Submitted:
12 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
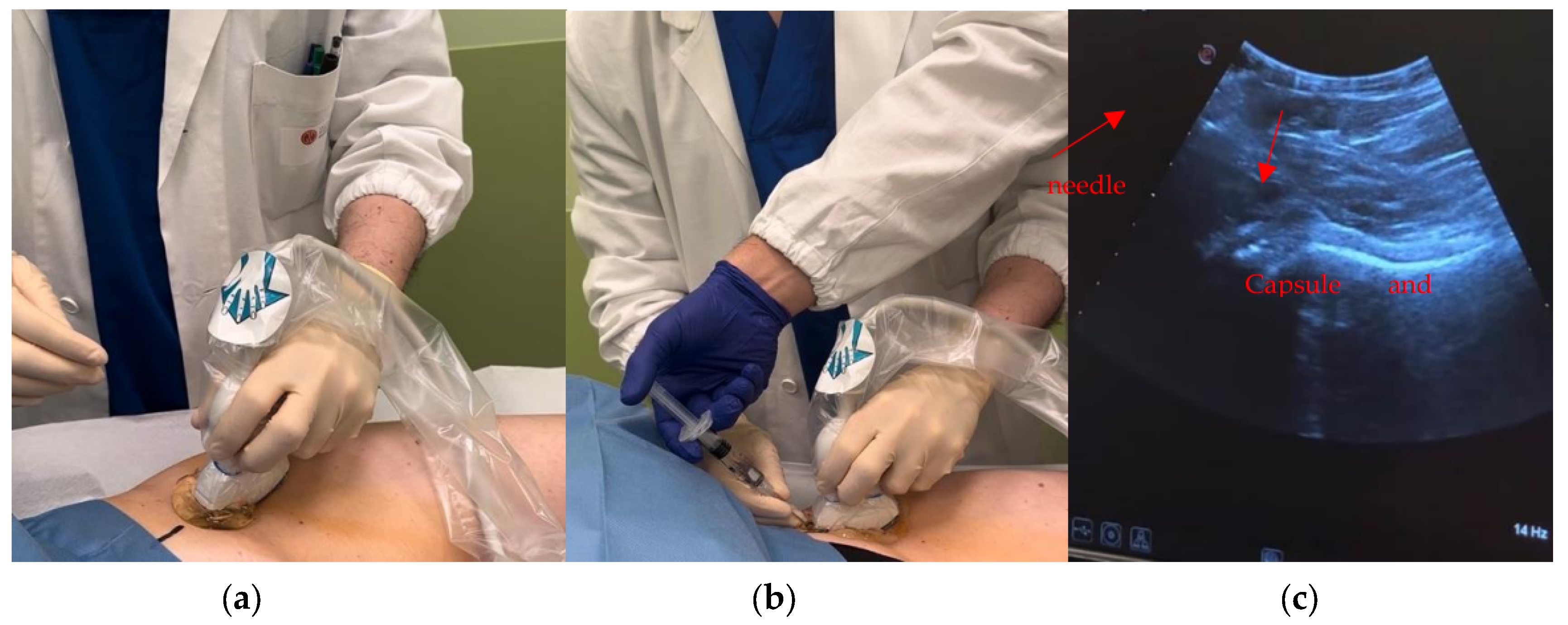
Brief Description of US Imaging
Procedure
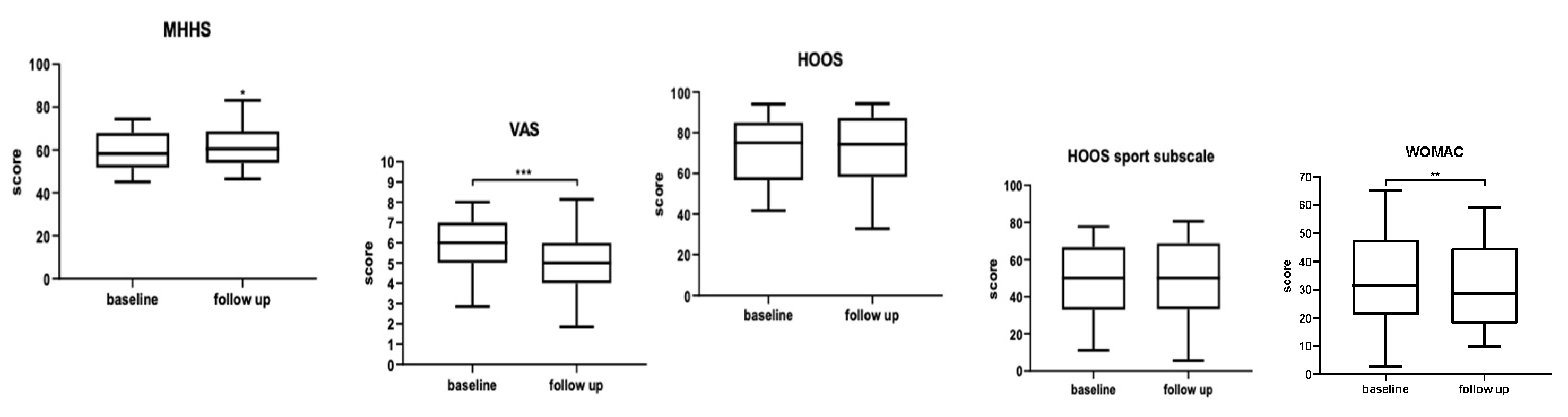
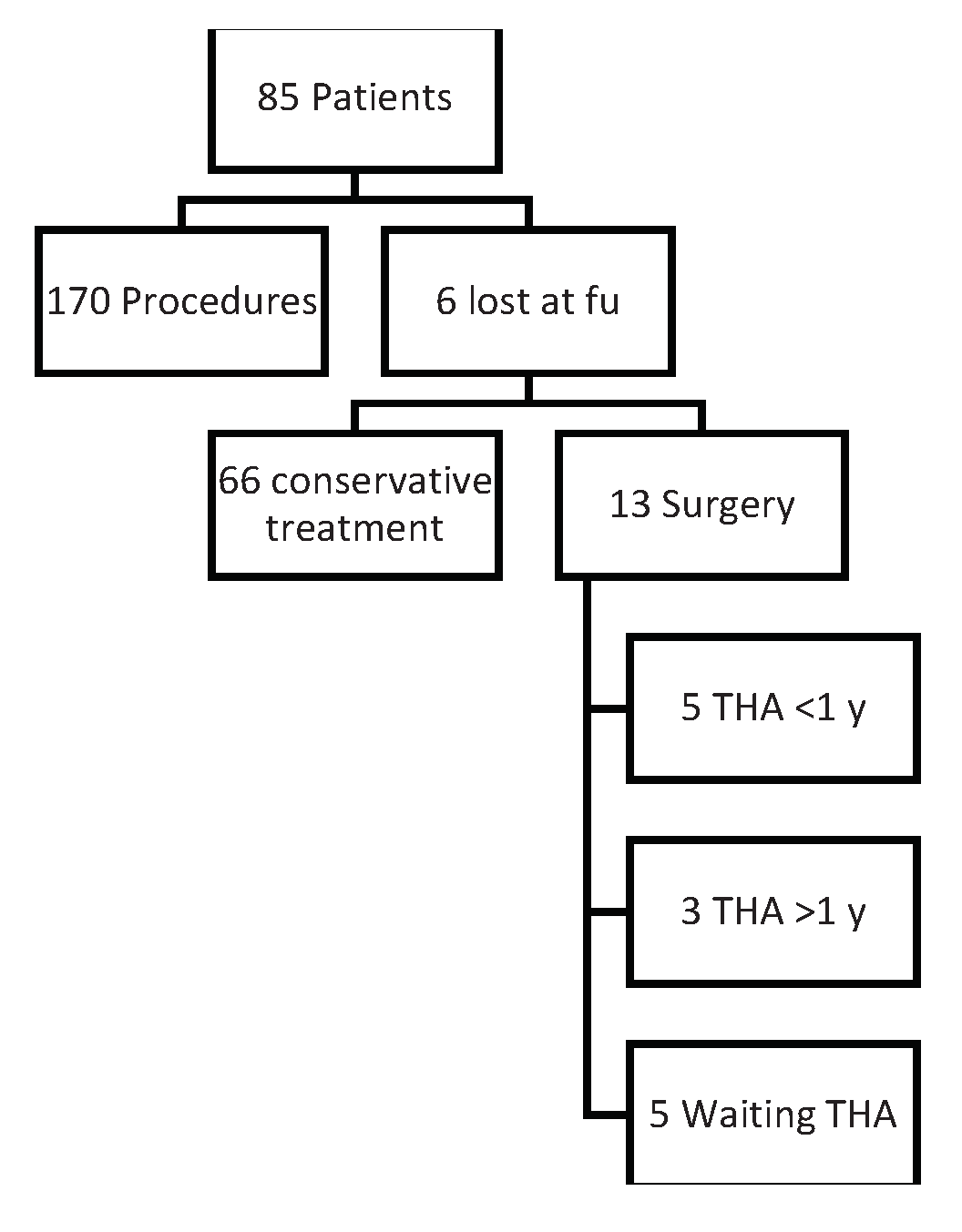
Results
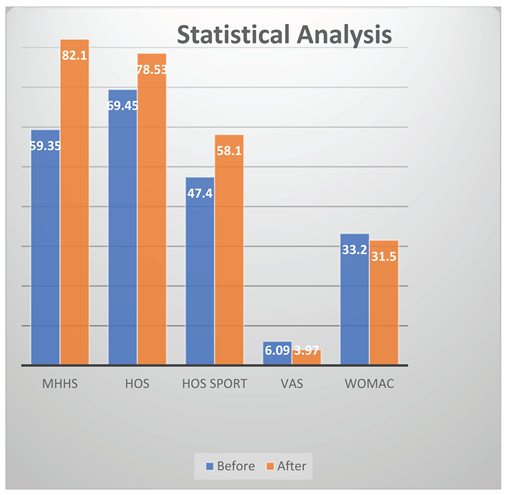
Discussion
Conclusion
Funding
Conflicts of Interest
References
- Moss, A.; Murphy, L.; Helmick, C.; Schwartz, T.; Barbour, K.; Renner, J.; Kalsbeek, W.; Jordan, J. Annual incidence rates of hip symptoms and three hip OA outcomes from a U.S. population-based cohort study: the Johnston County Osteoarthritis Project. Osteoarthr. Cartil. 2016, 24, 1518–1527. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Helmick, C.G.; Arnett, F.C.; Deyo, R.A.; Felson, D.T.; Giannini, E.H.; Heyse, S.P.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1998, 41, 778–799. [Google Scholar] [CrossRef]
- Murphy L, Helmick CG. The Impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs 2012, 31, 85–91. [CrossRef]
- Iagnocco, A. Imaging the joint in osteoarthritis: a place for ultrasound? Best Pr. Res. Clin. Rheumatol. 2010, 24, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hoeber, S.; Aly, A.-R.; Ashworth, N.; Rajasekaran, S. Ultrasound-guided hip joint injections are more accurate than landmark-guided injections: a systematic review and meta-analysis. Br. J. Sports Med. 2015, 50, 392–396. [Google Scholar] [CrossRef]
