciero, P.J.; Goran, M.I. Endurance Exercise in Aging Humans: Effects on Energy Metabolism. Exercise and Sport Sciences Reviews 1994, 22, 251. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J Clin Invest 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen Production and Action. Journal of the American Academy of Dermatology 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Angoff, R.; Himali, J.J.; Maillard, P.; Aparicio, H.J.; Vasan, R.S.; Seshadri, S.; Beiser, A.S.; Tsao, C.W. Relations of Metabolic Health and Obesity to Brain Aging in Young to Middle-Aged Adults. Journal of the American Heart Association 2022, 11, e022107. [Google Scholar] [CrossRef]
- Facchini, F.S.; Hua, N.; Abbasi, F.; Reaven, G.M. Insulin Resistance as a Predictor of Age-Related Diseases. The Journal of Clinical Endocrinology & Metabolism 2001, 86, 3574–3578. [Google Scholar] [CrossRef]
- Paolisso, G.; Gambardella, A.; Ammendola, S.; D’Amore, A.; Balbi, V.; Varricchio, M.; D’Onofrio, F. Glucose Tolerance and Insulin Action in Healthy Centenarians. Am J Physiol 1996, 270, E890–E894. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance Exercise as a Countermeasure for Aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef]
- Resnick, H.E.; Carter, E.A.; Aloia, M.; Phillips, B. Cross-Sectional Relationship of Reported Fatigue to Obesity, Diet, and Physical Activity: Results From the Third National Health and Nutrition Examination Survey. Journal of Clinical Sleep Medicine 2006, 02, 163–169. [Google Scholar] [CrossRef]
- Hobday, R.A.; Thomas, S.; O’Donovan, A.; Murphy, M.; Pinching, A.J. Dietary Intervention in Chronic Fatigue Syndrome. Journal of Human Nutrition and Dietetics 2008, 21, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet Components Associated with Perceived Fatigue in Breast Cancer Survivors: Diet, Fatigue and Breast Cancer. European Journal of Cancer Care 2013, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chase, E.; Chen, V.; Martin, K.; Lane, M.; Wooliscroft, L.; Adams, C.; Rice, J.; Silbermann, E.; Hollen, C.; Fryman, A.; et al. A Low-Fat Diet Improves Fatigue in Multiple Sclerosis: Results from a Randomized Controlled Trial. Mult Scler 2023, 29, 1659–1675. [Google Scholar] [CrossRef]
- Pommerich, U.M.; Brincks, J.; Christensen, M.E. Is There an Effect of Dietary Intake on MS-Related Fatigue? – A Systematic Literature Review. Multiple Sclerosis and Related Disorders 2018, 25, 282–291. [Google Scholar] [CrossRef]
- Bitarafan, S.; Harirchian, M.-H.; Nafissi, S.; Sahraian, M.-A.; Togha, M.; Siassi, F.; Saedisomeolia, A.; Alipour, E.; Mohammadpour, N.; Chamary, M.; et al. Dietary Intake of Nutrients and Its Correlation with Fatigue in Multiple Sclerosis Patients. Iran J Neurol 2014, 13, 28–32. [Google Scholar] [PubMed]
- Albrechtsen, M.T.; Langeskov-Christensen, M.; Jørgensen, M.L.K.; Dalgas, U.; Hansen, M. Is Diet Associated with Physical Capacity and Fatigue in Persons with Multiple Sclerosis? – Results from a Pilot Study. Multiple Sclerosis and Related Disorders 2020, 40, 101921. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; Cheek, J.J.; Fox, S.S.; Healy, H.S.; Schweizer, M.L.; Bao, W.; Kamholz, J.; Titcomb, T.J. Efficacy of Diet on Fatigue and Quality of Life in Multiple Sclerosis. Neurology 2023, 100, e357–e366. [Google Scholar] [CrossRef]
- Mousavi-Shirazi-Fard, Z.; Mazloom, Z.; Izadi, S.; Fararouei, M. The Effects of Modified Anti-Inflammatory Diet on Fatigue, Quality of Life, and Inflammatory Biomarkers in Relapsing-Remitting Multiple Sclerosis Patients: A Randomized Clinical Trial. International Journal of Neuroscience 2021, 131, 657–665. [Google Scholar] [CrossRef]
- Campagnolo, N.; Johnston, S.; Collatz, A.; Staines, D.; Marshall-Gradisnik, S. Dietary and Nutrition Interventions for the Therapeutic Treatment of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Systematic Review. Journal of Human Nutrition and Dietetics 2017, 30, 247–259. [Google Scholar] [CrossRef]
- Maric, D.; Brkic, S.; Mikic, A.N.; Tomic, S.; Cebovic, T.; Turkulov, V. Multivitamin Mineral Supplementation in Patients with Chronic Fatigue Syndrome. Medical science monitor: International medical journal of experimental and clinical research 2014, 20, 47. [Google Scholar]
- Bo, S.; Pisu, E. Role of Dietary Magnesium in Cardiovascular Disease Prevention, Insulin Sensitivity and Diabetes. Current Opinion in Lipidology 2008, 19, 50. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Reschke, J.E.; Kleckner, I.R.; Magnuson, A.; Amitrano, A.M.; Culakova, E.; Shayne, M.; Netherby-Winslow, C.S.; Czap, S.; Janelsins, M.C.; et al. The Effects of a Mediterranean Diet Intervention on Cancer-Related Fatigue for Patients Undergoing Chemotherapy: A Pilot Randomized Controlled Trial. Cancers 2022, 14, 4202. [Google Scholar] [CrossRef]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutrition and Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L.; Lamonica, G.; Dessì, M.; Spiga, C.; Astara, G.; et al. Efficacy of L-Carnitine Administration on Fatigue, Nutritional Status, Oxidative Stress, and Related Quality of Life in 12 Advanced Cancer Patients Undergoing Anticancer Therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef]
- Marx, W.; Teleni, L.; Opie, R.S.; Kelly, J.; Marshall, S.; Itsiopoulos, C.; Isenring, E. Efficacy and Effectiveness of Carnitine Supplementation for Cancer-Related Fatigue: A Systematic Literature Review and Meta-Analysis. Nutrients 2017, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, Inflammation, and ω-3 and ω-6 Fatty Acid Intake Among Breast Cancer Survivors. JCO 2012, 30, 1280–1287. [Google Scholar] [CrossRef]
- Barton, D.L.; Soori, G.S.; Bauer, B.A.; Sloan, J.A.; Johnson, P.A.; Figueras, C.; Duane, S.; Mattar, B.; Liu, H.; Atherton, P.J.; et al. Pilot Study of Panax Quinquefolius (American Ginseng) to Improve Cancer-Related Fatigue: A Randomized, Double-Blind, Dose-Finding Evaluation: NCCTG Trial N03CA. Support Care Cancer 2010, 18, 179–187. [Google Scholar] [CrossRef]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin Ginseng (Panax Quinquefolius) to Improve Cancer-Related Fatigue: A Randomized, Double-Blind Trial, N07C2. JNCI: Journal of the National Cancer Institute 2013, 105, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Tsai, C.-H.; Li, T.-C.; Yang, Y.-W.; Huang, W.-S.; Lu, M.-K.; Tseng, C.-H.; Huang, H.-C.; Chen, K.-F.; Hsu, T.-S.; et al. Effects of the Traditional Chinese Herb Astragalus Membranaceus in Patients with Poststroke Fatigue: A Double-Blind, Randomized, Controlled Preliminary Study. Journal of Ethnopharmacology 2016, 194, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutrition and Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- de Oliveira Campos, M.P.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.A.; Giglio, A.D. Guarana (Paullinia Cupana) Improves Fatigue in Breast Cancer Patients Undergoing Systemic Chemotherapy. The Journal of Alternative and Complementary Medicine 2011, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- da Costa Miranda, V.; Trufelli, D.C.; Santos, J.; Campos, M.P.; Nobuo, M.; da Costa Miranda, M.; Schlinder, F.; Riechelmann, R.; del Giglio, A. Effectiveness of Guaraná (Paullinia Cupana) for Postradiation Fatigue and Depression: Results of a Pilot Double-Blind Randomized Study. The Journal of Alternative and Complementary Medicine 2009, 15, 431–433. [Google Scholar] [CrossRef] [PubMed]
- de Sette, C.V.M.; Ribas de Alcântara, B.B.; Schoueri, J.H.M.; Cruz, F.M.; de Cubero, D.I.G.; Pianowski, L.F.; Peppone, L.J.; Fonseca, F.; del Giglio, A. Purified Dry Paullinia Cupana (PC-18) Extract for Chemotherapy-Induced Fatigue: Results of Two Double-Blind Randomized Clinical Trials. Journal of Dietary Supplements 2018, 15, 673–683. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Naidoo, R.; Cronin, J. High-Protein, Low-Fat, Short-Term Diet Results in Less Stress and Fatigue Than Moderate-Protein, Moderate-Fat Diet During Weight Loss in Male Weightlifters: A Pilot Study. International Journal of Sport Nutrition and Exercise Metabolism 2015, 25, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kaya Kaçar, H.; Avery, A.; Bennett, S.; McCullough, F. Dietary Patterns and Fatigue in Female Slimmers. Nutrition & Food Science 2020, 50, 1213–1227. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Porges, S.W. Vagal Tone: A Physiologic Marker of Stress Vulnerability. Pediatrics 1992, 90, 498–504. [Google Scholar] [CrossRef]
