Introduction
Even though disconnecting us from the outside world, sleep still hosts internal conscious sensory experiences, or dreams, triggered by the generation of an internal, virtual world [
1]. Strikingly, these experiences usually evoke the sensation of being awake, since similar features as in our external sensorium (characters, objects, colors, places, and sounds) are incorporated in a realistic manner [
2]. Moreover, similarly to waking experiences, dreams reflect our current concerns, interests and personality, and are highly rich in emotions [
3]. As soon as we fall asleep, we stop consciously perceiving sensory stimuli from the external world, and instead get invaded by internal thoughts and hallucinations that are often unrelated to our previous immediate experiences [
4].
Despite their realism, dreams, especially from rapid-eye-movement (REM) sleep, often feature bizarre and creative elements, mostly because they do not simply replay previous experiences [
5,
6,
7]. In a study examining dream reports and waking activities from participants over 14 days, Fosse et al. [
5] showed that while 65% of dream reports incorporate aspects of waking experiences, the exact replay of waking events was found in only 1-2 %. Instead, dreams are constructed from a combination of various isolated, sometimes non-obviously related episodic fragments, [
8,
9,
10]. This combination of unrelated memories results in REM dreams often appearing bizarre and creative in retrospect. For instance, a person may dream of attending a tea party hosted on the moon, where the guests are historical figures speaking in riddles—a bizarre experience. Creatively, a dream could involve designing a building that transforms itself based on the weather, showcasing innovative solutions to climate change challenges.
The observed novelty in our dreams raises the question of their potential function. How could such a virtual, hallucinatory and fantastic experience benefit our cognitive functions? It remains debated whether dreams have any functionality at all or whether they are mere epiphenomenal byproducts of sleep [
11,
12,
13]. Contemporary theories often relate dreaming to memory consolidation [e.g., see [
14,
15,
16] or emotional processing [e.g., see [
17,
18,
19]. A further strand of theories assumes that the combination of memories into a new, virtual scenario during dreaming serves to enhance creativity [e.g., see [
9,
20]. Motivated by anecdotal evidence of scientific discoveries originating in dreams—e.g., the benzene structure by Kekule (1865) or the chemical neurotransmission by Loewi (1936) [
1]—the role of dreams in creativity has been taken into wider consideration. It has been proposed that the creative associations between unrelated memories during dreaming could lead to the discovery of unexpected solutions which lies at the essence of creative problem-solving [
9]. Through this process, the dreamer is enabled to engage in creative experimentations that might once serve as a solution for potential future problems [
8,
21], such as e.g., rehearsing threat perception and avoidance. However, empirical studies report that dreams rarely contain practical solutions to real-life problems, in addition to the fact that most dreams are forgotten [
10,
22,
23,
24].
In contrast, in a recent computational study, we [the authors of [
25] argued that the creative aspect of dreams serves a more basic function than creativity itself, that is, dreaming facilitates learning semantic representations based on sensory experiences gathered during waking. In this study, we proposed a cortical architecture, where sensory inputs are perceived through feedforward pathways of sensory cortices, while dreams are generated through the feedback pathways. In particular, we show that the generation of dreams during REM sleep can be explained by an adversarial learning mechanism inspired by Generative Adversarial Networks [GANs, [
26] where feedback pathways trick feedforward pathways into believing that the dream comes from outside. Crucially, this mechanism leads to the acquisition of structured, semantic cortical representations, essential to perform downstream tasks such as object recognition.
In this article, we provide an accessible overview of this computational approach, thereby explaining how adversarial dreams could facilitate creativity and the learning of an internal representation of objects and concepts from the external world. We show how an adversarial dream implicitly assumes meta-structures in the brain (a `conductor’) that gate and represent information to interpret activity in early sensory cortices either as dreamed/imagined, or being evoked from the external world. This leads us to the notion of dream awareness, and more specifically to the awareness of the dream content and the dream state. We discuss the model in light of previous theories of dream origin, functions, and changes of dreams across lifespan.
1. A Computational Model for Creative Dreams
Even though dreaming is a universal phenomenon, characterizing its role is still, up to this day, a challenging task. For instance, it is difficult to assess whether the improvement of skills after a night of sleep is due to the occurrence of a certain dream, or to other physiological features of sleep such as hippocampal replay during NREM sleep [
27]. In other words, the potential effects of sleeping and dreaming are entangled and thus confounded, since they are naturally co-occurring. Nonetheless, there have been some attempts to investigate the effect of dreaming, notably through the use of pharmacological interventions to suppress REM sleep in participants [see, e.g., [
28]. Despite these efforts, disentangling the effects of dreams within sleep remains complex and largely unclear.
Here, computational models can help to quantify physiological features such as dreams and replay through simulations, and thereby decipher their contributions within a defined task. This was the challenge of our study that, through the construction of a “perturbed and adversarial dreaming” (PAD) model, suggests a role for dreams in learning to classify objects that come in a variety of versions in different visual scenes [