- Narouze, S.N. Ultrasound-Guided Interventional Procedures in Pain Management. Reg. Anesthesia Pain Med. 2010, 35, S55–S58. [Google Scholar] [CrossRef]
- Dodré, E.; Lefebvre, G.; Cockenpot, E.; Chastanet, P.; Cotten, A. Interventional MSK procedures: the hip. Br. J. Radiol. 2016, 89, 20150408. [Google Scholar] [CrossRef]
- Crowe, H.W. Osteo-Arthritis of the Hip-Joint Treated by Intra-Articular Injections. Proc. R. Soc. Med. 1947, 40, 486–487. [Google Scholar] [CrossRef]
- Leveaux, V.M.; Quin, C.E. Local Injection of Hydrocortisone and Procaine in Osteo-Arthritis of the Hip Joint. Ann. Rheum. Dis. 1956, 15, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, A.; Ekhtiari, S.; Bozzo, A.; Phillips, M.; Bhandari, M. Intra-articular saline injection is as effective as corticosteroids, platelet-rich plasma and hyaluronic acid for hip osteoarthritis pain: a systematic review and network meta-analysis of randomised controlled trials. Br. J. Sports Med. 2020, 55, 256–261. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.S.; Maricar, N.; Parkes, M.J.; Felson, D.T.; O’Neill, T.W. The efficacy of intra-articular steroids in hip osteoarthritis: A systematic review. Osteoarthr. Cartil. 2016, 24, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Houck, D.A.; Littlefield, C.P.; Kraeutler, M.J.; Potyk, A.G.; Mei-Dan, O.; Dragoo, J.L.; Frank, R.M.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Hip Osteoarthritis Yields Similarly Beneficial Short-Term Clinical Outcomes: A Systematic Review and Meta-analysis of Level I and II Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 38, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Adriaensen, M.; Albano, D.; Alcala-Galiano, A.; Allen, G.; Gómez, M.P.A.; Aringhieri, G.; Bazzocchi, A.; Beggs, I.; Chianca, V.; et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)—part VI, foot and ankle. Eur. Radiol. 2021, 32, 1384–1394. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons Management of Osteoarthritis of the Hip Evidence-Based Clinical Practice Guideline. aaos.org/oahcpg2. Am Acad Orthop Surg 2023.
- Tait, R.C.; Chibnall, J.T. Physician judgments of chronic pain patients. Soc. Sci. Med. 1997, 45, 1199–1205. [Google Scholar] [CrossRef]
- Wilson, M.G.; Michet, C.J.; Ilstrup, D.M.; Iii, L.J.M. Idiopathic Symptomatic Osteoarthritis of the Hip and Knee: A Population-Based Incidence Study. Mayo Clin. Proc. 1990, 65, 1214–1221. [Google Scholar] [CrossRef]
- A Balazs, E.; Denlinger, J.L. Viscosupplementation: a new concept in the treatment of osteoarthritis. J. Rheumatol. 1993, 39, 3–9. [Google Scholar]
- Harris, WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Ayeni, O.R.; Farrokhyar, F.; Crouch, S.; Chan, K.; Sprague, S.; Bhandari, M. Pre-operative intra-articular hip injection as a predictor of short-term outcome following arthroscopic management of femoroacetabular impingement. Knee Surgery, Sports Traumatol. Arthrosc. 2014, 22, 801–805. [Google Scholar] [CrossRef]
- Nilsdotter, A.K.; Lohmander, L.S.; Klässbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS) – validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003, 4, 10–10. [Google Scholar] [CrossRef] [PubMed]
- Bodendorfer, B.M.; Clapp, I.M.; DeFroda, S.F.; Malloy, P.; Alter, T.D.; Parvaresh, K.C.; Chahla, J.; Nho, S.J. The Natural Course of Recovery After Hip Arthroscopy for Femoroacetabular Impingement According to the International Hip Outcome Tool–12 and Hip Outcome Score Sports Subscale. Am. J. Sports Med. 2021, 49, 3250–3260. [Google Scholar] [CrossRef] [PubMed]
- Mujahed, T.; Hassebrock, J.D.; Makovicka, J.L.; Pollock, J.R.; Wilcox, J.G.; Patel, K.A.; Economopoulos, K.J. Preoperative Intra-articular Steroid Injections as Predictors of Hip Arthroscopy: 2-Year Outcomes. Orthop. J. Sports Med. 2021, 9. [Google Scholar] [CrossRef]
- Hunt, D.; