1. Introduction
With the current prolonged life span of cancer patients, the adverse effects of chemotherapy, especially cardiovascular disease, have gained enormous attention. Indeed, the incidence of cardiovascular events such as cardiac injury, cardiovascular toxicity or cardiovascular hypersensitivity is higher than malignant tumors’ recurrence rate [
1]. Whereas ischemic heart disease constitutes the most common cause of cardiac failure world-wide, the cardiovascular complications of chemotherapy tend to become the second most common leading cause of heart failure. Therefore, several mechanisms including ischemic heart disease, have been proposed to explain the association between anticancer treatments and myocardial disease [
2]. Cardiovascular dysfunction or cardiac function worsening during chemotherapy might be attributed to chemotherapeutic drugs or to radiation therapy. Indeed, in a recent report concerning patients with left-sided breast cancer who were received a higher mean heart dose of radiotherapy, the risk of ischemic heart disease was raised by 6.2% per Gy (gray Units of ionizing radiation dose, hazard ratio 1.062, 95% confidence interval 1.01–1.12; P = 0.012) [
3]. Additional significant risk factors were age, chronic kidney disease, and hyper-lipidaemia. The authors proposed that the left ventricle receiving 25 Gy (LV V25) ≥ 4% is the best parameter in predicting major myocardial ischemic events and was superior to mean heart dose of radiotherapy. Better heart protection in breast cancer radiotherapy could be achieve with the above dose of radiotherapy, though further validation studies warranted. Cardiovascular deterioration can be manifested as acute cardiac events including coronary spasm, acute myocardial infarction, hypotension, cardiac arrhythmias (bradycardia, tachyarrhythmias, atrio-ventricular blocks, QT prolongation, torsades de pointes), pericarditis, myocarditis, pericardial effusion and thrombo-embolism. Moreover, chronic conditions, such as hypertension, systolic and diastolic left ventricular dysfunction presenting clinically as heart failure or cardiomyopathy are additional pathologies. Drugs can affect the cardiovascular system either through direct effects on cardiac myocytes resulting in cardiomyopathy, or through an indirect impact such as hypertension, subsequently increasing the risk of heart disease [
4]. The two main public health issues that have the biggest effects on society and the economy are cancer and cardiovascular disease. Research in the area of long-term cancer survivorship care is progressing. Many survivors will endure a number of long-term consequences from their cancer and treatment. After cancer treatment, a cardiovascular risk assessment at the end of the course will determine which patients need long-term cardiology follow-up beyond the first year. Patients with cancer who are asymptomatic but have a high risk of cardiovascular events in the future need to be monitored for a long time
.
In this review, we provide an overview on cardiovascular dysfunction or cardiac function worsening and cardiovascular hypersensitivity, associated with the use of anticancer drugs.
2. Cardiac Toxicity
The term “cardiotoxicity,” which refers
to all side effects of cancer therapy, is widely used
in cardiovascular society. When a dose-related cardiovascular side effect continues even after the causing treatment is stopped, it is referred to as cardiotoxicity.
Acute toxicity is the
term used to describe negative effects from a single or brief exposure. Heart toxicity ultimately results in a fibrotic response that needs to be confirmed histologically—a process that hasn’t been carried out much up to this point [
5]. The phrase “cardiac dysfunction related to cancer treatment” has been used interchangeably. There is still much to learn about the definition, characterization, and pathophysiology of cardiac dysfunction during chemotherapy. The term cardiotoxicity lacks consensus in medical societies, especially when this term is used to describe acute adverse effects of chemotherapeutic monoclonal antibodies. Broadly speaking, it encompasses both the quantity of the material to which the body is exposed and the mode of exposure, such as skin absorption, oral ingestion, or respiratory tract inhalation.
Subchronic toxicity is the ability of a toxic substance to cause effects for more than one year but less than the lifetime of the exposed organism. Chronic toxicity refers to the ability of a substance or mixture of substances to exert its harmful effects over a prolonged period.
The definition of cardiotoxicity is important and has practical implications for how patients are managed. Unfortunately, there is no universal definition of cardiotoxicity. Although definitions reported in clinical trials differ, they all thematically define cardiotoxicity by a progressive decrease in left ventricular ejection fraction. Various organizations have defined cardiotoxicity differently using different threshold changes in left ventricular ejection fraction [
6].
The more detailed definition has been proposed by the Evaluation Committee that oversees clinical trials of trastuzumab that includes one or more of the following: Global or more severe diaphragmatic cardiomyopathy with reduced left ventricular ejection fraction, symptoms of heart failure, and cardiac signs, including an audible third heart sound associated with a galloping rhythm, tachycardia, or both.
With regard to echocardiography, the reduction in left ventricular ejection fraction is a mainstay index for deciding about drug-induced myocardial dysfunction. However, there is not an agreement with the cut-off index, ranging from ≤5% to ≤55% from baseline values [
7]. The European Society of Cardiology’s current guidelines [
8] for the non-invasive diagnosis of amyloid light-chain cardiac amyloidosis specifically strongly recommend the use of a multiparametric echocadiographic score of ≥ 8 to define left ventricular functional impairment during cancer therapy as follows: −
a) relative wall thickness >0.6 (3 points),
b) apex-to-basis ratio of systolic longitudinal strain >2.9 (3 points),
c) tricuspid annular plane systolic excursion which used as an objective measure of right-ventricular dysfunction (TAPSE) ≤19 mm (2 points),
d) left ventricular global longitudinal strain (GLS) ≥-13% (1 point) and
e) E/e’ ratio which is parameter for diastolic function assessment that is frequently used for heart failure with preserved ejection fraction evaluation (E/e’ ratio) >11 (1point)[
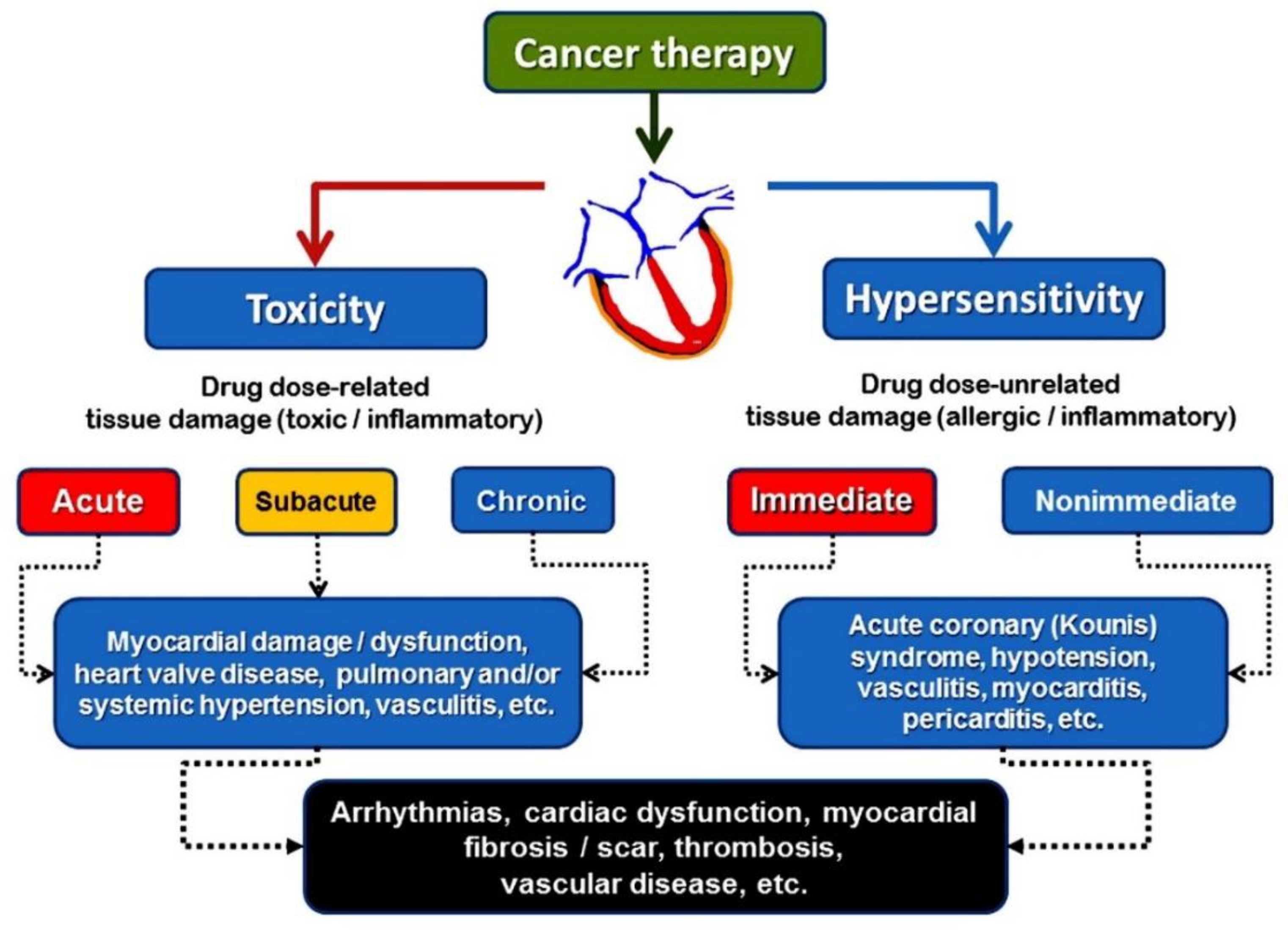
8] Cardiovascular hypersensitivity seems to be the pathophysiologic basis of acute coronary syndromes such as coronary vasospasm, acute myocardial infarction, Kounis syndrome and myocarditis caused by cancer therapy [
Figure 1].
3. Cardiovascular Hypersensitivity
An inflammatory response known as cardiovascular hypersensitivity is not dose-dependent, can occur at any point during treatment, even with very low drug dosages, and may be accompanied by tryptase and anti-drug antibodies. IgE antibodies are involved in anti-drug antibodies in hypersensitivity reactions, but the IgG isotype may also be involved. It is true that certain chemotherapy medications have been linked to IgE-mediated hypersensitivity reactions. It is possible that the classical skin manifestations are absent, and the tryptase measurements are misleading. Doctors need to be informed that a formula determines the minimum acute elevation in tryptase level that is clinically significant (peak mast cell tryptase should be >1.2× baseline tryptase + 2 mg/L) [
9]
In severe hypersensitivity (anaphylaxis), the skin manifestations may be absent, which can render the diagnosis difficult [
10]. Severe hypotension caused by decreased cardiac output from volume loss and plasma leakage has been linked to this. This lowers venous return and delays the release of anaphylactic mediators, which cause redness, rash, and/or itching on the skin.
Cardiovascular hypersensitivity rather than cardiac toxicity seems to be the pathophysiologic basis of acute coronary syndromes such as Kounis syndrome, myocarditis, and cardiac arrhythmias caused by cancer therapy. This is based on clinical and laboratory evidence. The chemotherapeutic agents that induce hypersensitivity reactions are shown in
Table 1
Cardiovascular hypersensitivity should be used in conjunction with cardiac toxicity to describe the adverse events elicited by various chemotherapeutic agents, including monoclonal antibodies, and should not be overlooked.
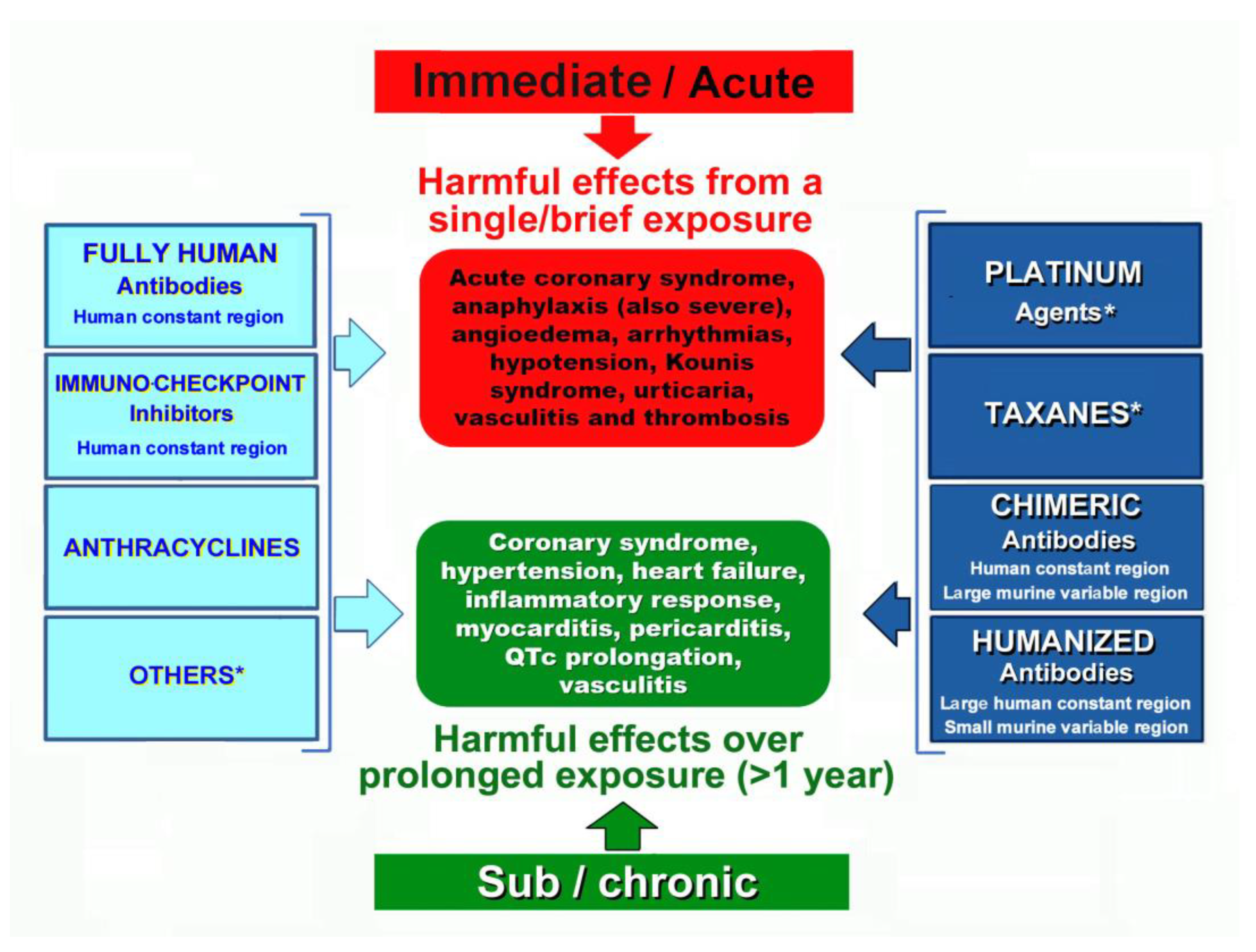
The hypersensitivity reactions to chemotherapeutic agents are classified [
11] as immediate, which occur in the first 6 hours after the administration of treatment, or nonimmediate, which usually occur days or weeks later
Figure 2.
4. The Kounis Hypersensitivity-Associated Acute Coronary Syndrome
Initial diagnosis of serum pathology was made for allergic, hypersensitive, anaphylactic, or anaphylactoid reactions linked to cardiovascular symptoms. These reactions were classified as acute carditis, morphologic cardiac reactions, or rheumatic carditis with an unclear etiology. The Kounis syndrome was later named after the initial thorough description of the allergic angina syndrome, which was first published in 1991 [
12]. It was described as a coronary spasm that was a sign of endothelial dysfunction or microvascular angina that resulted in an allergic acute myocardial infarction [
13,
14]. The inflammatory mediators released from mast cell degranulation and other interacting cells, such as T lymphocytes, macrophages, eosinophils, and platelets, during an allergic insult are the cause of this syndrome [
15]. Chymase, which functions as a converting enzyme, along with tryptase, histamine, and arachidonic acid products, can all contribute to the acute ischemic event by causing coronary spasm, atheromatous plaque erosion or rupture, and platelet activation in the Kounis syndrome cascade. Along with the coronary arteries, Kounis syndrome can also affect the cerebral, mesenteric, and peripheral arteries.
Its incidence in patients who experience an allergic, hypersensitive, anaphylactic, or anaphylactoid insult ranges from 1.1% to 3.4% [
16]. Initially believed to be an uncommon ailment, Kounis syndrome actually seems to be an underdiagnosed illness. Three types of this syndrome have been described so far [
17]:
1. Type I or MINOCA type (myocardial infarction with nonobstructive coronary arteries), which affects 76.6% of patients with normal or nearly normal coronary arteries and is brought on by histamine, chymase, or arachidonic acid products (leukotrienes, platelet-activating factor).
2. Type II, which affects 22.3% of patients with quiescent preexisting coronary disease and is brought on by the same factors as type I plus platelet activation.
3. Type III, which affects 5.1% of patients and is brought on by stent polymers, stent metals, eluted medications, dual antiplatelets, and environmental exposures in patients with stent thrombosis (subtype IIIa) and/or stent restenosis (subtype IIIb).
The Kounis syndrome may be triggered by a variety of medications including chemotherapeutic drugs, metals, foods, environmental exposures, and medical conditions
Table 2
5. Hypersensitivity to Monoclonal Antibodies
There are three types of monoclonal antibodies used in the treatment of neoplastic, hematologic, or inflammatory diseases that include chimeric, humanized, fully human, and immune checkpoint inhibitors.
a. Chimeric monoclonal antibodies. The term “chimeric” comes from the Greek mythological monstrous chimera. The chimera was one of the offspring of Typhon and Echidna and a sibling monster as Cerberus and the Lernaean Hydra appeared as a fire-breathing goat-headed lion with a serpent-headed tail. These antibodies consist of human constant regions and murine variable regions. They are used as second-line drugs for the treatment of neoplastic, hematologic, and other chronic systemic inflammatory disorders, such as rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, and systemic vasculitis. They bind to the epidermal growth factor receptor and block receptor-dependent signal transduction pathways such as anti-apoptosis, angiogenesis, and tumor metastasis. Both non-anti-TNF-α and anti-TNF-α chimeric monoclonal antibodies have been implicated in the development of immediate or delayed cardiac hypersensitivity reaction [
18]
. Chest pain, hypotension, severe anaphylaxis and acute hypersensitivity-associated coronary Kounis syndrome are the main adverse clinical manifestation [
19,
20,
21,
22]. Hypersensitivity reactions to rituximab may be due to either an immunoglobulin E (IgE) type I-mediated reaction or massive cytokine release reactions [
23].
b. Humanized monoclonal antibodies are produced by mouse-human hybrids and have the majority of human sequence, with only a small portion of mouse sequence in the complementarity-determining regions. In a recent report, the incidence and severity of anaphylaxis and hypersensitivity in trials of intravenous humanized monoclonal antibodies pertuzumab plus trastuzumab or the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection for HER2-positive breast cancer were analyzed. The authors recommended that if such reactions occur, treatment should be delayed or abandoned and that appropriate medical treatments should be administered [
24].
c. Fully human monoclonal antibodies are derived from human sequences in both the constant and variable regions and are designed to minimize immunogenicity and maximize compatibility with the human immune system. Nivolumab is the only fully human monoclonal antibody used in practice and is well tolerated, but patients should also be warned of the possibility of serious hypersensitivity reactions for which they should urgently see a physician for individualized evaluation [
25].