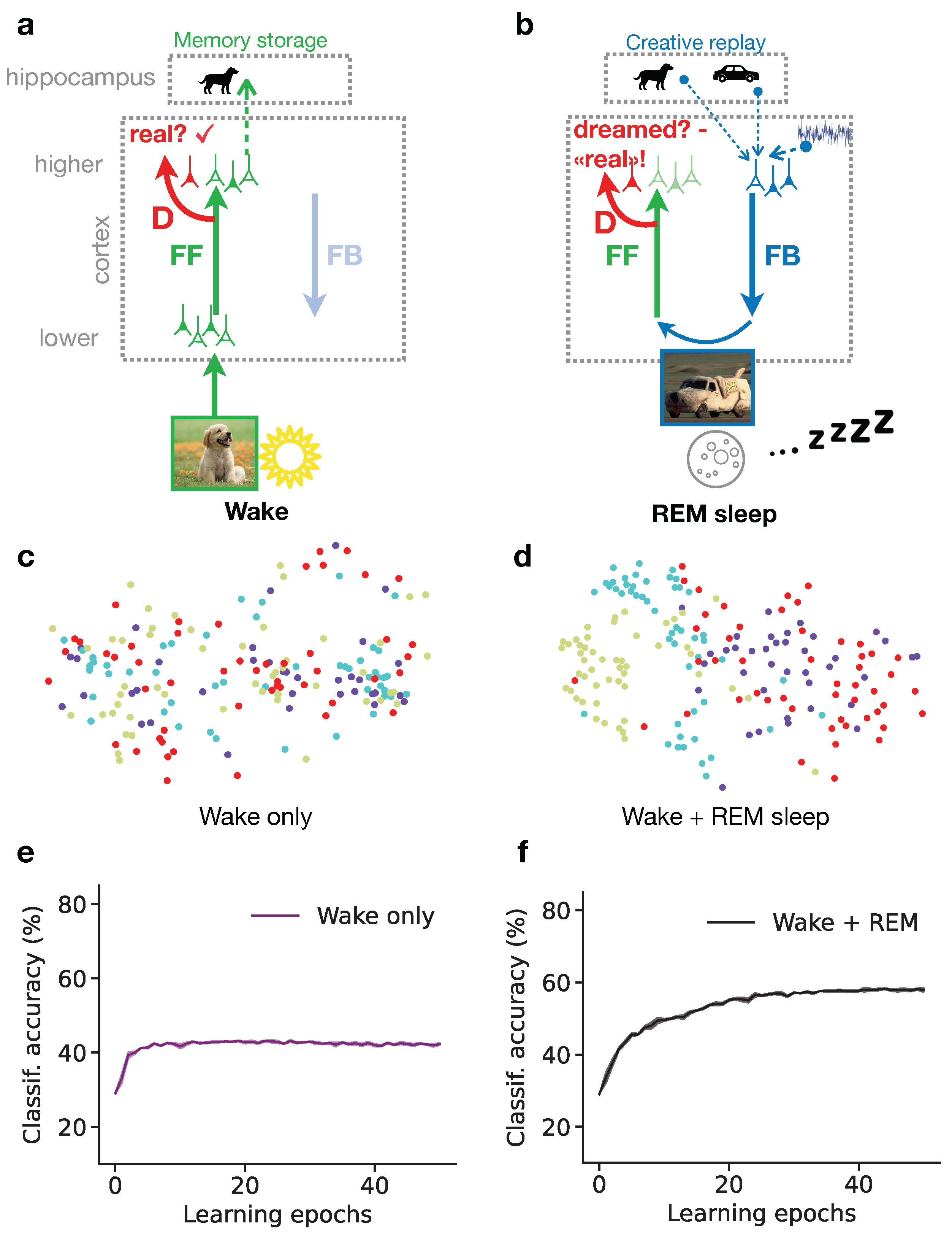
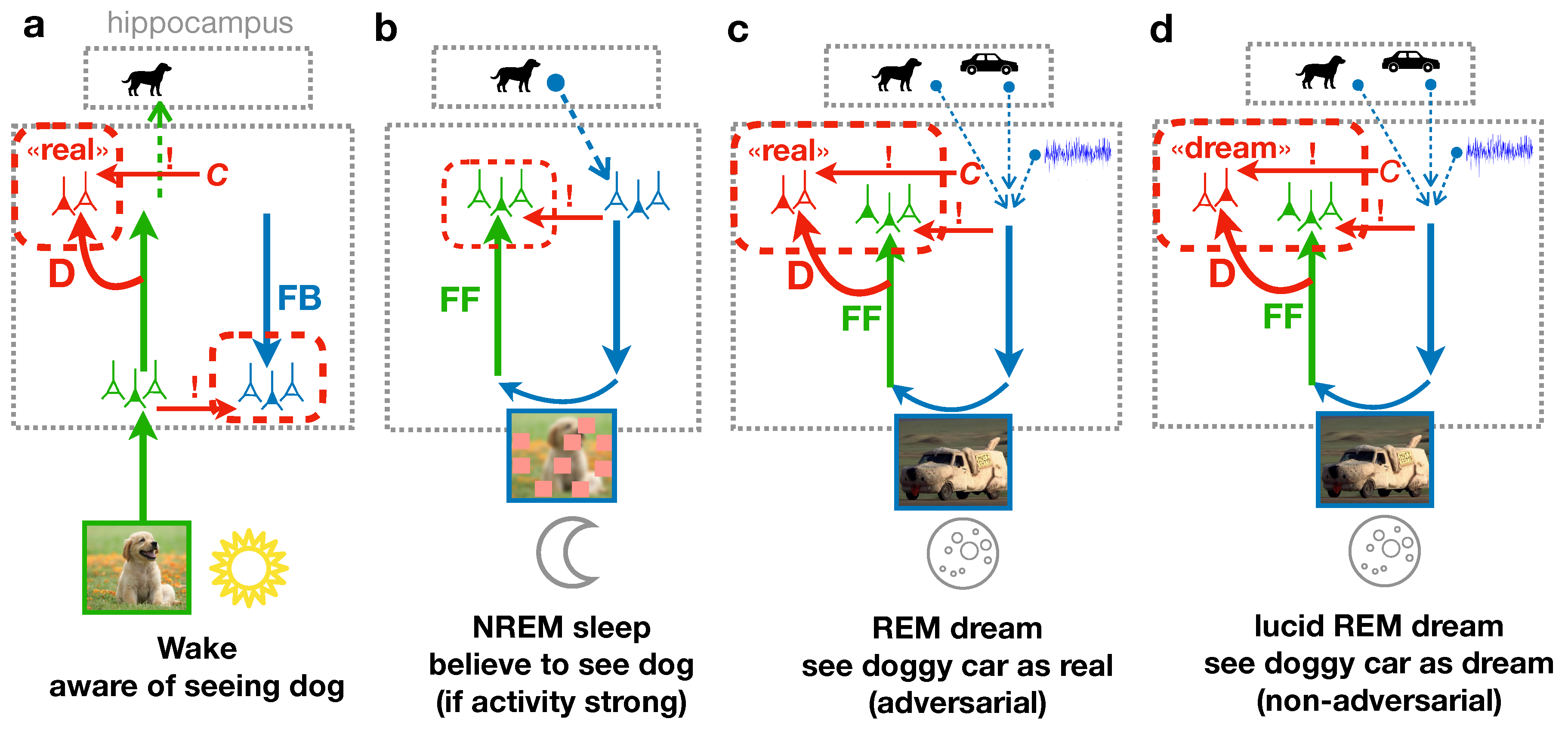
25]. The PAD model is composed of a cortical feedforward (FF) pathway with a discriminator network (D), and a feedback (FB) pathway that generates sensory activity from a hippocampal memory. The system can either be in a wake state, where external stimuli are directly driving the FF pathway, or in a dream state, where sensory activity is generated by a hippocampal memory replay fed into the FB pathway.
During wakefulness (fig:elifea), the cortex is exposed to diverse natural images that are processed along the cortical hierarchy through the FF pathway, until incorporated in high-level neuronal representations, for example in the Inferior-Temporal (IT) cortex [
29]. Snapshots of the activity in these representations are temporarily stored in the hippocampus as episodic memory, that, for instance during sleep, can be replayed into the cortical representation. During the forward processing of the sensory input, the discriminator network D learns to recognize that the sensory activity evoked by an image presentation is
real (fig:elifea, red `D’).
During the REM sleep phase (fig:elifeb), the representation stored from the previous day is replayed from the hippocampus (“dog” memory) along with past, sometimes unrelated memories (“car” memory) and some additional cortical background activity (which is modeled as noise). The resulting activity in higher cortical areas is representing the dream content. It is processed along the cortical FB pathway down to low-level sensory areas where details are added to the dream. Due to the combination of diverse memories, the dream might contain various unrelated elements, such as a car having the texture or shape of a dog. Yet, the mere superposition of the abstract representations in the higher cortical area does not entail instructions of how the FB pathway can succeed in generating a realistic sensory activity out of the novel combination. This is where the principle of GANs, i.e., adversarial learning, comes into play. The dream is processed through the FF pathway and the discriminator D. During the REM sleep, the discriminator learns that the internally generated sensory activity is dreamed. The FB pathway, however, learns to improve the realism of its generated activity, and with that makes the task for the discriminator to correctly classify the activity as dream more difficult. This FB pathway represents the adversarial learning, leading to the adversarial dreams that try to evoke a misclassification of the discriminator.
The described PAD model of sleep is trained by repeating many wake-sleep cycles, whereby a set of natural images is repeatedly processed by the FF and FB pathways and the discriminator D. Once training is completed, we can examine the quality of the learned high-level representations (fig:elifec-d) within the learned latent space of the model (the highest cortical representation). Note that these representations are not learned in a supervised manner with an explicit teaching signal that would indicate the ground truth category of the observed object. Instead, the representations are formed through unsupervised learning, leading to a clustered higher-level neuronal representation of visual objects. The structure of the latent space can be illustrated by Principal Component Analysis [PCA, [
30] applied to the activity in the last layer of the FF network. Projecting the activity vectors to the first two principal components, this procedure allows visualizing how the representations of various learned objects cluster in the latent space. The visualization is not part of the model, although PCA could itself be modeled by a neuronal network [
31].
Comparing PCA projections after different training regimes allows for investigating the effect of wakefulness versus REM dreams: If only the wake phase is present, the obtained PCA projection shows that representations from different object categories are entangled, indicating that wakefulness is not sufficient to construct structured semantic representations (fig:elifec). When both Wake and REM sleep phases are simulated (fig:elifed), the PCA projection shows relatively distinct clusters of latent representations according to the semantic category (“class identity”) of the corresponding images. The model thus tends to organize latent representations such that high-level, semantic clusters are distinguishable, potentially helping humans and other animals to discern different object categories from their sensorium. This is in particular important for animals, that do not receive explicit teaching signals in the way humans and their children do throughout development.
These results can be quantified by evaluating the performance of a linear decoder (classifier) trained on high-level cortical representations obtained throughout the model simulation. If the REM phase is included in the training, the accuracy of the classifier tends to be much higher than if only the Wake phase is simulated (fig:elifee-f). The results show that the generation of dreams during REM sleep is essential to organize high-level representations according to the semantics of the sensorium, suggesting that dreaming is an essential component of learning.
2. Semantization Requires More than Memory Replay
Previous cognitive theories of sleep, such as “semantization” theories [
32,
33], suggest that the commonalities between multiple experienced episodes are extracted during NREM sleep to form a cortical semantic representation. A cognitive model [
34] proposed that semantic formation is based on the invariant overlapping and statistical regularities between single replayed episodic memories. Areas of overlap are strengthened via Hebbian learning, allowing for an abstraction of shared elements among these memories, the so-called semantic “gist". For example, the reactivation of various memories of “cat experiences" facilitates the extraction and consolidation of the concept “cat" from repeating features with episodic memories (four legs, pointed ears, tail, etc.) in cortical representations.
