1. Introduction
Veronica, the largest genus of flowering plants in the family Plantaginaceae, comprises approximately 450 species [
1]. Within
Veronica, the subgenus
Pseudolysimachion encompasses over 30 species. In Korea, 10 species belonging to the
Pseudolysimachion genus are distributed throughout wild mountainous regions across the country. Among these,
P. nakaianum (Ohwi) T. Yamaz. is a perennial herb found in semi-shaded moist areas, and characterized by mauve flowers during the summer months; it is highly prized as an ornamental garden plant. To expand the cultivation of
P. nakaianum, the establishment of a mass-breeding system based on comprehensive surveys and research efforts is imperative.
Clonal propagation refers to the reproduction of genetically identical plants through nonsexual means [
2]. One conventional method for clonal propagation involves multiplying plant stem cuttings in large quantities within a short timeframe. Oh et al. [
3] investigated the impact of plant growth regulators, cutting media, cutting positions, and leaf sizes on the growth of
V. rotunda. Similarly, Song et al. [
4] examined the effects of shade, media, and concentrations of plant growth regulators on the rooting process of four
Veronica species (
V. glabrifolia Kitag.,
V. pusanensis Y. N. Lee,
V. glabrifolia Kitag. ×
V. spicata 'Alba', and
V. spicata ‘Ulster Blue Dwarf’ ×
V. longifolia) through stem cuttings. In comparison with conventional propagation methods, in vitro propagation enables rapid mass reproduction without being constrained by seasonal limitations. Moreover, in vitro propagation allows for plant multiplication through the culture of small plant pieces on a synthetic medium.
Cytokinins, commonly used in in vitro tissue culture across various plant species, play pivotal roles in plant growth and development [
5]. They stimulate cell division and shoot induction, and are crucial for mass tissue proliferation. Shahzad et al. [
6] observed that 6-benzylaminopurine (BAP) was the most effective cytokinin for shoot regeneration in
V. anagallis-aquatica, while in
V. spicata, BAP was shown to be the most effective in stimulating axillary shoot growth [
7]. In addition to cytokinins, the nature of the plant materials significantly influences the success of in vitro propagation [
8].
Veronica plants regenerate through adventitious shoots generated from shoot tips [
7] and nodal segments [
6]. While cotyledon and petiole explants have been utilized for shoot induction in very few species within the Plantaginaceae family (such as
Plantago camtschatica L. and
P. maritima L.), there have been no reports of adventitious shoot organogenesis within the
Pseudolysimachion genus thus far.
Hence, in this study, we present, for the first time, an efficient system for the rapid clonal propagation of P. nakaianum via in vitro caulogenesis using cotyledon, petiole, and leaf explants. Furthermore, we investigated the effects of five different types of cytokinins on shoot formation.
2. Materials and Methods
2.1. Plant Materials, Explant Sterilization, and In Vitro Culture Conditions
Seeds of
P. nakaianum were sourced from the Geumwon Mountains Forest Resources Management Office, Gyeongnam Institute of the Environment and Forest, Korea. Following a modified protocol outlined by Shin et al. [
9], the seeds were sterilized using several steps: immersion of seeds in 70% ethanol for 30 s followed by three rinses with sterile distilled water, then disinfection for 2 min in 1% sodium hypochlorite, and finally five rinses with sterile distilled water. For germination induction, the seeds were cultured in vitro on half-strength Murashige and Skoog (MS) medium [
10], supplemented with 20 g L
-1 sucrose and 3 g L
-1 Gelrite
™ (Duchefa Biochemie, Haarlem, The Netherlands) as a solidifying agent in 100 × 25 mm Petri dishes. After adjusting the pH of the medium to 5.7 using 0.1 N NaOH, Gelrite
™ was added to it followed by sterilization by autoclaving at 121 °C for 20 min. After two weeks of culture, some germinants were utilized for cotyledon-based shoot induction experiments, while others were transferred to square vessels (60 × 60 × 100 mm, SPL Life Sciences, Korea) containing 50 mL of half-strength MS medium supplemented with 30 g L
-1 sucrose and 3 g L
-1 Phytagel for shoot induction from petioles and leaves.
2.2. Effects of Cytokinins on Shoot Induction in Cotyledons
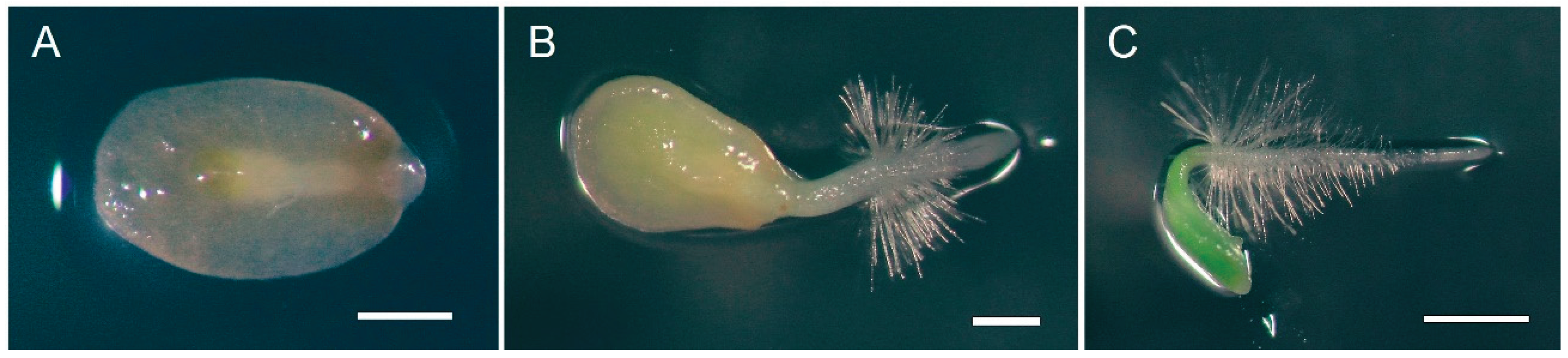
Cotyledons harvested randomly from two-week-old
P. nakaianum germinants (
Figure 1), cultured in vitro, were detached from the hypocotyls. Cotyledons derived from each explant were used to investigate the effects of various cytokinins on callus formation and the induction of adventitious shoot buds. A 2 mg L
-1 concentration of cytokinins [BAP, kinetin, thidiazuron (TDZ), zeatin, 2-isopentenyladenine (2iP)] was incorporated into half-strength MS medium supplemented with 0.05 mg L
-1 1-naphthaleneacetic acid (NAA), 30 g L
-1 sucrose, and 3 g L
-1 Gelrite
™ as a solidifying agent in 100 × 25 mm Petri dishes. The cultured cotyledon explants were exposed to a light intensity of 80 µmol m
-2 s
-1 provided by cool white fluorescent lamps at 25 ± 2°C under a 16-h photoperiod. Each experimental set comprised nine cultures and was replicated four times to ensure reproducibility. The adventitious shoot induction rate and the number of shoots per explant were analyzed after a six-week culture period.
2.3. Effects of Cytokinins on Shoot Induction in Petioles and Leaves
Two types of explants (petioles and leaves) derived from 8-week-old in vitro cultures were used in shoot induction experiments. The explants were uniformly cut to a length of 5 mm using a surgical knife, with the leaf explants positioned with the abaxial side in contact with the medium. The cytokinin treatments mirrored those described previously. Cultures were maintained under consistent environmental conditions as detailed in section 2.2. Each experiment was replicated three times to enhance reproducibility and comprised ten explants per Petri dish. The adventitious shoot induction rate and the number of shoots per explant were recorded at the end of the six-week-long cultivation period.
2.4. Adventitious Shoot Elongation, Rooting, and Acclimatization of Regenerated Plants
Multiple shoots originating from the explants were individually separated and transferred to square vessels containing 50 mL of half-strength MS medium supplemented with 30 g L-1 sucrose and 3 g L-1 GelriteTM. After eight weeks, completely regenerated P. nakaianum plantlets, characterized by well-developed shoot and root systems, were transplanted into a dome-covered tray filled with a mixture of peat moss, perlite, and decomposed granite soil (1:1:1). Subsequently, the trays were placed in a culture room featuring a 16-h photoperiod and light intensity of 24 µmol m-2 s-1 provided by cool white fluorescent lamps at 25 ± 1°C. After 14 days, the plantlets underwent gradual exposure during acclimatization, achieved by opening the vents in the dome covering the tray. The survival rate of the plants was assessed after acclimatization for 4 weeks.
2.5. Statistical Analysis
Following verification of normality for all collected data (including callus formation, shoot induction, and number of shoots) using the Shapiro-Wilk test, the significance of treatment effects on callus formation, shoot induction, and number of shoots was assessed through analysis of variance (ANOVA) using the statistical programming environment R (version 4.2.1). Significant differences between means were determined using Tukey’s honest significant difference test at a significance level of 5%.
3. Results
3.1. Effects of Cytokinins on Shoot Induction in Cotyledons
Table 1 presents the outcomes of cytokinin applications on callus formation, shoot induction, and the number of shoots per cotyledon explant of
P. nakaianum. Callus initiation commenced on the cut surfaces of cotyledons after 1 week of culture, and the percentage of callus formation was calculated accordingly. There was a significant impact of cytokinin type on callus formation (
p ≤ 0.0001). Cotyledon explants cultured on a medium containing 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA exhibited the highest percentage of callus formation (97.2 ± 2.8%), whereas no callus formation was observed on media supplemented with BAP. By the third week of culture, adventitious shoots emerged from the cotyledon explants (
Figure 2). The shoot induction and the number of shoots per cotyledon explant were evaluated after six weeks of culture. Cytokinins notably influenced the induction of adventitious shoots in cotyledons (
p = 0.0005). The medium supplemented with 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA emerged as the optimal cytokinin treatment condition, not only for adventitious shoot induction in cotyledons (61.1 ± 9.6%), but also for a high frequency of shoots per cotyledon explant (3 ± 0.6). Nonetheless, results from Tukey’s test revealed no significant differences between the TDZ and zeatin treatments.
3.2. Effects of Cytokinins on Shoot Induction in Petioles and Leaves
Table 2 shows the effects of cytokinins on callus formation, shoot induction, the number of shoots per petiole, and leaf explants of
P.
nakaianum. Shoot induction from petiole explants was more effective than adventitious shoot induction from leaf explants in
P.
nakaianum (
Figure 3). The ANOVA results showed that the cytokinin type had a statistically significant effect on shoot induction in petioles (
p = 0.0046). Petiole explants on medium containing 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA showed not only the highest percentage of callus formation (83.3 ± 3.3%), but also the highest percentage of shoot induction (26.7 ± 8.8). The ANOVA results showed that cytokinin type had a statistically significant effect on callus formation in leaves (
p ≤ 0.0001). Leaf explants on medium containing 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA showed the highest percentage of callus formation (100%), although there were no significant differences between TDZ and zeatin (90 ± 10%) in Tukey’s test. No callus formation was observed in the leaf explants in media containing BA and kinetin. Shoot induction from leaf explants on medium containing TDZ was quite low (3.3 ± 3.3%), and in other treatments, adventitious shoots were not induced. In this study, we proved that petiole explants on half-strength MS medium supplemented with 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA were suitable for adventitious shoot induction and enhanced frequency of shoot generation in
P.
nakaianum, whereas, leaf explants were not appropriate.
3.3. Adventitious Shoot Elongation, Rooting, and Acclimatization of Regenerated Plants
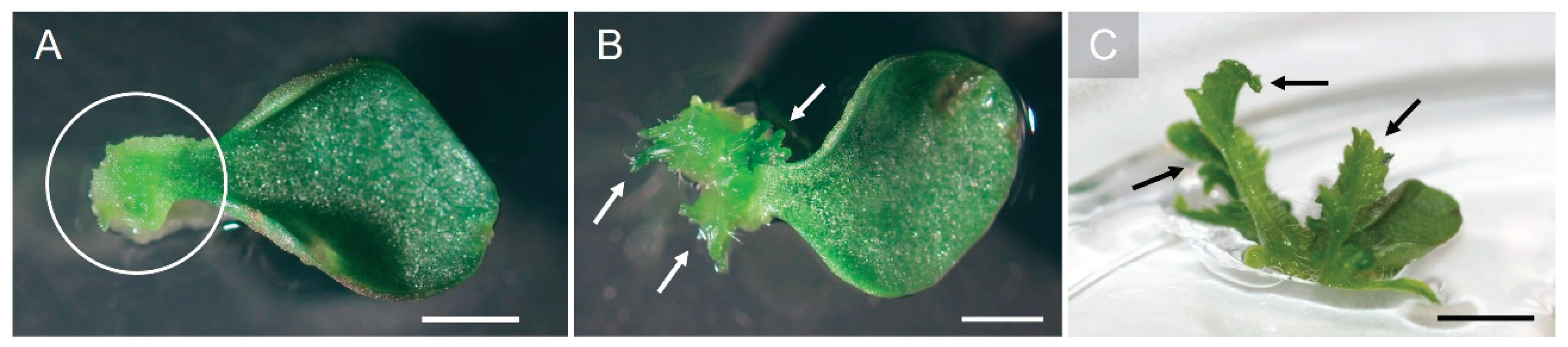
Shoots induced from both cotyledon and petiole explants were elongated using half-strength MS medium for six weeks. Well-rooted
P. nakaianum plantlets, approximately 5 cm in length with 6–10 leaves, cultivated in vitro (
Figure 4A), were washed with tap water to remove the solid medium from the roots before being transplanted into dome-covered trays filled with a mixture of peat, decomposed granite, and perlite in a 1:1:1 ratio (
Figure 4B). Subsequently, the plantlets were placed in a culture room maintained at 25 ± 2°C under a 16-h photoperiod with a light intensity of approximately 80 µmol m
-2 s
-1 (provided by cool white fluorescent lamps) to ensure optimal humidity levels in the culture environment. After 14 days, the plastic cover was removed, and the plantlets were transplanted into plastic pots filled with a mixture of coco peat, decomposed granite, peat, and perlite in a 5:1:1:3 ratio for eight weeks. The plants exhibited approximately 70% survival after four weeks of growth under ex vitro conditions (
Figure 4C). To our knowledge, no comprehensive studies have been previously undertaken on the regeneration of
P. nakaianum using in vitro culture systems.
4. Discussion
Shoot induction stands as a primary function of cytokinins in tissue culture [
11]. Our investigation identified 2 mg L
-1 TDZ as the optimal cytokinin treatment for inducing shoot organogenesis in
P. nakaianum. Since the cytokinin activity of TDZ was discovered in 1982, the hormone has been widely utilized to stimulate shoot formation and enhance axillary shoot proliferation, demonstrating particular efficacy in recalcitrant woody species [
12]. As a phenylurea substitute, TDZ has found application across numerous plant species [
13]. Consistent with our findings, previous studies have reported the regeneration of adventitious shoots from cotyledons of
Fraxinus americana L. (white ash) using 10 µM TDZ [
14],
Rubus sp. using 5 µM TDZ [
15], and
Prunus persica L. (peach),
P. domestica L. (plum), and
P. cerasus L. (sour cherry) using 5-12.5 µM TDZ [
16]. According to Huetteman and Preece [
17], cotyledons from mature or immature seeds from in vitro cultures are the most common explants for the adventitious regeneration of woody plants with TDZ. Moreover, Bano et al. [
18] noted that shoot induction from cotyledon explants proved more effective than from hypocotyl explants in
Brassica juncea. Similarly, Chi et al. [
19] observed earlier shoot formation in cotyledons of
Brassica campestris than in hypocotyls. In our study, while adventitious shoots were induced in cotyledon explants using TDZ, the same was not observed in hypocotyl explants.