d. Immune checkpoint inhibitors are drugs which inhibit proteins that stop the immune system from attacking the cancer cells. They act by targeting immunologic receptors on the surface of T-lymphocytes. These drugs can also cause hypersensitivity reactions. Such reactions are mild to moderate and include skin rash, itching, headache, fever and nausea. While serious reactions are rare, they can be fatal without proper treatment. The incidence of such reactions with the use of Avelumab was found to be 23.1% and desensitization protocols have been suggested to prevent reactions [
26]. The use of immune checkpoint inhibitors has been associated with myocarditis and acute myocardial infarction. Myocardial biopsies performed in six patients with immune checkpoint inhibitor-induced myocarditis revealed mast cells, hypereosinophilia, shrunken and irregular cardiac myocytes, but not associated with fibrosis [
27]. The cytoplasm of mast cells is eosinophilic and contains varying amounts of lysosomes. Therefore, eosinophilic and histiocytic myocarditis are types of drug-induced hypersensitivity myocarditis [
28] which have been originally described by one of the authors of this paper. In a recent study of 3684 Asian Chinese patients receiving immune checkpoint inhibitors, 24 of them developed a myocardial infarction within the first 90 days [
29]. Ιn this study sensitivity analyzes excluded patients with myocardial infarction-related death and the authors did not describe the patients’ clinical signs and symptoms. Therefore, the Kounis hypersensitivity myocardial infarction cannot be excluded. Moreover, Cemiplimab is the only immune checkpoint inhibitor approved by the European Medicine Agency and about 4% of patients have developed hypersensitivity reactions with its use. Severe hypersensitivity reaction to Cemiplimab in a patient with cutaneous squamous cell carcinoma was successfully treated with a desensitization protocol [
30].
6. Hypersensitivity to Cytotoxic Agents and the Kounis syndrome
Platinum-based chemotherapeutic agents, including cisplatin, carboplatin, oxaliplatin, and Nedaplatin (Japan), heptaplatin (Korea), and lobaplatin (China), impede DNA replication, thereby suppressing cancer cell division and proliferation. Every variety of platinum agent shares an identical platinum core, with a cross-reactivity of roughly 45%. All these agents have been incriminated to induce hypersensitivity reactions [
6,
31] usually of type I (Immediate type), but rarely also type II (Cytotoxic type), type III (immune complex type) and type IV (delayed type). The range of hypersensitivity to cisplatin is 5–14%, to carboplatin it is 9–40%, and to oxaliplatin it is 10–25%.
Severe cardiac hypersensitivity reactions, such as acute hypersensitivity-induced Kounis-type myocardial infarction, can occur in response to platinum agents and manifest as the usual symptoms of IgE/mast cell-mediated hypersensitivity reactions [
32,
33,
34,
35,
36,
37].
Taxanes such as Cabazitaxel, Docetaxel, Nab-Paclitaxel and Paclitaxel are complex alkaloid esters that provide broad-spectrum anticancer activity of solid malignancies. Their mechanism of action consists in the inhibition of cell division, chromatid separation and growth which ultimately leads to cell death. Hypersensitivity reactions are common and range from mild, severe and fatal unresponsive to initial therapy. Approximately 30% of patients receiving taxanes have developed hypersensitivity reactions. Proposed mechanisms include IgE-mediated anaphylaxis with elevated tryptase levels, direct activation of mast cells and/or basophils, and complement activation [
5]. Cross-reactivity in various types of taxanes increases to 50%. Nab-Paclitaxel which is similar to paclitaxel contains human albumin particles instead of Cremophor El and hypersensitivity reactions are less. Kounis syndrome associated with hypersensitivity has occurred in treated patients with paclitaxel administration [
38,
39]. Moreover, drug-coated balloons are used widely as a form of endovascular treatment for peripheral arterial disease. These balloons contain anti-proliferative drugs such as paclitaxel which improve vessel patency by reducing neointimal hyperplasia and restenosis. Indeed, acute coronary syndrome of Kounis type secondary to anaphylaxis after inflation of a paclitaxel-coated balloon used to treat recurrent superficial femoral artery stenosis have been reported [
40,
41]
7. Other Anticancer Medicines Inducing Hypersensitive Reactions and Kounis syndrome
a. Alkylating agents have the ability to attach an alkyl group to DNA. Alkylated DNA makes cancer cells much more sensitive to damaged DNA and in this way their proliferation is prevented. The alkylating agents, cyclophosphamide and ifosfamidem are occasionally associated with hypersensitivity reactions [
31].
b. Antimetabolites, that are also called nucleotide synthesis inhibitors, disrupt DNA synthesis by substituting for the natural metabolite or interfering with production of a major nucleotide metabolite. The antimetabolite Capecitabine is an orally available prodrug that is converted to 5-fluorouracil within tumor tissues and is used to treat metastatic colorectal and breast cancer. Cardiac manifestations include angina, acute coronary syndrome, arrhythmias, myocarditis, and heart failure. It is not known whether these side effects are attributable to the accumulation of toxic metabolites or hypersensitivity. However, in a report of capecitabine-induced cardiac arrest due to ventricular fibrillation, the underlying pathology supported by immunological investigation showed Kounis type I hypersensitivity-related syndrome [
42,
43,
44,
45,
46]. Moreover, 5-fluorouracil has been also reported to induced vasospasm of Kounis syndrome type [
47,
48,
49].
c. Anthracyclines are chemotherapeutic agents that block DNA replication and transcription of genes by binding DNA-forming adducts and crosslinks. Moreover, they can prevent the activity of DNA helicase and interfere with DNA strand separation. They can cause both cardiac toxicity and cardiovascular hypersensitivity. Cancer patients can suffer hypersensitivity reactions up to 45% during treatment if left without premedication [
31,
50]. However, with formulations such as pegylated liposomal doxorubicin or liposomal daunorubicin, the incidence of hypersensitivity reactions is around 9–14% [
31,
51]. The Kounis hypersensitivity-associated myocardial infarction has been reported as side effect of Epirubicin [
52].
d. Enzyme inhibitors have been used as anticancer drugs because inhibit enzymes that involved in DNA replication and RNA transcription. The following 4 enzymes PFK-2/FBPase-2, ATIC, LTA
4H and Jmjd6 are promising targets for the development of new anti-cancer drugs [
53]. Irinotecan and Topotecan are both enzyme inhibitors used alone or combined with other agents to treat a variety of tumors. They belong to DNA topoisomerase I inhibitor class of drugs [
54,
55]. Hypersensitivity reactions have been already reported with the use of both drugs [
56,
57]. Topotecan was injected to sub-Tenon’s space in a fibrin sealant which was used as an adjunct to laser therapy for small retinoblastoma tumors in 25 children (77 injections). Two children developed severe hypersensitivity reactions on their third sub-Tenon’s injection of topotecan in fibrin sealant. The authors of this report suggested that such reactions could be the result of both the drug and fibrin glue [
58]
. e. Protein kinase inhibitors are a new approach to cancer treatment with targeted therapy. They can also cause both cardiac toxicity and cardiovascular hypersensitivity. These agents inhibit the protein kinase enzymes which have the ability to phosphorylate proteins and modulate their function [
59]. This dysfunction of protein kinase enzymes constitutes the basis of many cancers but also of several cardiovascular manifestations, such as ischemia/reperfusion injury, left ventricular remodeling, angiogenesis and atherogenesis. Hypersensitivity reaction to protein kinase inhibitors can occur and desensitization strategies have been applied [
60]. Urticaria and angioedema have been reported during their use. Some cases of Stevens-Johnson syndrome (flu-like symptoms, painful rash and blisters) have been described with regorafenib which is a vascular endothelial growth factor receptor inhibitor, Palbociclib which is a cyclin-dependent protein kinase inhibitor and ribociclib [
11]. Cross-reactivity between dabrafenib and vemurafenib has been reported, possibly due to their similar chemical structure [
11].
f. Tyrosine kinase inhibitors are small molecules or peptides which have the ability to inhibit either cytosolic or receptor tyrosine kinases. They inhibit tyrosine kinases which activate a variety of proteins via signal transduction cascades. These agents phosphorylate specific amino acids and modify cell growth, migration, differentiation, apoptosis, and death. The protein activation or inhibition causes dysregulation of signal cascades, which can lead to malignancy and other pathologic conditions. Therefore, blocking these initial signals via tyrosine kinase inhibitors can prevent the aberrant action of the mutated or dysfunctional tyrosine kinases. In a study of 78 consecutive patients suffering from chronic myeloid leukemia who were treated with the third generation tyrosine kinase inhibitor, 3 patients experienced myocardial infarction (3.8%) [
61]. Imatinib was also reported to induce or promote cardiac dysfunction manifesting with cardiac arrythmias but overall cardiac side effects are rare [
62]. The potential mechanisms of these side effects are unclear but hypersensitivity reactions can not be excluded [
63].
8. Transcatheter Arterial Chemoembolization for Cancer Patients and Kounis syndrome
A minimally invasive, nonsurgical technique called transcatheter embolization is used to reduce or eliminate a tumor’s blood supply [
64]. Particle embolization and the injection of chemotherapy medications are combined in transarterial chemoembolization. In this manner, the tumor’s size is decreased, its symptoms are lessened, and its growth is postponed. The most often utilized single agents in transarterial chemoembolization are the chemotheraputic drugs oxorubicin, cisplatin, and epirubicin. As the most prevalent malignant tumor of the liver, hepatocellular carcinoma is specifically treated using this technique. However, these medications are combined with lipiodol, also known as iodized oil, which is a vehicle for the delivery of chemotherapeutic medications that remain in tumor nodules following injection into particular hepatic artery branches. This procedure is often complicated syndrome called postembolization syndrome. The symptoms of this syndrome include fever, vomiting, nausea, and abdominal pain and may affect 60% to 70% of individuals undergoing transarterial chemoembolization. Hepatic insufficiency and, in rare cases, lipoidol embolism to the brain and lung are also serious side effects. Transcatheter arterial chemoembolization is most useful as a liver transplant bridge and as the initial line of treatment for inoperable hepatocellular carcinoma. Additionally, Kounis syndrome was reported in a 79-year-old man with hepatocellular cancer 30 minutes following successful transcatheter arterial chemoembolization [
65]. After receiving an injection of ethylester of iodinated poppy-seed oil fatty acid, iopamidol, and an iodinated contrast medium, the patient started to complain of anterior chest pain and eventually lost consciousness. An inferior myocardial infarction was diagnosed by a twelve-lead ECG that displayed full atrioventricular block with junctional escape rhythm and considerable ST segment elevation in the II, III, and aVF leads. On the other hand, the right coronary artery angiography showed no signs of blockage or stenosis. This patient may have experienced a coronary artery spasm after receiving two allergenic substances, one of which may have been a chemotheraputic medication (the authors did not specify which one) combined with iodinated contrast media. Indeed, the theory that patients who are simultaneously exposed to more than one allergens may exhibit more symptoms than those who are only sensitive to one allergen is supported by clinical research [
66]. When a patient is concurrently exposed to the appropriate antigens, IgE antibodies with varying specificities can have cumulative effects and in small, even subthreshold, quantities can cause the release of cell mediators [
67].
9. Conclusion
Since the treatment of cancer and cardiovascular illness has become tightly associated, it is anticipated that the incidence of major cardiovascular problems associated with cancer treatment would rise over time. It is crucial to have dedicated cardiovascular clinics to treat cancer patients with heart failure or cardiac hypersensitivity. These clinics should offer professional pre-treatment evaluation, monitoring, and treatment to ensure that cancer treatment proceeds as planned.
The relevant medical faculties of cardiology, allergy and oncology have issued excellent guidelines for the treatment and prevention of cancer therapy-related cardiovascular toxicity criteria and hypersensitivity reactions of chemotherapeutic agents. However, the Spanish multidisciplinary research network for allergic diseases has issued guidelines on the hypersensitivity reactions to cancer chemotherapy emphasizing the need for diagnosis, management and desensitization procedures without referring to cardiotoxicity [
68]. Likewise, the European Network on Drug Allergy and Drug Allergy Interest Group of the European Academy of Allergy and Clinical Immunology have organized a task force to provide data and recommendations regarding the allergological work on the field of hypersensitivity reactions to chemotherapy without referring to cardiotoxicity [
69,
70,
71]. In the 2022 European Society of Cardiology (ESC) Guidelines on cardio-oncology, in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS), the hypersensitivity reactions and the Kounis hypersensitivity associated coronary syndrome are absent despite that in the medical literature there are many reports emphasizing cardiovascular hypersensitivity and the Kounis syndrome as severe side effects in cancer therapy [
8]. We believe that closed coordination and understanding among all these excellent organizations is necessary for both researchers and practicing physicians.
For the benefit of patients and society, cardiac toxicity, cardiovascular hypersensitivity, and Kounis syndrome are crucial triplets in cardio-oncology that every general practitioner and specialist should be aware of.