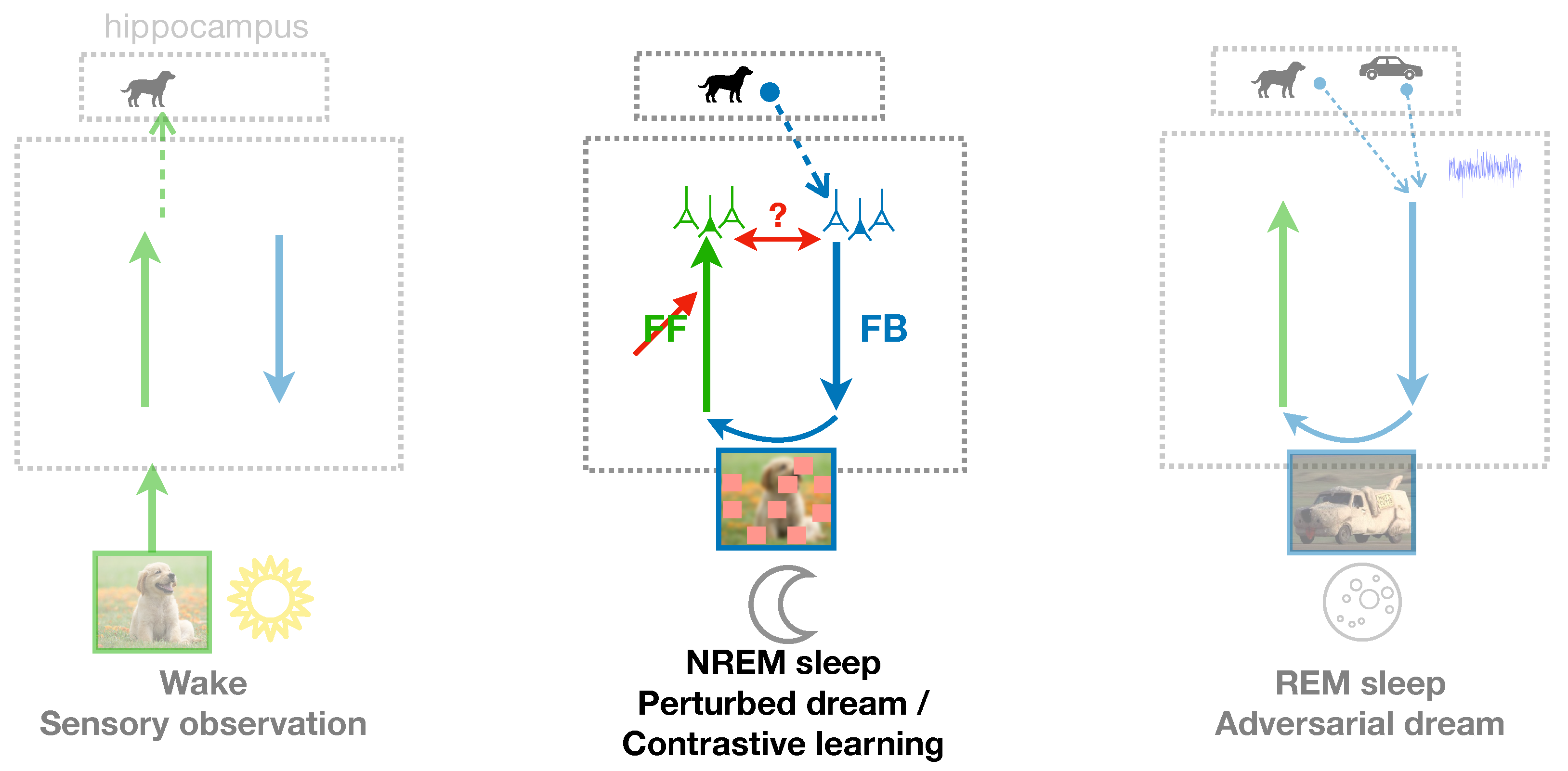
The PAD model offers a further perspective on the semantization process during sleep. It suggests that the semantization of cortical representations is more likely induced by REM dreams, featuring combinations of episodic memories, than by the replay of single episodic memories during NREM sleep. We incorporated NREM sleep into our model by a phase where individual hippocampal memories are replayed without combination with other memories. Instead, the activity in the sensory area after processing through the FB pathway is perturbed by randomized patches (see fig:fullmodel and section on NREM sleep below). We find that NREM sleep in our model has little or no impact on the acquisition of semantic representations—even when adversarial learning based on individual memory replays is enabled in the FF/D and FB pathway (see Deperrois et al. [
25] for details). The simulations indicate that, to extract semantic concepts from sensory data, the brain must go beyond merely replaying previous experiences as classically accounted for in NREM sleep [
35]. It appears that novel but realistic contents must be internally created from simultaneous multiple memories that explore the boundaries between object categories, and help to form a representation of these objects based on contents and content differences. The suggested role of creative REM dreams may help to refine cognitive theories about sleep function and to delineate the role of NREM and REM sleep in memory semantization.
The described creative memory replay can be seen in the light of the overfitted brain hypothesis, which proposes that “nightly dreams evolved to combat the brain’s overfitting during its daily learning” [
24]. More specifically, the overfitted brain hypothesis posits that the creation of corrupted sensory activity from stochastic memory replay during sleep increases the generalizability of the object representations learned during the day. The stochastic memory replay aligns with the NREM phase in our model. In our NREM phase, hippocampal memories are fed back into the cortex and randomly perturbed, in order to train the cortical representations in the FF pathway to become noise resistant. Yet, as we showed, the random perturbations in single memories alone are not as efficient in separating objects in the cortical representation as when memories are paired in a creative replay during REM [
25], fig:elifed]. The PAD model therefore suggests that sleep increases generalizability with both, stochastic replay during NREM and – going beyond the overfitted brain hypothesis [
24] – also with creative replay during REM sleep.
3. Adversarial dreams on the edge between fantasy and reality
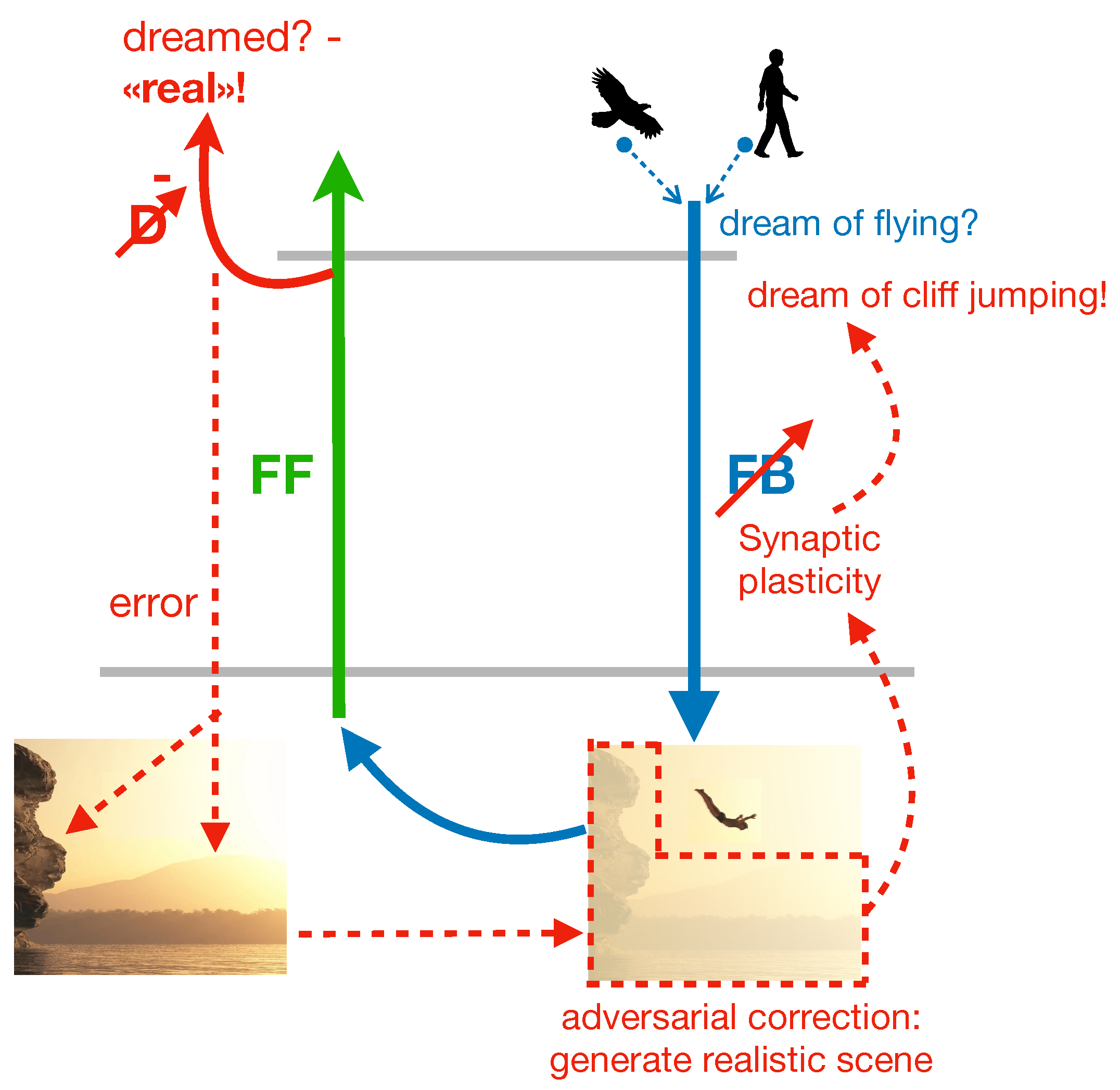
Aside from proposing a role for dream function, the adversarial dreaming principle of the PAD model suggests a mechanism for how internal sensory activity can be generated in the brain, and how an equilibrium between fantastic and realistic aspects can be maintained, potentially beyond the stage of dreaming. Following the PAD framework, we assume that different memories, for example a memory of an eagle and a human (the dreamer), are concurrently replayed from the hippocampus and combined in high-level cortical areas (fig:cliff). The combined activity is sent through the FB pathway, leading to the generation of a dream representing a flying human, which is, while being novel and creative, not very convincing in terms of realism. This lack of realism allows the discriminator to easily detect that the sensory activity is dreamed and not real.