- Fang, R.; Ye, S.; Huangfu, J.; Calimag, D.P. Music Therapy Is a Potential Intervention for Cognition of Alzheimer’s Disease: A Mini-Review. Transl Neurodegener 2017, 6, 2. [Google Scholar] [CrossRef]
- Matsumura, T.; Muraki, I.; Ikeda, A.; Yamagishi, K.; Shirai, K.; Yasuda, N.; Sawada, N.; Inoue, M.; Iso, H.; Brunner, E.J.; et al. Hobby Engagement and Risk of Disabling Dementia. J Epidemiol 2023, 33, 456–463. [Google Scholar] [CrossRef]
- Sommerlad, A.; Sabia, S.; Livingston, G.; Kivimäki, M.; Lewis, G.; Singh-Manoux, A. Leisure Activity Participation and Risk of Dementia. Neurology 2020, 95, e2803–e2815. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Tsutsumimoto, K.; Doi, T.; Nakakubo, S.; Kurita, S.; Makizako, H.; Shimada, H. Relationships between Cognitive Leisure Activities and Cognitive Function in Older Adults with Depressive Symptoms: A Cross-Sectional Study. BMJ Open 2020, 10, e032679. [Google Scholar] [CrossRef] [PubMed]
- Muscari, A.; Giannoni, C.; Pierpaoli, L.; Berzigotti, A.; Maietta, P.; Foschi, E.; Ravaioli, C.; Poggiopollini, G.; Bianchi, G.; Magalotti, D.; et al. Chronic Endurance Exercise Training Prevents Aging-Related Cognitive Decline in Healthy Older Adults: A Randomized Controlled Trial. International Journal of Geriatric Psychiatry 2010, 25, 1055–1064. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip Strength and the Risk of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Frontiers in Aging Neuroscience 2021, 13. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. Quantitative Measures of Healthy Aging and Biological Age. Healthy Aging Res 2015, 4, 26. [Google Scholar] [CrossRef]
- Hirase, T.; Okubo, Y.; Delbaere, K.; Menant, J.C.; Lord, S.R.; Sturnieks, D.L. Risk Factors for Falls and Fall-Related Fractures in Community-Living Older People with Pain: A Prospective Cohort Study. International Journal of Environmental Research and Public Health 2023, 20, 6040. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.; Hunter, G. Determinants of Musculoskeletal Frailty and the Risk of Falls in Old Age. J Musculoskelet Neuronal Interact 2006, 6, 167–173. [Google Scholar]
- Zech, A.; Hübscher, M.; Vogt, L.; Banzer, W.; Hänsel, F.; Pfeifer, K. Balance Training for Neuromuscular Control and Performance Enhancement: A Systematic Review. Journal of Athletic Training 2010, 45, 392–403. [Google Scholar] [CrossRef]
- Stathokostas, L.; McDonald, M.W.; Little, R.M.D.; Paterson, D.H. Flexibility of Older Adults Aged 55–86 Years and the Influence of Physical Activity. Journal of Aging Research 2013, 2013, e743843. [Google Scholar] [CrossRef]
- Araújo, C.G.S. de Flexibility Assessment: Normative Values for Flexitest from 5 to 91 Years of Age. Arq. Bras. Cardiol. 2008, 90, 280–287. [Google Scholar] [CrossRef]
- Wolters, S.; Schumacher, B. Genome Maintenance and Transcription Integrity in Aging and Disease. Frontiers in Genetics 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H.J. DNA Damage, Aging, and Cancer. New England Journal of Medicine 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Sanders, J.L.; Newman, A.B. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? Epidemiologic Reviews 2013, 35, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat Rev Genet 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. In Metabolism in Cancer; Cramer, T., A. Schmitt, C., Eds.; Recent Results in Cancer Research; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-42118-6. [Google Scholar]
- Chow, M.T.; Möller, A.; Smyth, M.J. Inflammation and Immune Surveillance in Cancer. Seminars in Cancer Biology 2012, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.R. Brain Aging: Models, Methods, and Mechanisms; CRC Press, 2007; ISBN 978-1-00-061155-7.