Author Contributions
Conceptualization, N.G.K., M.Y.H., C. d. G., V. M., C. G., S.F.A., P.P., S.N.K., M.A., G.T., I.K.; data curation, N.G.K., M.Y.H, C.d.G.; writing—original draft preparation,N.G.K., M.Y.H., C.d,G,; writing—review and editing, N.G.K., M.Y.H., C.d.G., S.N.K. B.M. S.F.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang Y, Wang Y, Han X, Sun J, Li C, Adhikari BK, Zhang J, Miao X, Chen Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front Cardiovasc Med 2022; 9: 727487. [CrossRef]
- Palmieri V, Vietri MT, Montalto A, Montisci A, Donatelli F, Coscioni E, Napoli C. Cardiotoxicity, cardioprotection, and prognosis in survivors of anticancer treatment undergoing cardiac surgery: Unmet needs. Cancers (Basel). 2023; 15: 2224. [CrossRef]
- Lai TY, Hu YW, Wang TH, Chen JP, Shiau CY, Huang PI, Lai IC, Tseng LM, Huang N, Liu CJ. Association of radiation dose to cardiac substructures with major ischaemic events following breast cancer radiotherapy. Eur Heart J. 2023; 44: 4796-4807. [CrossRef]
- Morelli MB, Bongiovanni C, Da Pra S, Miano C, Sacchi F, Lauriola M, D’Uva G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front Cardiovasc Med 2022; 9: 847012. [CrossRef]
- Terada CI, Onoue K, Fujii T, Itami H, Morita K, Uchiyama T, Takeda M, Nakagawa H, Nakano T, Baba Y, Amemiya K, Saito Y, Hatakeyama K, Ohbayashi C. Histopathological and epigenetic changes in myocardium associated with cancer therapy-related cardiac dysfunction. ESC Heart Fail 2022; 9: 3031-3043. [CrossRef]
- Kounis NG, Koniari I, Hahalis G. Cardio-oncology, Immuno-oncology, Onco-cardiology and Onco-immunology. Int J Cardiol 2016; 223: 254-257. [CrossRef]
- Gavila J, Seguí MÁ, Calvo L, López T, Alonso JJ, Farto M, Sánchez-de la Rosa R. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol 2017; 19: 91-104. [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022; 43: 4229-4361. Erratum in: Eur Heart J. 2023 May 7;44(18):1621. [CrossRef]
- Baretto RL, Beck S, Heslegrave J, Melchior C, Mohamed O, Ekbote A, Huissoon AP, Krishna MT. Validation of international consensus equation for acute serum total tryptase in mast cell activation: A perioperative perspective. Allergy 2017; 72: 2031-2034. [CrossRef]
- Kounis NG, Cervellin G, Koniari I, Bonfanti L, Dousdampanis P, Charokopos N, Assimakopoulos SF, Kakkos SK, Ntouvas IG, Soufras GD, Tsolakis I. Anaphylactic cardiovascular collapse and Kounis syndrome: systemic vasodilation or coronary vasoconstriction? Ann Transl Med 2018; 6: 332. [CrossRef]
- Vega A, Jimenez-Rodriguez TW, Barranco R, Bartra J, Diéguez MC, Doña I, Fernández-Rivas M, Gandolfo-Cano M, Gastaminza-Lasarte G, González-Mancebo E, de la Hoz Caballer B, Sánchez-Morillas L, Torres MJ, Berges-Gimeno MP, Muñoz-Cano R. Hypersensitivity Reactions to Cancer Chemotherapy: Practical Recommendations of ARADyAL for Diagnosis and Desensitization. J Investig Allergol Clin Immunol 2021; 31: 364-384. [CrossRef]
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 1991; 45: 121-128. [CrossRef]
- Rich MW. Is vasospastic angina an inflammatory disease? Am J Cardiol 2005; 96: 1612.
- Zavras GM, Papadaki PJ, Kokkinis CE, Kalokairinov K, Kouni SN, Batsolaki M, Gouvelou-Deligianni GV, Koutsojannis C. Kounis syndrome secondary to allergic reaction following shellfish ingestion. Int J Clin Pract 2003; 57: 622-4. [CrossRef]
- Kounis NG, Koniari I, Velissaris D, Tzanis G, Hahalis G. Kounis Syndrome-not a single-organ arterial disorder but a multisystem and multidisciplinary disease. Balkan Med J 2019; 36: 212-221. [CrossRef]
- Kounis NG, Mplani V, de Gregorio C, Koniari I. Attack the ATAK; A Challenging Contemporary Complex: Pathophysiologic, Therapeutic, and Preventive Considerations. Balkan Med J 2023; 40: 308-311. [CrossRef]
- Kounis NG, Koniari I, Tsigkas G, Davlouros P. Humanized Monoclonal Antibodies Against IgE Antibodies as Therapy for IgE-Mediated Coronary Syndromes: Are We There Yet? Can J Cardiol 2020; 36: 816-819. [CrossRef]
- Dupont M, Carlier C, Gower-Rousseau C, Barbier-Lider P, Botsen D, Brasseur M, Burgevin A, Chourbagi C, D’Almeida R, Hautefeuille V, Hentzien M, Lambert A, Lamuraglia M, Lavau-Denes S, Lopez A, Parent D, Slimano F, Brugel M, Bouché O. Incidence and associated factors of cetuximab-induced hypersensitivity infusion reactions in 1392 cancer patients treated in four French areas: a possible association with Lyme disease? BMC Cancer 2022; 22: 1219. [CrossRef]
- Gori T, Münzel T. A case of coronary hypersensitivity (Kounis) syndrome associated with mid-ventricular ballooning pattern, intracoronary thrombosis and troponin elevation. Int J Cardiol 2011; 149: 377-8. [CrossRef]
- Mazarakis A, Tsigkas G, Soufras GD, Kounis NG: Anti chimeric antibodies against chimeric monoclonal antibodies may result in Kounis hypersensitivity associated acute coronary syndrome. J Crohns Colitis. 2012. [CrossRef]
- Kounis NG, Soufras GD, Kounis GN. Antibodies against antibodies inducing Kounis syndrome. Int J Cardiol 2013; 168: 4804-5. [CrossRef]
- Diaz-Rodriguez PE, Molina-Lopez VH, Gonzalez Burgos BA, Nieves-La Cruz C. A Rare Case of Kounis Syndrome Secondary to Infliximab. Cureus 2023; 15: e44704. [CrossRef]
- Brili S, Bei E, Kounis NG, Chrysohoou C, Antoniou CK, Kontopidou F, Bonfanti L, Cervellin G, Tousoulis DT, Tsioufis C. Hypertensive crisis and pulmonary edema following rituximab-induced anaphylaxis. Acta Biomed 2021; 92: e2021115. [CrossRef]
- Swain SM, Tan AR, Gianni L, Kuemmel S, Dang CT, Schneeweiss A, O’Shaughnessy J, Liu H, Aguila C, Heeson S, Macharia H, Yang K, Restuccia E, Loibl S. Incidence and severity of anaphylaxis and hypersensitivity in trials of intravenous pertuzumab plus trastuzumab or the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection for HER2-positive breast cancer. Eur J Cancer 2023; 178: 70-81. [CrossRef]
- Pîrlog CF, Paroșanu AI, Slavu CO, Olaru M, Popa AM, Iaciu C, Niță I, Moțatu P, Horia C, Manolescu LSC, Nițipir C. Nivolumab Hypersensitivity Reactions a Myth or Reality in Solid Tumors-A Systematic Review of the Literature. Curr Oncol 2022; 29: 9428-9436. [CrossRef]
- Banini M, Salvestrini V, Vultaggio A, Perlato M, Mecheri V, Cerbai C, Scotti V, Matucci A, Mangoni M, Livi L, Bonomo P. Desensitization Protocol for Cemiplimab-Related Infusion Reaction in Cutaneous Squamous Cell Carcinoma: A Case Report and Literature Review. Curr Oncol 2023; 30 :6699-6707. [CrossRef]
- Sobol I, Chen CL, Mahmood SS, Borczuk AC. Histopathologic Characterization of Myocarditis Associated with Immune Checkpoint Inhibitor Therapy. Arch Pathol Lab Med 2020; 144: 1392-1396. [CrossRef]
- Kounis NG, Zavras GM, Soufras GD, Kitrou MP. Hypersensitivity myocarditis. Ann Allergy 1989; 62:71–74. [CrossRef]
- Chan JSK, Tang P, Lee TTL, Chou OHI, Lee YHA, Li G, Leung FP, Wong WT, Liu T, Tse G. Association between immune checkpoint inhibitors and myocardial infarction in Asians: A population-based self-controlled case series. Cancer Med 2023; 12: 9541-9546. [CrossRef]
- Banini M, Salvestrini V, Vultaggio A, Perlato M, Mecheri V, Cerbai C, Scotti V, Matucci A, Mangoni M, Livi L, Bonomo P. Desensitization Protocol for Cemiplimab-Related Infusion Reaction in Cutaneous Squamous Cell Carcinoma: A Case Report and Literature Review. Curr Oncol 2023; 30: 6699-6707. [CrossRef]
- Galateanu B, Pușcașu AI, Tircol SA, Tanase BC, Hudita A, Negrei C, Burcea-Dragomiroiu GT, Negreanu L, Vacaroiu IA, Ginghină O. Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions. Int J Mol Sci 2023; 24: 3886. [CrossRef]
- Baroni M, Todd S, Pattarino F, Doni F. Carboplatin-induced Kounis syndrome. J Cardiol Cases. 2011; 4: e58-e61. [CrossRef]
- Tambe V, Tambe A, Goodman A, Shepherd Z. Carboplatin-Induced Kounis Syndrome. Am J Ther. 2020; 27: e647-e652. [CrossRef]
- Oneglia C, Kounis NG, Beretta G, Ghizzoni G, Gualeni A, Berti M. Kounis syndrome in a patient with ovarian cancer and allergy to iodinated contrast media: report of a case of vasospastic angina induced by chemotherapy. Int J Cardiol. 2011; 149: e62-e65. [CrossRef]
- Chang PH, Hung MJ, Yeh KY, Yang SY, Wang CH. Oxaliplatin-induced coronary vasospasm manifesting as Kounis syndrome: a case report. J Clin Oncol. 2011; 29: e776-8. [CrossRef]
- Albanes M, Raffaele Didonna, Nada Chaoul, Federica Mazzone, Marco Zurlo, Fortunato Iacovelli, Francesco Monitillo, Flavio Rimmaudo, Marco Tucci, Mauro Cives, Camillo Porta, Vito Procacci.A challenge in emergency department: a case report of oxaliplatin-induced Kounis syndrome. Precis Cancer Med 2023; 6: 18.
- Kounis NG, Cervellin G, Lippi G. Cisplatin-induced bradycardia: Cardiac toxicity or cardiac hypersensitivity and Kounis syndrome? Int J Cardiol 2016; 202: 817-8. [CrossRef]
- Wang B, Sethwala A, Gurvitch R. Type I Kounis syndrome from paclitaxel infusion. J Cardiol Case Rep 2020; 3: 1-2. [CrossRef]
- Wang B, Sethwala A, Gurvitch R. Type 1 Kounis syndrome after paclitaxel infusion in a patient treated for lung adenocarcinoma. Intern Med J. 2021; 51: 448-449. [CrossRef]
- Narroway HG, Katib N, Gomes ML, Varcoe RL, Thomas SD. Kounis Syndrome after Angioplasty of the Superficial Femoral Artery with Paclitaxel-Coated Balloon. Ann Vasc Surg 2020; 69: 450.e17-450.e22. [CrossRef]
- Lake E, Twigg M, Farquharson F. Acute hypersensitivity reaction to femoral drug-coated balloons. Vasa 2017; 46: 223-225. [CrossRef]
- Kido K, Adams VR, Morehead RS, Flannery AH. induced ventricular fibrillation arrest: Possible Kounis syndrome. J Oncol Pharm Pract 2016; 22: 335-340. [CrossRef]
- Kounis NG, Tsigkas GG, Almpanis G, Mazarakis A. Kounis syndrome is likely culprit of coronary vasospasm induced by capecitabine. J Oncol Pharm Pract 2012; 18: 316-8. [CrossRef]
- Scott PA, Ferchow L, Hobson A, Curzen NP. Coronary spasm induced by capecitabine mimicks ST elevation myocardial infarction. Emerg Med J 2008; 25: 699-700. [CrossRef]
- Coughlin S, Das S, Lee J, Cooper J. Capecitabine induced vasospastic angina. Int J Cardiol 2008; 130: e34-6. [CrossRef]
- Tsiamis E, Synetos A, Stefanadis C. Capecitabine may induce coronary artery vasospasm. Hellenic J Cardiol 2012; 5): 320-3.
- Canale ML, Camerini A, Stroppa S, Porta RP, Caravelli P, Mariani M, Balbarini A, Ricci S. A case of acute myocardial infarction during 5-fluorouracil infusion. J Cardiovasc Med (Hagerstown). 2006; 7: 835-7. [CrossRef]
- Tajik R, Saadat H, Taherkhani M, Movahed MR. Angina induced by 5-fluorouracil infusion in a patient with normal coronaries. Am Heart Hosp J 2010; 8: E111-2. [CrossRef]
- Karabay CY, Gecmen C, Aung SM, Guler A, Candan O, Batgerel U, Kalayci A, Kirma C. Is 5-fluorouracil-induced vasospasm a Kounis syndrome? A diagnostic challenge. Perfusion 2011; 26: 542-5. [CrossRef]
- Rahman A, Treat J, Roh JK, Potkul LA, Alvord WG, Forst D, Woolley PV. A phase I clinical trial and pharmacokinetic evaluation of liposome-encapsulated doxorubicin. J Clin Oncol 1990; 8: 1093-100. [CrossRef]
- Zhuang W, Lai X, Mai Q, Ye S, Chen J, Liu Y, Wang J, Li S, Huang Y, Qin T, Hu H, Wu J, Yao H. Biomarkers of PEGylated Liposomal Doxorubicin-Induced Hypersensitivity Reaction in Breast Cancer Patients Based on Metabolomics. Front Pharmacol 2022; 13: 827446. [CrossRef]
- Liang HZ, Zhao H, Gao J, Cao CF, Wang WM. Epirubicin-induced Kounis syndrome. BMC Cardiovasc Disord 2021; 21: 133. [CrossRef]
- Teixeira CSS, Sousa SF. Current Status of the Use of Multifunctional Enzymes as Anti-Cancer Drug Targets. Pharmaceutics 2021; 14:10. [CrossRef]
- Jang JY, Kim D, Kim ND. Recent Developments in Combination Chemotherapy for Colorectal and Breast Cancers with Topoisomerase Inhibitors. Int J Mol Sci 2023; 24: 8457. [CrossRef]
- Fu DJ, Wang T. Targeting NEDD8-activating enzyme for cancer therapy: developments, clinical trials, challenges and future research directions. J Hematol Oncol. 2023; 16: 87. [CrossRef]
- Pagani M, Bavbek S, Alvarez-Cuesta E, Berna Dursun A, Bonadonna P, Castells M, Cernadas J, Chiriac A, Sahar H, Madrigal-Burgaleta R, Sanchez Sanchez S. Hypersensitivity reactions to chemotherapy: an EAACI Position Paper. Allergy 2022; 77: 388-403. [CrossRef]
- Abu-Amna M, Hassoun G, Hadad S, Haim N, Bar-Sela G. Successful Desensitization Protocol for Hypersensitivity Reaction Caused by Irinotecan in a Patient with Metastatic Colorectal Cancer. Clin Colorectal Cancer 2015;14(4): e49-51. [CrossRef]
- Astudillo PP, Durairaj P, Chan HS, Héon E, Gallie BL. Hypersensitivity to sub-Tenon’s topotecan in fibrin adhesive in patients with retinoblastoma. J AAPOS. 2015 Feb;19(1):86-7. [CrossRef]
- Grela-Wojewoda A, Pacholczak-Madej R, Adamczyk A, Korman M, Püsküllüoğlu M. Cardiotoxicity Induced by Protein Kinase Inhibitors in Patients with Cancer. Int J Mol Sci 2022; 23: 2815. [CrossRef]
- Chillari KA, Britnell SR, Brown JN, Hammond JM. Desensitization to protein kinase inhibitors: A systematic review. Ann Allergy Asthma Immunol 2017; 119: 9-15. [CrossRef]
- Chan O, Talati C, Isenalumhe L, Shams S, Nodzon L, Fradley M, Sweet K, Pinilla-Ibarz J. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv 2020; 4: 530-538. [CrossRef]
- Akin C, Arock M, Valent P. Tyrosine kinase inhibitors for the treatment of indolent systemic mastocytosis: Are we there yet? J Allergy Clin Immunol 2022; 149: 1912-1918. [CrossRef]
- Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther 2023; 8: 262. [CrossRef]
- Guan YS, He Q, Wang MQ. Transcatheter arterial chemoembolization: history for more than 30 years. ISRN Gastroenterol. 2012; 2012: 480650. [CrossRef]
- Iyonaga T, Ichiki T, Watanabe T, Masuda S, Yamamoto M, Akatsuka Y, Taira Y. A case of Kounis syndrome associated with transcatheter arterial chemoembolization for hepatocellular carcinoma. J Cardiol Cases 2015; 12: 106-109. [CrossRef]
- Nopp A, Johansson SG, Lundberg M, Oman H. Simultaneous exposure of several allergens has an additive effect on multisensitized basophils. Allergy 2006; 61: 1366-1368. [CrossRef]
- Kounis NG, Mazarakis A, Almpanis G, Gkouias K, Kounis GN, Tsigkas G. The more allergens an atopic patient is exposed to, the easier and quicker anaphylactic shock and Kounis syndrome appear: Clinical and therapeutic paradoxes. J Nat Sci Biol Med 2014; 5: 240-4. [CrossRef]
- Vega A, Jimenez-Rodriguez TW, Barranco R, Bartra J, Diéguez MC, Doña I, Fernández-Rivas M, Gandolfo-Cano M, Gastaminza-Lasarte G, González-Mancebo E, de la Hoz Caballer B, Sánchez-Morillas L, Torres MJ, Berges-Gimeno MP, Muñoz-Cano R. Hypersensitivity Reactions to Cancer Chemotherapy: Practical Recommendations of ARADyAL for Diagnosis and Desensitization. J Investig Allergol Clin Immunol 2021; 31:364-384. [CrossRef]
- Pagani M, Bavbek S, Alvarez-Cuesta E, Berna Dursun A, Bonadonna P, Castells M, Cernadas J, Chiriac A, Sahar H, Madrigal-Burgaleta R, Sanchez Sanchez S. Hypersensitivity reactions to chemotherapy: an EAACI Position Paper. Allergy 2022;77:388-403. [CrossRef]
- Barroso B, Gómez-López A, Betancor D, Valverde-Monge M, Sastre J. Reply to “European academy of allergy and clinical immunology, food allergy, anaphylaxis guidelines group. EAACI guidelines: Anaphylaxis (2021 update)”. Allergy 2024 Jan 12. [CrossRef]
- Gent DG, Rebecca D. The 2022 European Society of Cardiology Cardio-oncology Guidelines in Focus. Eur Cardiol 2023; 18: e16. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).