The adversarial learning of the FB pathway will restore the realism in this scene. The key point of adversarial dreaming is that the FB pathway attempts to fool the discriminator into believing that the dream actually is a sensory experience. There are two ways to implement this fooling: either by reverting the synaptic plasticity in the FB pathway and keep the correct target for the discriminator (as described and simulated in Deperrois et al. [
25]), or keep the plasticity in the FB pathway untouched, while reverting the target for D. It is this second version that we suggest here as a biological implementation. The reason is that the target imposed on the discriminator may provide an awareness signal for the content, as we elaborate later (see fig:awereness). The REM phase still needs a sign-switch in the synaptic plasticity, and this is now on the synapses of the discriminator network during REM sleep (fig:cliff). In fact, while D learns during wakefulness to correctly classify externally generated sensory activity as
real, it should learn to correctly classify internally generated sensory activity as
dreamed during the REM phase. A sign-switch of synaptic plasticity (necessary in the D network when the target remains
real during the REM phase) was experimentally observed by the action of acetylcholine [
38] that is also involved in the regulation of REM sleep [
21].
In detail, the “adversarial game” during the REM sleep starts by imposing the wrong target
real! on the discriminator output. This typically leads to an error between the target and what the input from D would like to produce. To still improve the discriminator, plasticity in D is inverted, as just explained. But plasticity in the FB pathway remains the same, so that the FB network now tries to produce a sensory activity that becomes more real, as requested by the target
real! for D. The FB pathway is also told how to improve because the error from D, that carries the instruction to improve the realism in the sensory activity, is propagating down through D and the FF pathway to the FB network (for a biological form error backpropagation see Sacramento et al. [
39]). In other words, the FB pathway adapts such that the internally generated activity in the sensory area will more likely fool the discriminator into believing that the activity is real and produced from the outside. As a consequence, after thousands of wake-sleep cycles, the dream is thus better blended into the reality of the outside world. The same computational principles may also apply to improve creative imagination during wakefulness (see Discussion).
To come back to our example, we consider the experience of dreaming about flying through the clouds, resulting from combining the hippocampal memory of a bird and a human (fig:cliff). The dream could be easily unmasked as “fake” by the discriminator D. Yet, sometimes humans engage in sports such as cliff jumping, whereby it appears as if the jumper is flying. Accordingly, the realism of the dream could be increased by adding visual details such as some cliffs and water. On the other hand, the omission of these details signifies errors that must be corrected to enhance the dream’s plausibility. In this case, the synaptic connections of the FB pathway are modified to generate a more realistic, plausible dream. Consequently, in a subsequent REM phase, we might find ourselves leaping off a cliff to fly over the sea. The novel dreams created during the REM phase in our model will also change the early cortical activity produced during wakefulness when `mind-wandering’ through the latent representation. This in turn may influence our future actions. For instance, we may go cliff jumping the next day after the REM dream has generated the corresponding scene.
Adversarial learning might thus explain how dreams, originating in a creative combination of memories, can be constrained to become more realistic and make them compatible with our waking experiences and actions. This is in line with Hobson’s pioneer activation-synthesis theory that claims that REM dreams result from the brain “making the best of a bad job in producing even partially coherent dream imagery from the relatively noisy signals sent up to it from the brain stem” [
40], p. 1347]. In the spirit of this citation, the modeled generative process during REM sleep starts with a noise signal added to random combinations of memories and tries to produce a coherent and realistic cortical activity through adversarial learning. We will next see how the resulting balance between fantastic and creative dream elements can be useful to trigger creative insights.
4. Adversarial Dreaming at the Heart of Creativity?
Adversarial dreaming may also influence creativity during wakefulness. As a consequence of adversarial dreaming, the FB projection can lead to the generation of sensory activity patterns that have unlikely been evoked by previously experienced stimuli (novel combination of episodic memories), but that nevertheless may be possible in reality (increased realism through the adversarial game). This kind of constrained simulation may have functional benefits in terms of creative thinking and gaining insights in general.
Novel cognitive insights were argued to result from a period of “incubation”, where non-obvious, remote associations from memory (or knowledge) elements are brought into our thinking [
41,
42]. These associations can sometimes be compatible with reality, in which case they can provide a solution to a complex problem through a creative insight [
43], “Aha moments”] that deliberate reasoning alone may not provide. Dreaming appears to be an ideal stage to promote novel associations and eventually enhance creative insights, as previously suggested [
9,
44,
45,
46]. More recent studies show that subjects with narcolepsy, characterized by falling asleep directly into REM sleep and having a higher percentage of REM sleep [
47], show higher creative potential [
36].
Creative problem-solving is also promoted by the twilight stage before sleep, as allegedly exploited by the great inventor
Thomas Edison [
37]. While napping down, he held two balls that would drop and awaken him as he entered deep sleep, enabling him to capture thoughts about his inventions that would otherwise be lost. Experimental examination confirmed this anecdotal evidence and revealed that creative problems are easier solved on the verge of falling asleep and being awakened from the first stage of NREM sleep (N1), as opposed to being awakened from the second stage of NREM sleep (N2) or staying awake throughout [
37]. Creative problem-solving is further fostered by a 60-minute nap as opposed to a 60-minute rest [
48]. In a similar line, Ritter et al. [
49] found that creative performance is already boosted by a simple eye-closure as opposed to keeping eyes opened. To relate these findings to our model of creative dreaming and imagination, we postulate that during the described creative stages our adversarial processes are triggered. The specific form of memory replay and synaptic plasticity arising in the adversarial learning may be induced through differential activation of neuromodulators, as it is also observed during the transition across sleep stages [
50], and in shaping the memory replay in general [
51].
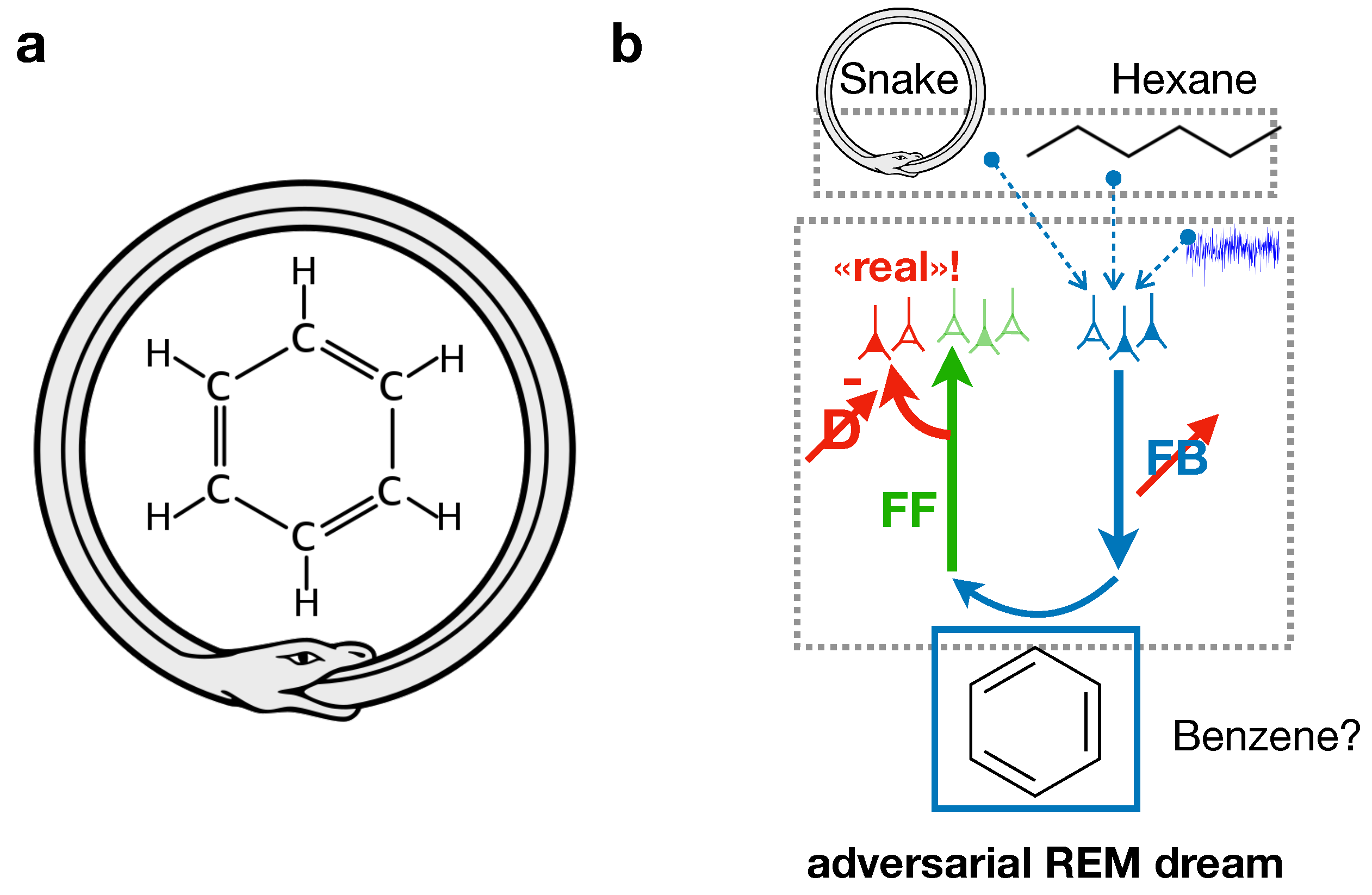
Beyond Edison’s nap technique, anecdotal evidence exists from August Kekulé who discovered the Benzene structure through a dream in which he combined two non-related concepts, a snake biting its tail, and the carbohydrate molecular chain [
1]. Here, the adversarial dreaming framework could explain the occurrence of such insights (fig:benzenestructure). The memories of a depiction of the ouroboros symbol (a snake eating its tail) and the hexane molecule could be randomly replayed from the hippocampus and combined in high-level areas during REM sleep. Propagating this activity down through the FB pathway, the adversarial learning mechanism could allow the generation of a dreamed molecule that contains aspects of the ouroboros, such as its cyclic shape. By forcing this dream to be realistic against the discriminator judgement, the dreamed molecule could be harmonized with known properties of chemistry, so that it might exist in reality. Naturally, not all creative combinations experienced during dreaming are useful, and their usefulness is ultimately determined by how compatible they are with the actual external world.
This suggests that additional steps may be involved, such as experimentally verifying the occurred insight—reflected in Kekulé ’s words: “Let us learn to dream, gentlemen, and then perhaps we shall learn the truth... but let us beware of publishing our dreams before they have been put to the proof by the waking understanding.” (account of his famous dream of the benzene structure, as quoted in Olah [
52], p. 54)
5. NREM: Improving Memory Robustness by Perturbed Replay
The suggested adversarial REM dreams represent part of the full PAD model that also includes NREM sleep. For the past decades, NREM sleep has been associated with memory consolidation [
35,
53,
54] and memory semantization [
32,
34]. The main mechanism hypothesized to drive these consolidation processes is the reactivation of hippocampal representations observed during slow-wave sleep, the deepest stage of NREM sleep[
55]. Accordingly, replaying hippocampal memories allows a transfer to cortical networks for long-term retention via Hebbian learning [
54], possibly through an abstraction of semantic concepts by discarding spatiotemporal details and keeping the commonalities between replayed memories [
32,
33,
34].
The PAD model also incorporates hippocampal replay during NREM sleep and suggests a complementary role of REM sleep in memory consolidation and semantization. While memory replay has been extensively associated with memory consolidation, as such, it is not obvious how the reactivation of previous memories alone could improve cortical representations. Here we argue that non-creative dreams might be beneficial if some perturbations are applied, making the recognition of dreamed objects more challenging. During NREM phases in the PAD model, a single episodic memory is retrieved from the hippocampus (instead of multiple memories in the case of REM) (fig:fullmodel, middle), resulting in dreaming of a sensory input previously experienced. Additionally, this dream is perturbed by some randomized occlusions in the sensory area, and the FF pathway is trained to map the perturbed dream to the initially replayed latent activity in the higher cortical area. Such a learning paradigm is reminiscent of the sleep phase in the Wake-Sleep algorithm [
56]. In this algorithm, the FF recognition pathway is trained during the Sleep phase so that it inverts the FB generative pathway. The generative FB pathway is itself trained during the Wake phase so that it learns to reproduce the sensory activity from the internal representation generated by the FF pathway.
By replaying and perturbing previously experienced inputs, the PAD model shows that learned cortical representations are more robust to perturbations that might occur in the visual field when an object is partially hidden by obstacles (see
Figure 5c&d in Deperrois et al. [
25]). While REM dreams tend to semanticize cortical representations through their creative process, NREM dreams make them more robust to environmental noise. Together, NREM and REM dreams act complementary to construct semantic, robust representations.
In the PAD model the REM phase is the main driver of semantization. Future modeling work could identify other elements so that the NREM phase, while still replaying a single memory at a time, could contribute more to memory semantization, not only to making memories robust against noise. As recently suggested [
57], NREM sleep could, for instance, host a contrastive learning process to push object representation farther apart and hence improve semantization.
Contrastive learning [
58,
59,
60] is in fact a training procedure used in recent artificial intelligence models as a way to efficiently learn semantic representations. The main idea is to construct a latent space such that similar sensory inputs (positive examples) are represented close together, while dissimilar sensory inputs (negative examples) are pushed apart within the latent space. During the NREM phase, positive examples can be obtained by systematically perturbing the activity generated in the sensory area out of the single memory replay, for instance by mimicking the view point of the object, and with this creating two different instances from the same hippocampal memory. These two instances of the low-level object representation are then learned to be mapped close to each other in the high-level representation. Negative examples in a NREM memory replay can be obtained by pushing the projection of the FF pathway to the higher cortical area away from previous hippocampal memories replays in this higher area [
57].
6. Adversarial Processes Explaining Dream Awareness?
Our model for unsupervised learning of object representations out of naturalistic sensory inputs – what we call semantization – implicitly assumes various meta-structures that gate the learning process. A first meta-structure pops up when considering the classical Wake-Sleep algorithm [
56] described above as a model for NREM sleep (that we extended by random perturbations in the replay). For instance, when replaying a memory from the hippocampus into the FB pathway at the level of the higher cortical area, this activity serves as a target for the activity that is re-created through the FB→FF loop back to the same higher cortical area (red `?’ in fig:fullmodel). One way to biologically implement the hippocampal replay as a target or “teaching signal” for the FF neurons is to “nudge” these neurons in the soma, while the synapses projecting from the FF pathway to their dendrites learn to reproduce the somatic nudging [
61]. When the somatic nudging is strong enough, the match between the dendritic input and somatic activity may elicit a dendritic calcium spike, and with this also the perception [
62]. It was argued that during sleep, when such dendritic calcium spikes arise, the dreamer is becoming aware of the content of the dream [
63], although not necessarily of the fact that this is a dream (fig:awerenessb). Becoming aware of the dreaming content is what dreams differentiate from the more general state of sleep without dreaming.
During the Wake, the FB neurons in the sensory area are nudged by the FF sensory neurons (that are themselves driven by the sensory input). A possible match between the teacher signal an the top-down signal through the FB pathway may elicit a dendritic calcium spike in these FB neurons, and again signal the awareness of the sensory content (Takahashi et al. [
62], fig:awerenessa). Crucially, and different to NREM sleep, the output of the discriminator network D receives an additional nudging input from the conductor
C, signaling the target
real. This conductor is itself a neuronal population representing the meta-information whether the sensory activity should be perceived as externally or internally generated. We postulate that the teaching signal in the FB neurons from the low-level FF neurons makes us aware of seeing an object (content-awareness), while the teaching signal in the D neurons from the conductor makes us aware that the object is real (state-awareness, upper red in part in fig:awerenessa). The same conductor was suggested to be also involved in forming consciousness Benitez et al. [
64].
During REM sleep, the adversarial learning also requires activity in the discriminator network. As we suggest here, the conductor adversarially sets the target real for the output of the discriminator D (while it reverts the plasticity in D so that this still can learn that the sensory activity is dreamed). With the real-target being backpropagated, the FB pathway is told how to improve the realism of the sensory activity it generates. As in Wake, the conductor signal in the discriminator neurons tells the subject that the sensory activity is (incorrectly) generated from outside and is considered as real (state-awareness). In addition, subjects may become aware of the dream content because the FF neurons in the higher cortical areas are nudged by the FB neurons, initiated by the memory replay (content-awareness, fig:awerenessc). It is tempting to postulate that the double nudging of the FF neurons and the specific real-discriminator neurons during REM sleep is the reason that subjects perceive the sensory activity as real during sleep, and remember more often REM dreams, as compared to NREM activity where the D neurons do not receive a teaching signal. According to the PAD model we become aware of a REM dream because it is adversarial (fig:awerenessc).
Lucid dreaming in this model is explained by nudging the higher cortical FF neurons with the target activity set by the FB pathway (as in NREM and REM), but the discriminator neurons are now nudged to encode
dreamed, corresponding to the true (non-adversarial) state of the sensory activity (fig:awerenessd). The teaching signal
dreamed for the discriminator neurons may give the subject the awareness that its current state is the dream, as in fact happening in lucid dreaming [
65]. To keep a functional benefit of lucid dreaming, the plasticity in the FB pathway has to be turned of, as in NREM sleep (fig:fullmodel), so that the FB pathway is not unlearned by reverting the discriminator target. Correspondingly, plasticity in the discriminator network should not be reverted (and instead can be as in the Wake state).
If during lucid dreaming the described plasticity modulations are not correctly implemented, while the target in D is still switched from
real to
dream, negative consequences of the dream experience are expected. With a discriminator target being
dream and plasticity in the FB pathway remaining turned on, random combinations of memory replays would be pushed further away from any realism, easy detectable by D as dream. In fact, failed induction of lucid dreaming, potentially explained by an unilateral switch of the discriminator target alone without stopping FB plasticity, may lead to harrowing dysphoric dreams [
66]. However, if the switch in the D-label is correctly synchronised with the required plasticity changes, the awareness of the dream and the dream content during lucid dreaming may help to consolidate specifically selected memories, as also exploited in therapy therapeutic applications [
66].
Overall, the meta-structure of a discriminator and a conductor coming with adversarial dreaming opens a door to differentiate between (
i) sensory activity that we become aware of, or not, during wakefulness, (
) the memory replay during sleep without dreaming, likely happening in NREM sleep, (
) the awareness of the dream content while sleeping, but not being aware of the dream state itself, as in REM sleep, and (
) the awareness of the dream content and the dream state, as in lucid dreaming. These awareness modulations, and in particular the state-awareness of the dream, is potentially modulated by the same ratio of acetylcholine over noradrenaline and serotonin that is also shown to tune NREM sleep and other metacognitive processes [
21,
67].
Discussion
In this perspective article we have reviewed the PAD (Perturbed & Adversarial Dream) model—a novel proposal for the formation, function, and interplay of NREM and REM dreams. This model is based on the concept of GANs (Generative Adversarial Networks, Goodfellow et al. [
68]), which have been proposed to be implemented in the brain [
69]. GANs come with a discriminator network that tells the internally generated sensory activity apart from the externally induced sensory activity. Such a discriminator network may be realized in the brain by a reality monitoring region located in the anterior prefrontal cortex [
70].
The PAD model suggests that during REM sleep new sensory contents are created out of a combination of stored hippocampal memories, shaped by an adversarial game between FB and FF pathways improving the realism of the dream. While the proposed adversarial mechanism has shown benefits for learning semantic representations
in silico, we have also discussed its potential implications for higher-level cognitive functions, such as enhancing creative insight. We suggested a complementary role of non-creative dreams, mostly occurring during NREM sleep, in improving the robustness of cortical representations against variations in the sensory inputs. While our model focuses on the role of sleep and dreams in memory semantization and creativity enhancement, dreams are also assumed to be involved in processing emotions and motivation [
71] that are not explicitly addressed here. Nevertheless, the suggested functional structure of REM and NREM sleep aligns with various empirical findings and implicates some promising directions for empirical studies in human subjects. In general, the PAD model with the `neuronal conductor’ that orchestrates the learning in the various sleep stages offers a framework to study metacognition. It may explain how the awareness of dream contents in REM versus NREM sleep, and how the phenomenon of lucid dreaming as a state-awareness, may arise.
Empirical Justifications and Predictions
The PAD model generates empirical predictions that can be organized along three distinct lines. First, REM dreams should facilitate the emergence of semantic representations. Second, REM dreams should enhance creative insights. And third, dreams can only enhance creative insights if they are balanced in terms of fantastic and realistic elements—if a dream is overly detached from, or too close to reality, it cannot help creative problem-solving. The PAD model predicts, that the adversarial game between FF and FB pathways results in an equilibrium of fantastic and realistic elements, beneficial for creative insight. Before we further elaborate on empirical findings and suggest possible studies, it is worth pointing out that evaluating the effects of REM dreaming encompasses several significant problems. Measuring the effects of dreaming is complicated by the fact that dreaming naturally occurs only during sleep. This co-occurrence renders it hard to disentangle the unique effects of dreaming versus sleeping. Additionally, it is difficult to directly attest whether a subject is dreaming or not, or determine what the subject is dreaming about (although a real-time dialogue between experimenter and dreamer is possible [
72] and images can be reconstructed from fMRI activity [
73]). Finally, sleep deprivation is not only experimentally challenging but also ethically delicate.
Semantization. The first line of experimental investigation arises from the model’s prediction that REM dreams facilitate the emergence of semantic representations. We propose to employ a category learning task in which subjects must acquire representations for novel objects. As a dependent measure, it is evaluated how well the acquired representations can be generalized to previously unseen images of the objects. Immediately after completing the task, the performance of one group of participants is evaluated, while the performance of another group is evaluated after a night’s sleep. In addition to a night’s sleep after the learning task, the third group of participants receives a pharmacological agent known for inhibiting REM sleep, such as e.g., an anti-depressant. [e.g., see [
28,
74]. This design would allow us to investigate the potential effects of REM dreams on the semantization process of cortical representations. However, as pointed out above, such a design does not allow to disentangle the effects of dreaming vs. sleeping. Nevertheless, evidence that REM sleep improves category learning, compared to no sleep and REM-inhibited sleep, would be consistent with the PAD model’s predictions. Given the difficulties arising from the entanglement of sleeping and dreaming, we further suggest testing the experimental predictions of the PAD model by investigating the effects of mental imagery—another process that internally generates visual experience. The cognitive process of
mental imagery is assumed to cause perception-like experience of visual stimuli in the absence of corresponding external stimulation [e.g., [
75,
76]. In contrast to dreaming, mental imagery is voluntarily triggered, its content is relatively controllable [
77], and there is no entanglement with sleep. These characteristics render it comparably more suitable for testing the effects of internally generated experiences on category learning. Considering that mental imagery shares the same neuronal substrates as dreaming [
2,
77], we suggest that mental imagery is a valid proxy process to test the predictions of the PAD model on semantization. We propose to employ a category learning task as described above. During the task, some subjects are asked to perform additional mental imagery training sessions, whereby the objects to be learned (for instance a `doggy car’) need to be imagined. In parallel, a control group of subjects would perform the category learning task without engaging in any mental imagery. The PAD model predicts that subjects who internally generate additional visual input by mental imagery (postulated to activate the adversarial process of improving the realism of the imagination), learn representations that become easier to linearly separate (as compared to the control group).
Finally, while model simulations suggest, that the creative combination of episodic memories during REM dreams facilitates semantization, this should not be the case for the non-creative replay during NREM sleep. Yet, the PAD model predicts that dreams during NREM increase the robustness of learned representations. After completing the category learning task, participants proceed to sleep. They are then awakened from sleep upon the onset of REM sleep, as indicated by polysomnographic recordings. Upon awakening, participants are tasked with categorizing perturbed versions of the images from the categorization task (e.g., added noise, blurring, or occlusions). According to the PAD model, we hypothesize that classification accuracy will be higher for participants who have experienced a phase of NREM sleep compared to a control group who have not undergone a NREM sleep phase.
Creativity and REM dreams. The second line of experimental investigation arises from the central claim that creativity is nurtured by REM dreams, as has previously been shown [
9,
20]. Further evidence that adversarial learning could be involved arises from the observation that prefrontal networks implicated in reality monitoring [
78] are generally deactivated during REM sleep [
79], while leaving the question open whether specific REM activation sites may also be related to daydreaming and creative visual imagery during wakefulness [
80].
We also postulate a difference between NREM dreams (non-creative; only replay of memories) and REM dreams (creative; recombination of memories). In this line, semantic analysis of dream protocols showed that REM dreams are likely composed of more minimal-story-units than NREM dreams [
81], consistent with the model assumption that REM dreams are composed of a mixture of episodic memories. Moreover, the analysis of dream protocols by non-semantic word graphs has shown that REM dreams are more complex and have a larger connectedness (although they are graph-theoretically less random-like) than NREM dreams [
82].
Since creative problem solving is also shown to be boosted in the first NREM sleep stage (N1, Lacaux et al. [
37]) by exploiting Edison’s technique, or by simply closing the eyes [
49] during active imagination, one may wonder whether an adversarial process postulated during REM sleep is likewise in play during the N1 stage or the imagination with closed eyes. To test such a hypothesis one may look at similarities in the local characterization of REM vs. N1 sleep stages in MEG signals [
83,
84], for instance. While similarities in metacognitive processes during REM sleep and wakefulness have been linked to similarities in EEG signals [
21,
67], linking adversarial and creative processes to specific brain signals, however, will remain a challenge.
REM dreams becoming more realistic. The PAD model posits that REM dreams become more realistic during our learning process and during the refinement of cortical representations in general. In the model, the formation of a good representation requires 10 to 40 thousand Wake-NREM-REM cycles (with 4 cycles/night corresponding to roughly 8–27 years). In the human brain, the formation of cortical representations of objects and concepts spans over childhood into adolescence [e.g., see [
85,
86,
87]. Hence, when applied to a real-world scenario, the learning process covered by the model may extend beyond the period during which humans acquire representations. It has been reported that children’s recurrent dreams are more likely to contain monsters and ghoulish creatures, while with aging and maturation, recurrent dreams are more likely to represent personal competences [
88]. Likewise, the frequency of nightmares decreases from early adolescence to late adulthood [
89], and so does the frequency of dream recalls [
90]. The hypothesis that REM dreams become more realistic with age has yet to be tested. Several questionnaires could be employed to quantitatively assess the reduction of bizarreness of dreams across time, like the bizarreness score [
91], in combination with the dream frequency scale [
92] or the Creative Achievement Questionnaire [
93].
Conclusion
Inspired by modern artificial intelligence, the PAD model connects cortical structures and dream phenomenology to a functional model of sleep. The model complements the memory consolidation theory during sleep with a creative process that combines memories to form new contents. Adversarial dreaming during REM sleep allows for exploring, testing and structuring the newly formed cortical representations while keeping these representations compatible with wake experiences. Adversarial dreams may improve our creative abilities by reenacting the past to generate novel virtual experiences that later may enrich reality. Adversarial processes include a discriminator network to tell apart externally from internally generated sensory activity, together with a `conductor’ that orchestrates the teaching signals for the involved networks (FF, D, and FB) during wakefulness, NREM and REM sleep. The framework also offers to introduce a notion of content-awareness and state-awareness. These notions help to delineate sleep from dream and lucid dreaming, and could explain why we become aware of REM dreams, but not so much of NREM dreams. The suggested experimental approaches may help to validate these concepts, and hopefully help to elucidate the mystery of sleep, including its variation of awareness.