- Wild, K.; Howieson, D.; Webbe, F.; Seelye, A.; Kaye, J. Status of Computerized Cognitive Testing in Aging: A Systematic Review. Alzheimer’s & Dementia 2008, 4, 428–437. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Amieva, H.; Dartigues, J.-F.; Jacqmin-Gadda, H. Sensitivity of Four Psychometric Tests to Measure Cognitive Changes in Brain Aging-Population–Based Studies. American Journal of Epidemiology 2007, 165, 344–350. [Google Scholar] [CrossRef]
- Belleville, S.; Fouquet, C.; Hudon, C.; Zomahoun, H.T.V.; Croteau, J. Consortium for the Early Identification of Alzheimer’s disease-Quebec Neuropsychological Measures That Predict Progression from Mild Cognitive Impairment to Alzheimer’s Type Dementia in Older Adults: A Systematic Review and Meta-Analysis. Neuropsychol Rev 2017, 27, 328–353. [Google Scholar] [CrossRef]
- Butler, M.; McCreedy, E.; Nelson, V.A.; Desai, P.; Ratner, E.; Fink, H.A.; Hemmy, L.S.; McCarten, J.R.; Barclay, T.R.; Brasure, M.; et al. Does Cognitive Training Prevent Cognitive Decline? Ann Intern Med 2018, 168, 63–68. [Google Scholar] [CrossRef]
- Kouzuki, M.; Kato, T.; Wada-Isoe, K.; Takeda, S.; Tamura, A.; Takanashi, Y.; Azumi, S.; Kojima, Y.; Maruyama, C.; Hayashi, M.; et al. A Program of Exercise, Brain Training, and Lecture to Prevent Cognitive Decline. Annals of Clinical and Translational Neurology 2020, 7, 318–328. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, I.-C.; Hsiung, C.A. Reading Activity Prevents Long-Term Decline in Cognitive Function in Older People: Evidence from a 14-Year Longitudinal Study. International Psychogeriatrics 2021, 33, 63–74. [Google Scholar] [CrossRef]
- Fallahpour, M.; Borell, L.; Luborsky, M.; Nygård, L. Leisure-Activity Participation to Prevent Later-Life Cognitive Decline: A Systematic Review. Scandinavian Journal of Occupational Therapy 2016, 23, 162–197. [Google Scholar] [CrossRef] [PubMed]
- Van den Noort, M.; Vermeire, K.; Bosch, P.; Staudte, H.; Krajenbrink, T.; Jaswetz, L.; Struys, E.; Yeo, S.; Barisch, P.; Perriard, B.; et al. A Systematic Review on the Possible Relationship Between Bilingualism, Cognitive Decline, and the Onset of Dementia. Behavioral Sciences 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Muiños, M.; Ballesteros, S. Does Dance Counteract Age-Related Cognitive and Brain Declines in Middle-Aged and Older Adults? A Systematic Review. Neuroscience & Biobehavioral Reviews 2021, 121, 259–276. [Google Scholar] [CrossRef]
- Marioni, R.E.; Proust-Lima, C.; Amieva, H.; Brayne, C.; Matthews, F.E.; Dartigues, J.-F.; Jacqmin-Gadda, H. Social Activity, Cognitive Decline and Dementia Risk: A 20-Year Prospective Cohort Study. BMC Public Health 2015, 15, 1089. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; Hutt, R.; Yi Tsui, C.K.; Zorokong, K.; Marfeo, E. Meditation-Based Interventions for Adults With Dementia: A Scoping Review. The American Journal of Occupational Therapy 2020, 74, 7403205010p1–7403205010p14. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.; Zhu, L.; Chen, D.; Cai, K.; Liu, Z.; Chen, A. Moderate Exercise Combined with Enriched Environment Enhances Learning and Memory through BDNF/TrkB Signaling Pathway in Rats. International Journal of Environmental Research and Public Health 2021, 18, 8283. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Jones, T.W.; Cassidy, S.; Siervo, M.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. The Effect of Age on the Relationship between Cardiac and Vascular Function. Mech Ageing Dev 2016, 153, 1–6. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-Predicted Maximal Heart Rate Revisited. J Am Coll Cardiol 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Sharma, G.; Goodwin, J. Effect of Aging on Respiratory System Physiology and Immunology. Clin Interv Aging 2006, 1, 253–260. [Google Scholar] [CrossRef]
- Tolep, K.; Higgins, N.; Muza, S.; Criner, G.; Kelsen, S.G. Comparison of Diaphragm Strength between Healthy Adult Elderly and Young Men. Am J Respir Crit Care Med 1995, 152, 677–682. [Google Scholar] [CrossRef]
- Berry, R.B.; Pai, U.P.; Fairshter, R.D. Effect of Age on Changes in Flow Rates and Airway Conductance after a Deep Breath. Journal of Applied Physiology 1990, 68, 635–643. [Google Scholar] [CrossRef]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Chapter Six - Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. In Progress in Molecular Biology and Translational Science; Bouchard, C., Ed.; Molecular and Cellular Regulation of Adaptation to Exercise; Academic Press, 2015; Vol. 135, pp. 129–151.
- Meinild Lundby, A.-K.; Jacobs, R.A.; Gehrig, S.; de Leur, J.; Hauser, M.; Bonne, T.C.; Flück, D.; Dandanell, S.; Kirk, N.; Kaech, A.; et al. Exercise Training Increases Skeletal Muscle Mitochondrial Volume Density by Enlargement of Existing Mitochondria and Not de Novo Biogenesis. Acta Physiologica 2018, 222, e12905. [Google Scholar] [CrossRef]
- Lundby, C.; Jacobs, R.A. Adaptations of Skeletal Muscle Mitochondria to Exercise Training. Experimental Physiology 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Kasch, F.W.; Boyer, J.L.; Camp, S.V.; Nettl, F.; Verity, L.S.; Wallace, J.P. Cardiovascular Changes with Age and Exercise. Scandinavian Journal of Medicine & Science in Sports 1995, 5, 147–151. [Google Scholar] [CrossRef]
- Skinner, J.S.; Gaskill, S.E.; Rankinen, T.; Leon, A.S.; Rao, D.C.; Wilmore, J.H.; Bouchard, C. Heart Rate versus %VO2max: Age, Sex, Race, Initial Fitness, and Training Response--HERITAGE. Med Sci Sports Exerc 2003, 35, 1908–1913. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B.; et al. International Variation in the Prevalence of COPD (The BOLD Study): A Population-Based Prevalence Study. The Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, Y.; Matsumoto, H.; Nagasaki, T.; Kanemitsu, Y.; Murase, K.; Ito, I.; Oguma, T.; Muro, S.; Asai, K.; Tabara, Y.; et al. Mouth Breathing, Another Risk Factor for Asthma: The Nagahama Study. Allergy 2016, 71, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Triana, B.E.G.; Ali, A.H.; León, I.B.G. Mouth Breathing and Its Relationship to Some Oral and Medical Conditions: Physiopathological Mechanisms Involved. Revista Habanera de Ciencias Médicas 2016, 15, 200–212. [Google Scholar]
- Lee, Y.-C.; Lu, C.-T.; Cheng, W.-N.; Li, H.-Y. The Impact of Mouth-Taping in Mouth-Breathers with Mild Obstructive Sleep Apnea: A Preliminary Study. Healthcare 2022, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Home Oxygen Therapy. Available online: https://www.nhs.uk/conditions/home-oxygen-treatment/ (accessed on 27 October 2023).
- Pioli, G.; Barone, A.; Giusti, A.; Oliveri, M.; Pizzonia, M.; Razzano, M.; Palummeri, E. Predictors of Mortality after Hip Fracture: Results from 1-Year Follow-Up. Aging Clin Exp Res 2006, 18, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Evans, W.J. Changes in Skeletal Muscle with Aging: Effects of Exercise Training. Exerc Sport Sci Rev 1993, 21, 65–102. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Engelhardt, M. Strength and Muscle Mass Loss with Aging Process. Age and Strength Loss. Muscles Ligaments Tendons J 2014, 3, 346–350. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Duque, G. Vitamin D in the Aging Musculoskeletal System: An Authentic Strength Preserving Hormone. Molecular Aspects of Medicine 2005, 26, 203–219. [Google Scholar] [CrossRef]
- Aschbacher, K.; Epel, E.; Wolkowitz, O.M.; Prather, A.A.; Puterman, E.; Dhabhar, F.S. Maintenance of a Positive Outlook during Acute Stress Protects against Pro-Inflammatory Reactivity and Future Depressive Symptoms. Brain, Behavior, and Immunity 2012, 26, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Lipowski, M. Level of Optimism and Health Behavior in Athletes. Med Sci Monit 2012, 18, CR39–CR43. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, D.K.; Brunning, S. Personality, Preventive Health Behaviour and Comparative Optimism about Health Problems. J Health Psychol 1999, 4, 193–208. [Google Scholar] [CrossRef]
- Mulkana, S.S.; Hailey, B.J. The Role of Optimism in Health-Enhancing Behavior. American Journal of Health Behavior 2001, 25, 388–395. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Scheier, M.F.; Greenhouse, J.B. Optimism and Physical Health: A Meta-Analytic Review. Annals of Behavioral Medicine 2009, 37, 239–256. [Google Scholar] [CrossRef]
- Mannix, M.M.; Feldman, J.M.; Moody, K. Optimism and Health-Related Quality of Life in Adolescents with Cancer. Child: Care, Health and Development 2009, 35, 482–488. [Google Scholar] [CrossRef]
- Kim, E.S.; Chopik, W.J.; Smith, J. Are People Healthier If Their Partners Are More Optimistic? The Dyadic Effect of Optimism on Health among Older Adults. Journal of Psychosomatic Research 2014, 76, 447–453. [Google Scholar] [CrossRef]
- Isenberg, C. An Examination of Regret as Expressed in the Life Reflections of Older Adults: Predictors of Regret Intensity and Frequency, and Association with Well-Being. phd, Concordia University, 2007.
- Jokisaari, M. Regrets and Subjective Well-Being: A Life Course Approach. Journal of Adult Development 2004, 11, 281–288. [Google Scholar] [CrossRef]
- Brassen, S.; Gamer, M.; Peters, J.; Gluth, S.; Büchel, C. Don’t Look Back in Anger! Responsiveness to Missed Chances in Successful and Nonsuccessful Aging. Science 2012, 336, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Bono, G.; McCullough, M.E.; Root, L.M. Forgiveness, Feeling Connected to Others, and Well-Being: Two Longitudinal Studies. Pers Soc Psychol Bull 2008, 34, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Allemand, M.; Hill, P.L.; Ghaemmaghami, P.; Martin, M. Forgivingness and Subjective Well-Being in Adulthood: The Moderating Role of Future Time Perspective. Journal of Research in Personality 2012, 46, 32–39. [Google Scholar] [CrossRef]
- Macaskill, A. Differentiating Dispositional Self-Forgiveness from Other-Forgiveness: Associations with Mental Health and Life Satisfaction. Journal of Social and Clinical Psychology 2012, 31, 28–50. [Google Scholar] [CrossRef]
- Robustelli, B.L.; Whisman, M.A. Gratitude and Life Satisfaction in the United States and Japan. J Happiness Stud 2018, 19, 41–55. [Google Scholar] [CrossRef]
- Kong, F.; Ding, K.; Zhao, J. The Relationships Among Gratitude, Self-Esteem, Social Support and Life Satisfaction Among Undergraduate Students. J Happiness Stud 2015, 16, 477–489. [Google Scholar] [CrossRef]
- Unanue, W.; Gomez Mella, M.E.; Cortez, D.A.; Bravo, D.; Araya-Véliz, C.; Unanue, J.; Van Den Broeck, A. The Reciprocal Relationship Between Gratitude and Life Satisfaction: Evidence From Two Longitudinal Field Studies. Frontiers in Psychology 2019, 10. [Google Scholar] [CrossRef]
- Mayungbo, O. AGREEABLENESS, CONSCIENTIOUSNESS AND SUBJECTIVE WELLBEING. PEOPLE: International Journal of Social Sciences 2016, 2, 68–87. [Google Scholar] [CrossRef]
- Gale, C.R.; Booth, T.; Mõttus, R.; Kuh, D.; Deary, I.J. Neuroticism and Extraversion in Youth Predict Mental Wellbeing and Life Satisfaction 40 Years Later. Journal of Research in Personality 2013, 47, 687–697. [Google Scholar] [CrossRef]
- Duckworth, A.; Weir, D.; Tsukayama, E.; Kwok, D. Who Does Well in Life? Conscientious Adults Excel in Both Objective and Subjective Success. Frontiers in Psychology 2012, 3. [Google Scholar]
- Stephan, Y. Openness to Experience and Active Older Adults’ Life Satisfaction: A Trait and Facet-Level Analysis. Personality and Individual Differences 2009, 47, 637–641. [Google Scholar] [CrossRef]
- Fors Connolly, F.; Johansson Sevä, I. Agreeableness, Extraversion and Life Satisfaction: Investigating the Mediating Roles of Social Inclusion and Status. Scandinavian Journal of Psychology 2021, 62, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Ph.D, N.R. If Only: How to Turn Regret Into Opportunity; Clarkson Potter/Ten Speed/Harmony, 2005; ISBN 978-0-7679-2023-0.
- Davis, D.E.; Choe, E.; Meyers, J.; Wade, N.; Varjas, K.; Gifford, A.; Quinn, A.; Hook, J.N.; Van Tongeren, D.R.; Griffin, B.J.; et al. Thankful for the Little Things: A Meta-Analysis of Gratitude Interventions. Journal of Counseling Psychology 2016, 63, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Smallen, D. Practicing Forgiveness: A Framework for a Routine Forgiveness Practice. Spirituality in Clinical Practice 2019, 6, 219–228. [Google Scholar] [CrossRef]
- Jackson, J.J.; Hill, P.L.; Payne, B.R.; Roberts, B.W.; Stine-Morrow, E.A.L. Can an Old Dog Learn (and Want to Experience) New Tricks? Cognitive Training Increases Openness to Experience in Older Adults. Psychol Aging 2012, 27, 286–292. [Google Scholar] [CrossRef]
- Schippers, M.C.; Ziegler, N. Life Crafting as a Way to Find Purpose and Meaning in Life. Frontiers in Psychology 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Javaras, K.N.; Williams, M.; Baskin-Sommers, A.R. Psychological Interventions Potentially Useful for Increasing Conscientiousness. Personality Disorders: Theory, Research, and Treatment 2019, 10, 13–24. [Google Scholar] [CrossRef]
- Suragarn, U.; Hain, D.; Pfaff, G. Approaches to Enhance Social Connection in Older Adults: An Integrative Review of Literature. Aging and Health Research 2021, 1, 100029. [Google Scholar] [CrossRef]
- Csikszentmihalyi, M. Flow. The Psychology of Optimal Experience. New York (HarperPerennial) 1990. 1990.
- Magsi, H.; Malloy, T. Underrecognition of Cognitive Impairment in Assisted Living Facilities. Journal of the American Geriatrics Society 2005, 53, 295–298. [Google Scholar] [CrossRef]
- Hayajneh, A.A.; Rababa, M.; Alghwiri, A.A.; Masha’al, D. Factors Influencing the Deterioration from Cognitive Decline of Normal Aging to Dementia among Nursing Home Residents. BMC Geriatrics 2020, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; McCann, J.J.; Li, Y.; Aggarwal, N.T.; Gilley, D.W.; Evans, D.A. Nursing Home Placement, Day Care Use, and Cognitive Decline in Alzheimer’s Disease. Am J Psychiatry 2007, 164, 910–915. [Google Scholar] [CrossRef]
- González-Colaço Harmand, M.; Meillon, C.; Rullier, L.; Avila-Funes, J.-A.; Bergua, V.; Dartigues, J.-F.; Amieva, H. Cognitive Decline After Entering a Nursing Home: A 22-Year Follow-Up Study of Institutionalized and Noninstitutionalized Elderly People. Journal of the American Medical Directors Association 2014, 15, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, P.; Díaz de Bustamante, M.; Aparicio Mollá, S.; Arenas, M.C.; Jiménez-Armero, S.; Lacosta Esclapez, P.; González-Espinoza, L.; Bermejo Boixareu, C. Functional, Cognitive, and Nutritional Decline in 435 Elderly Nursing Home Residents after the First Wave of the COVID-19 Pandemic. Eur Geriatr Med 2021, 12, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Sefton, N.; Craig, K.; Meadows, S.; Neher, J.O. Quality of Life in Older Persons with Dementia Living in Nursing Homes. afp 2008, 77, 1011–1012. [Google Scholar]
- Brandriet, L.M. Changing Nurse Aide Behavior to Decrease Learned Helplessness in Nursing Home Elders. Gerontology & Geriatrics Education 1996, 16, 3–19. [Google Scholar] [CrossRef]
- Powers, E.T.; Powers, N.J. Should Government Subsidize Caregiver Wages? Some Evidence on Worker Turnover and the Cost of Long-Term Care in Group Homes for Persons With Developmental Disabilities. Journal of Disability Policy Studies 2011, 21, 195–209. [Google Scholar] [CrossRef]
- Janevic, M.R.; M Connell, C. Racial, Ethnic, and Cultural Differences in the Dementia Caregiving Experience: Recent Findings. The Gerontologist 2001, 41, 334–347. [Google Scholar] [CrossRef]
- Katz, M.B. Poorhouses and the Origins of the Public Old Age Home. Milbank Mem Fund Q Health Soc 1984, 62, 110–140. [Google Scholar] [CrossRef]
- A History of Elder Care. Bayview Healthcare St. Augustine 2015.
- Karakaya, M.G.; Bilgin, S.Ç.; Ekici, G.; Köse, N.; Otman, A.S. Functional Mobility, Depressive Symptoms, Level of Independence, and Quality of Life of the Elderly Living at Home and in the Nursing Home. Journal of the American Medical Directors Association 2009, 10, 662–666. [Google Scholar] [CrossRef]
- Samuel, L.J.; Szanton, S.L.; Wolff, J.L.; Ornstein, K.A.; Parker, L.J.; Gitlin, L.N. Socioeconomic Disparities in Six-Year Incident Dementia in a Nationally Representative Cohort of U.S. Older Adults: An Examination of Financial Resources. BMC Geriatrics 2020, 20, 156. [Google Scholar] [CrossRef]
- Nicholas, L.H.; Langa, K.M.; Bynum, J.P.W.; Hsu, J.W. Financial Presentation of Alzheimer Disease and Related Dementias. JAMA Internal Medicine 2021, 181, 220–227. [Google Scholar] [CrossRef]
- Martenson, C.; Taggart, A. Prosper!: How to Prepare for the Future and Create a World Worth Inheriting; RDA Press, LLC, 2015; ISBN 1-937832-77-5.
- Paul, L.A.; Hystad, P.; Burnett, R.T.; Kwong, J.C.; Crouse, D.L.; van Donkelaar, A.; Tu, K.; Lavigne, E.; Copes, R.; Martin, R.V.; et al. Urban Green Space and the Risks of Dementia and Stroke. Environmental Research 2020, 186, 109520. [Google Scholar] [CrossRef] [PubMed]
- Astell-Burt, T.; Navakatikyan, M.A.; Feng, X. Urban Green Space, Tree Canopy and 11-Year Risk of Dementia in a Cohort of 109,688 Australians. Environment International 2020, 145, 106102. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the Evidence on Modifiable Risk Factors for Cognitive Decline and Dementia: A Population-Based Perspective. Alzheimer’s & Dementia 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Britt, K.C.; Richards, K.C.; Acton, G.; Hamilton, J.; Radhakrishnan, K. Association of Religious Service Attendance and Neuropsychiatric Symptoms, Cognitive Function, and Sleep Disturbances in All-Cause Dementia. Int J Environ Res Public Health 2023, 20, 4300. [Google Scholar] [CrossRef]
- Upenieks, L.; Zhu, X. Life Course Religious Attendance and Cognitive Health at Midlife: Exploring Gendered Contingencies. Research on aging 2023, 1640275231188998. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Rolls, E.T.; Cheng, W.; Kang, J.; Dong, G.; Xie, C.; Zhao, X.-M.; Sahakian, B.J.; Feng, J. Associations of Social Isolation and Loneliness With Later Dementia. Neurology 2022, 99, e164–e175. [Google Scholar] [CrossRef]
- Minematsu, A. The Frequency of Family Visits Influences the Behavioral and Psychological Symptoms of Dementia (BPSD) of Aged People with Dementia in a Nursing Home. Journal of Physical Therapy Science - J PHYS THER SCI 2006, 18, 123–126. [Google Scholar] [CrossRef]
- Sahathevan, R. Chapter 18 - Dementia: An Overview of Risk Factors. In Diet and Nutrition in Dementia and Cognitive Decline; Martin, C.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2015; ISBN 978-0-12-407824-6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