The choice of plant materials for in vitro tissue culture depends on the specific objectives. Seed materials are often unsuitable for maintaining and propagating plant species owing to segregation based on phenotypic traits. Hence, cotyledon explants may not ensure uniformity. Leaves, stems, petioles, and other plant parts are preferred explants for heterozygous plant species. Thus, we induced adventitious shoots on the petioles and leaves of
P. nakaianum. Interestingly, our study found better shoot organogenesis in petioles than in leaves, whereas Zeng et al. [
20] reported similar regeneration capacities in leaves and petioles. Other studies using petiole explants have shown a higher ability to induce tetraploidy compared to that using leaves [
21]. Additionally, Saunders and Shin [
22] noted minimal callus formation on petiole explants incubated in light, whereas our results demonstrated both callus formation and adventitious shoot initiation in most petiole explants on medium containing 2 mg L
-1 TDZ and 0.05 mg L
-1 NAA. However, there is currently no definitive understanding of why shoot induction is more successful in petiole explants than leaf explants of
P. nakaianum.
For effective in vitro tissue culture, the age of the plant material is as critical as the type of material used. Liu et al. [
23] showed that extremely young and old petioles were unsuitable for bud regeneration in
Jatropha curcas L. Since our study utilized petioles of varying ages randomly, comparisons among petioles of different ages were not feasible. Thus, additional comparative studies are warranted to elucidate this aspect further.
5. Conclusions
We have successfully developed an effective method for in vitro plant regeneration of P. nakaianum utilizing cotyledons and petioles with various cytokinin treatments. To our knowledge, this is the first report of plantlet regeneration from cotyledons and petioles via shoot organogenesis pathways in P. nakaianum. This established protocol offers a valuable approach for adventitious shoot regeneration, which holds promise for facilitating systematic genetic transformation aimed at enhancing traditional breeding methods.
Author Contributions
Conceptualization and writing—original draft, C.H.A.; formal analysis and methodology, J.M.K., J.M.S., J.W.S., and S.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We extend our gratitude to the Geumwon Mountain Forest Resources Management Office, Gyeongnam Institute of the Environment and Forest in Korea, for generously providing the P. longifolium seeds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Albach, D.C.; Martínez-Ortega, M.M.; Delgado, L.; Weiss-Schneeweiss, H.; Özgökce, F.; Fischer, M.A. Chromosome numbers in Veroniceae (Plantaginaceae): Review and several new counts. Ann. Mo. Bot. Gard. 2008, 95, 543–566. [Google Scholar] [CrossRef]
- Ahloowalia, B.S. Tissue culture / clonal propagation, in vitro. In Encycl. Appl. Plant Sci., Vol. 1; Thomas, B., Ed.; Academic Press: USA, 2003; pp. 1360–1364. [Google Scholar] [CrossRef]
- Oh, H.J.; Lee, S.Y.; Shin, U.S.; Kim, H.C.; Kim, S.Y. Several factors affecting growth of Veronica rotunda var. subintegra (Nakai) T. Yamaz. stem cutting. Korean J. Plant Res. 2021, 34, 270–277. [Google Scholar] [CrossRef]
- Song, C.Y.; Moon, J.Y.; Sung, J.W.; Park, B.S.; Nam, J.I.; Kim, J.M. Effects of shading rate, rooting media and plant growth regulators on rooting of Veronoca L. by cuttings. J. Pract. Agric. Fish. Res. 2022, 24, 35–44. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinins. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Parveen, S.; Fatema, M. Development of a regeneration system via nodal segment culture in Veronica anagallis-aquatica L. – An amphibious medicinal plant. J. Plant Interact. 2011, 6, 61–68. [Google Scholar] [CrossRef]
- Stapfer, R.E.; Heuser, C.W.; Deneke, C.F. Rapid multiplication of Veronica ‘Red Fox’ propagated in vitro. Hortic. Sci. 1985, 20, 866–867. [Google Scholar] [CrossRef]
- Mallón, R.; Rodríguez-Oubiña, J.; González, M.L. Shoot regeneration from in vitro-derived leaf and root explants of Centaurea ultreiae. Plant Cell Tissue Organ Cult. 2011, 106, 523–530. [Google Scholar] [CrossRef]
- Shin, J.W.; Chi, Y.H.; Kim, J.M.; Lee, J.W.; Chae, J.H.; Yang, C.H.; Baek, D.S.; Baek, S.G.; Yoon, H.W.; Lee, H.N.; Seo, J.M.; Kim, Y.R.; Nam, J.I.; Ahn, C.H. Effects of culture medium composition on the in vitro growth of stem cuttings of Kirengeshoma koreana Nakai, a rare species in Korea. J. People Plants Environ. 2022, 25, 637–643. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Maruyama, E.; Ishii, K.; Kinoshita, I.; Ohba, K.; Saito, A. Micropropagation of Guazuma crinita Mart. by root and petiole culture. In Vitro Cell. Dev. Biol. Plant 1997, 33, 131–135. [Google Scholar] [CrossRef]
- Lu, C.Y. The Use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Wilhelm, E. Micropropagation of juvenile sycamore maple via adventitious shoot formation by use of thidiazuron. Plant Cell Tissue Organ Cult. 1999, 57, 57–60. [Google Scholar] [CrossRef]
- Bates, S.; Preece, J.E.; Navarrete, N.E.; Van Sambeek, J.W.; Gaffney, G.R. Thidiazuron stimulates shoot organogenesis and somatic embryogenesis in white ash (Fraxinus americana L.). Plant Cell Tissue Organ Cult. 1992, 31, 21–29. [Google Scholar] [CrossRef]
- Fiola, J.A.; Hassan, M.A.; Swartz, H.J.; Bors, R.H.; McNicols, R. Effect of thidiazuron, light fluence rates and kanamycin on in vitro shoot organogenesis from excised Rubus cotyledons and leaves. Plant Cell Tissue Organ Cult. 1990, 20, 223–228. [Google Scholar] [CrossRef]
- Mante, S.; Scorza, R.; Cordts, J.M. Plant regeneration from cotyledons of Prunus persica, Prunus domestica, and Prunus cerasus. Plant Cell Tissue Organ Cult. 1989, 19, 1–11. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Bano, R.; Khan, M.H.; Rashid, H.; Khan, R.S.; Munir, I.; Swati, Z.A.; Chaudhry, Z. Callogenesis and organogenesis in three genotypes of Brassica juncea affected by explant source. Pak. J. Bot. 2010, 42, 3925–3932. [Google Scholar]
- Chi, G.L.; Barfield, D.G.; Sim, G.E.; Pua, E.C. Effect of AgNO3 and aminoethoxyvinylglycine on in vitro shoot and root organogenesis from seedling explants of recalcitrant Brassica genotypes. Plant Cell Rep. 1990, 9, 195–198. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Z.; Kang, X. Adventitious shoot regeneration from leaf, petiole and root explants in triploid (Populus alba × P. glandulosa)× P. Tomentosa. Plant Cell Tissue Organ Cult. 2019, 138, 121–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Qi, S.; Wang, X.; Zhao, J.; Zhang, J.; Li, B.; Zhang, Y.; Liu, X.; Yuan, W. In vitro tetraploid induction from leaf and petiole explants of hybrid sweetgum (Liquidambar styraciflua × Liquidambar formosana). Forests 2017, 8, 264. [Google Scholar] [CrossRef]
- Saunders, J.W.; Shin, K. Germplasm and physiologic effects on induction of high-frequency hormone autonomous callus and subsequent shoot regeneration in sugarbeet. Crop Sci. 1986, 26, 1240–1245. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, X.; Hui, W.; Liu, T.; Chen, X.; Li, J.; Zhuang, C.; Yang, Y.; Liu, Z. Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of Jatropha curcas L. Biotechnol. Biotechnol. Equip. 2015, 29, 479–488. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).