Acknowledgments
The authors thank Federico Benitez for helpful discussions and a careful reading of the manuscript.
References
- Mazzarello, P. What dreams may come? Nature 2000, 408, 523–523. [Google Scholar] [CrossRef] [PubMed]
- Nir, Y.; Tononi, G. Dreaming and the brain: from phenomenology to neurophysiology. Trends in Cognitive Sciences 2010, 14, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.A.; Stenstrom, P. What are the memory sources of dreaming? Nature 2005, 437, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Mildner, J.N.; Tamir, D.I. Spontaneous thought as an unconstrained memory process. Trends in neurosciences 2019, 42, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Fosse, M.J.; Fosse, R.; Hobson, J.A.; Stickgold, R.J. Dreaming and Episodic Memory: A Functional Dissociation? Journal of Cognitive Neuroscience 2003, 15, 1–9. [Google Scholar] [CrossRef]
- Schwartz, S. Are life episodes replayed during dreaming? Trends in Cognitive Sciences 2003, 7, 325–327. [Google Scholar] [CrossRef]
- Wamsley, E.J. Dreaming and Offline Memory Consolidation. Current Neurology and Neuroscience Reports 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S. Dream to Predict? REM Dreaming as Prospective Coding. Frontiers in Psychology 2016, 6. [Google Scholar] [CrossRef]
- Lewis, P.A.; Knoblich, G.; Poe, G. How Memory Replay in Sleep Boosts Creative Problem-Solving. Trends in Cognitive Sciences 2018, 22, 491–503. [Google Scholar] [CrossRef]
- Zadra, A.; Stickgold, R. When brains dream: Exploring the science and mystery of sleep; WW Norton, 2021.
- Wamsley, E.J. Dreaming and offline memory consolidation. Current neurology and neuroscience reports 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Flanagan, O. Deconstructing dreams: The spandrels of sleep. The Journal of Philosophy 1995, 92, 5–27. [Google Scholar] [CrossRef]
- Flanagan, O.J. Dreaming souls: Sleep, dreams, and the evolution of the conscious mind; Oxford University Press on Demand, 2000.
- Diekelmann, S.; Born, J. The memory function of sleep. Nature Reviews Neuroscience 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Nielsen, T.A.; Stenstrom, P. What are the memory sources of dreaming? Nature 2005, 437, 1286–1289. [Google Scholar] [CrossRef]
- Wamsley, E.J.; Stickgold, R. Memory, sleep, and dreaming: Experiencing consolidation. Sleep medicine clinics 2011, 6, 97–108. [Google Scholar] [CrossRef]
- Walker, M.P.; van Der Helm, E. Overnight therapy? The role of sleep in emotional brain processing. Psychological bulletin 2009, 135, 731. [Google Scholar] [CrossRef]
- Scarpelli, S.; Bartolacci, C.; D’Atri, A.; Gorgoni, M.; De Gennaro, L. The functional role of dreaming in emotional processes. Frontiers in psychology 2019, 10, 459. [Google Scholar] [CrossRef]
- Levin, R.; Nielsen, T. Nightmares, bad dreams, and emotion dysregulation: A review and new neurocognitive model of dreaming. Current Directions in psychological science 2009, 18, 84–88. [Google Scholar] [CrossRef]
- Cai, D.J.; Mednick, S.A.; Harrison, E.M.; Kanady, J.C.; Mednick, S.C. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences 2009, 106, 10130–10134. [Google Scholar] [CrossRef]
- Hobson, J.A. REM sleep and dreaming: towards a theory of protoconsciousness. Nature Reviews Neuroscience 2009, 10, 803–813. [Google Scholar] [CrossRef]
- Malcolm-Smith, S.; Solms, M. Incidence of Threat in Dreams: A Response to Revonsuo’s Threat Simulation Theory. Dreaming 2004, 14, 220. [Google Scholar] [CrossRef]
- Zadra, A.; Desjardins, S.; Marcotte, E. Evolutionary function of dreams: A test of the threat simulation theory in recurrent dreams. Consciousness and Cognition 2006, 15, 450–463. [Google Scholar] [CrossRef]
- Hoel, E. The overfitted brain: Dreams evolved to assist generalization. Patterns 2021, 2, 100244. [Google Scholar] [CrossRef]
- Deperrois, N.; Petrovici, M.A.; Senn, W.; Jordan, J. Learning cortical representations through perturbed and adversarial dreaming. eLife 2022, 11, e76384. [Google Scholar] [CrossRef]
- Goodfellow, I.J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks, 2014, [arXiv:stat.ML/1406.2661].
- Nadasdy, Z.; Hirase, H.; Czurkó, A.; Csicsvari, J.; Buzsáki, G. Replay and Time Compression of Recurring Spike Sequences in the Hippocampus. The Journal of Neuroscience 1999, 19, 9497–9507. [Google Scholar] [CrossRef] [PubMed]
- Oudiette, D.; Dealberto, M.J.; Uguccioni, G.; Golmard, J.L.; Merino-Andreu, M.; Tafti, M.; Garma, L.; Schwartz, S.; Arnulf, I. Dreaming without REM sleep. Consciousness and Cognition 2012, 21, 1129–1140. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Kourtzi, Z.; Kanwisher, N. The lateral occipital complex and its role in object recognition. Vision Research 2001, 41, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. LIII. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Baldi, P.; Hornik, K. Neural networks and principal component analysis: Learning from examples without local minima. Neural Networks 1989, 2, 53–58. [Google Scholar] [CrossRef]
- Nadel, L.; Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology 1997, 7, 217–227. [Google Scholar] [CrossRef]
- Winocur, G.; Moscovitch, M.; Bontempi, B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal–neocortical interactions. Neuropsychologia 2010, 48, 2339–2356. [Google Scholar] [CrossRef]
- Lewis, P.A.; Durrant, S.J. Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Sciences 2011, 15, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Klinzing, J.G.; Niethard, N.; Born, J. Mechanisms of systems memory consolidation during sleep. Nature Neuroscience 2019, 22, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Lacaux, C.; Izabelle, C.; Santantonio, G.; De Villèle, L.; Frain, J.; Lubart, T.; Pizza, F.; Plazzi, G.; Arnulf, I.; Oudiette, D. Increased creative thinking in narcolepsy. Brain 2019, 142, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Lacaux, C.; Andrillon, T.; Bastoul, C.; Idir, Y.; Fonteix-Galet, A.; Arnulf, I.; Oudiette, D. Sleep onset is a creative sweet spot. Science Advances 2021, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.E.; Placzek, A.N.; Dani, J.A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology 2007, 74, 1120–1133. [Google Scholar] [CrossRef]
- Sacramento, J.a.; Ponte Costa, R.; Bengio, Y.; Senn, W. Dendritic cortical microcircuits approximate the backpropagation algorithm. Advances in Neural Information Processing Systems; Bengio, S.; Wallach, H.; Larochelle, H.; Grauman, K.; Cesa-Bianchi, N.; Garnett, R., Eds. Curran Associates, Inc., 2018, Vol. 31.
- Hobson, J.A.; McCarley, R.W. The brain as a dream state generator: an activation-synthesis hypothesis of the dream process. The American journal of psychiatry 1977. [Google Scholar]
- Dijksterhuis, A.; Meurs, T. Where creativity resides: The generative power of unconscious thought. Consciousness and cognition 2006, 15, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Baird, B.; Smallwood, J.; Mrazek, M.D.; Kam, J.W.; Franklin, M.S.; Schooler, J.W. Inspired by distraction: Mind wandering facilitates creative incubation. Psychological science 2012, 23, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Kounios, J.; Beeman, M. The Aha! moment: The cognitive neuroscience of insight. Current directions in psychological science 2009, 18, 210–216. [Google Scholar] [CrossRef]
- Walker, M.P.; Liston, C.; Hobson, J.A.; Stickgold, R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research 2002, 14, 317–324. [Google Scholar] [CrossRef]
- Cai, D.J.; Mednick, S.A.; Harrison, E.M.; Kanady, J.C.; Mednick, S.C. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 10130–10134. [Google Scholar] [CrossRef]
- Llewellyn, S. Crossing the invisible line: De-differentiation of wake, sleep and dreaming may engender both creative insight and psychopathology. Consciousness and Cognition 2016, 46, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Rompré, S.; Gagnon, J.F.; Vendette, M.; Petit, D.; Montplaisir, J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep 2007, 30, 844–849. [Google Scholar] [CrossRef]
- Müri, R.M.; Camenzind, M.; Chiffi, K.; Stuber, I.; Eberhard-Moscicka, A.K. To Nap or to Rest? The Influence of a Sixty-Minute Intervention on Verbal and Figural Convergent and Divergent Thinking. Clinical and Translational Neuroscience 2023, 7, 20. [Google Scholar] [CrossRef]
- Ritter, S.M.; Abbing, J.; van Schie, H.T. Eye-Closure Enhances Creative Performance on Divergent and Convergent Creativity Tasks. Frontiers in Psychology 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Appelbaum, L.; De Lecea, L. Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 2018, 43, 937–952. [Google Scholar] [CrossRef]
- Atherton, L.A.; Dupret, D.; Mellor, J.R. Memory trace replay: The shaping of memory consolidation by neuromodulation. Trends in Neurosciences 2015, 38, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A. A life of magic chemistry: Autobiographical reflections of a nobel prize winner; John Wiley & Sons, 2002.
- McClelland, J.L.; McNaughton, B.L.; O’Reilly, R.C. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review 1995, 102, 419–457. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nature Reviews Neuroscience 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Girardeau, G.; Zugaro, M. Hippocampal ripples and memory consolidation. Current opinion in neurobiology 2011, 21, 452–459. [Google Scholar] [CrossRef]
- Hinton, G.; Dayan, P.; Frey, B.; Neal, R. The "wake-sleep" algorithm for unsupervised neural networks. Science 1995, 268, 1158–1161. [Google Scholar] [CrossRef]
- Deperrois, N.; Petrovici, M.A.; Senn, W.; Jordan, J. Learning beyond sensations: how dreams organize neuronal representations. Neuroscience and Biobehavioral Reviews 2023, 157. [Google Scholar] [CrossRef] [PubMed]
- Le-Khac, P.H.; Healy, G.; Smeaton, A.F. Contrastive Representation Learning: A Framework and Review. IEEE Access 2020, 8, 193907–193934. [Google Scholar] [CrossRef]
- Chen, T.; Zhai, X.; Ritter, M.; Lucic, M.; Houlsby, N. Self-Supervised GANs via Auxiliary Rotation Loss. 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR); IEEE: Long Beach, CA, USA, 2019; pp. 12146–12155. [Google Scholar] [CrossRef]
- Ericsson, L.; Gouk, H.; Loy, C.C.; Hospedales, T.M. Self-Supervised Representation Learning: Introduction, advances, and challenges. IEEE Signal Processing Magazine 2022, 39, 42–62. [Google Scholar] [CrossRef]
- Urbanczik, R.; Senn, W. Learning by the Dendritic Prediction of Somatic Spiking. Neuron 2014, 81, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Ebner, C.; Sigl-Glöckner, J.; Moberg, S.; Nierwetberg, S.; Larkum, M.E. Active dendritic currents gate descending cortical outputs in perception. Nature Neuroscience 2020, 23, 1277–1285. [Google Scholar] [CrossRef]
- Aru, J.; Siclari, F.; Phillips, W.A.; Storm, J.F. Apical drive—A cellular mechanism of dreaming? Neuroscience & Biobehavioral Reviews 2020, 119, 440–455. [Google Scholar] [CrossRef]
- Benitez, F.; Pennartz, C.; Senn, W. The conductor model of consciousness , our neuromorphic twins , and the human-AI deal. PsyArXiv, 1–23. [CrossRef]
- Baird, B.; Mota-Rolim, S.A.; Dresler, M. The cognitive neuroscience of lucid dreaming. Neuroscience & Biobehavioral Reviews 2019, 100, 305–323. [Google Scholar] [CrossRef]
- Mallett, R.; Sowin, L.; Raider, R.; Konkoly, K.R.; Paller, K.A. Benefits and concerns of seeking and experiencing lucid dreams: Benefits are tied to successful induction and dream control. SLEEP Advances 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Gott, J.A.; Stücker, S.; Kanske, P.; Haaker, J.; Dresler, M. Acetylcholine and metacognition during sleep. Consciousness and Cognition 2024, 117, 103608. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press, 2016. http://www.deeplearningbook.org.
- Gershman, S.J. The Generative Adversarial Brain. Frontiers in Artificial Intelligence 2019, 2. [Google Scholar] [CrossRef]
- Simons, J.S.; Garrison, J.R.; Johnson, M.K. Brain Mechanisms of Reality Monitoring. Trends in Cognitive Sciences 2017, 21, 462–473. [Google Scholar] [CrossRef]
- Smith, M.R.; Antrobus, J.S.; Gordon, E.; Tucker, M.A.; Hirota, Y.; Wamsley, E.J.; Ross, L.; Doan, T.; Chaklader, A.; Emery, R.N. Motivation and affect in REM sleep and the mentation reporting process. Consciousness and Cognition 2004, 13, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Konkoly, K.R.; Appel, K.; Chabani, E.; Mangiaruga, A.; Gott, J.; Mallett, R.; Caughran, B.; Witkowski, S.; Whitmore, N.W.; Mazurek, C.Y.; Berent, J.B.; Weber, F.D.; Türker, B.; Leu-Semenescu, S.; Maranci, J.B.; Pipa, G.; Arnulf, I.; Oudiette, D.; Dresler, M.; Paller, K.A. Real-time dialogue between experimenters and dreamers during REM sleep. Current Biology 2021, 31, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nishimoto, S. High-resolution image reconstruction with latent diffusion models from human brain activity. bioRxiv, 2022; 2022.11.18.517004. [Google Scholar]
- Boyce, R.; Williams, S.; Adamantidis, A. REM sleep and memory. Current Opinion in Neurobiology 2017, 44, 167–177. [Google Scholar] [CrossRef]
- Currie, G.; Ravenscroft, I. Recreative minds: Imagination in philosophy and psychology; Oxford University Press on Demand, 2002.
- Pearson, J.; Kosslyn, S.M. Mental imagery. Frontiers in Psychology 2013, 4, 3389. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J. The human imagination: the cognitive neuroscience of visual mental imagery. Nature Reviews Neuroscience 2019, 20, 624–634. [Google Scholar] [CrossRef]
- Siegel, J.M. Sleep viewed as a state of adaptive inactivity. Nature Reviews Neuroscience 2009, 10, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Muzur, A.; Pace-schott, E.F.; Hobson, J.A. The prefrontal cortex in sleep. Trends in Cognitive Sciences 2002, 6, 475–481. [Google Scholar] [CrossRef]
- Uitermarkt, B.D.; Bruss, J.; Hwang, K.; Boes, A.D. Rapid eye movement sleep patterns of brain activation and deactivation occur within unique functional networks. Human Brain Mapping 2020, 41, 3984–3992. [Google Scholar] [CrossRef]
- Nielsen, T.; Kuiken, D.; Hoffmann, R.; Moffitt, A. REM and NREM Sleep Mentation Differences: A Question of Story Structure? Tore. Sleep and Hypnosis 2001, 3, 9–17. [Google Scholar] [CrossRef]
- Marti, J.M.; Andriano, D.W.; Mota, N.B.; Mota-Rolim, S.A.; Araujo, J.F.; Solms, M.; Ribeiro, S. Structural differences between REM and non- REM dream reports assessed by graph analysis. PLoS ONE 2020, 15, 1–20. [Google Scholar] [CrossRef]
- Ioannides, A.A.; Liu, L.; Poghosyan, V.; Kostopoulos, G.K. Using MEG to understand the progression of light sleep and the emergence and functional roles of spindles and K-complexes. Frontiers in Human Neuroscience 2017, 11, 1–24. [Google Scholar] [CrossRef]
- Brancaccio, A.; Tabarelli, D.; Bigica, M.; Baldauf, D. Cortical source localization of sleep-stage specific oscillatory activity. Scientific Reports 2020, 10, 28–30. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Golarai, G.; Gabrieli, J. Developmental neuroimaging of the human ventral visual cortex. Trends in cognitive sciences 2008, 12, 152–162. [Google Scholar] [CrossRef]
- Ayzenberg, V.; Behrmann, M. Development of visual object recognition. Nature Reviews Psychology 2024, 3, 73–90. [Google Scholar] [CrossRef]
- Huber, L.S.; Geirhos, R.; Wichmann, F.A. The developmental trajectory of object recognition robustness: children are like small adults but unlike big deep neural networks. Journal of vision 2023, 23, 4–4. [Google Scholar] [CrossRef]
- Gauchat, A.; Séguin, J.R.; McSween-Cadieux, E.; Zadra, A. The content of recurrent dreams in young adolescents. Consciousness and Cognition 2015, 37, 103–111. [Google Scholar] [CrossRef]
- Scarpelli, S.; Bartolacci, C.; D’Atri, A.; Gorgoni, M.; De Gennaro, L. Mental sleep activity and disturbing dreams in the lifespan. International Journal of Environmental Research and Public Health 2019, 16. [Google Scholar] [CrossRef]
- Mangiaruga, A.; Scarpelli, S.; Bartolacci, C.; De Gennaro, L. Spotlight on dream recall: The ages of dreams. Nature and Science of Sleep 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Yu, C.; Shen, H. Bizarreness of lucid and non-lucid dream: effects of metacognition. Frontiers in psychology 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Schredl, M.; Erlacher, D. Lucid dreaming frequency and personality. Personality and Individual Differences 2004, 37, 1463–1473. [Google Scholar] [CrossRef]
- Carson, S.H.; Peterson, J.B.; Higgins, D.M. Reliability, validity, and factor structure of the creative achievement questionnaire. Creativity research journal 2005, 17, 37–50. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).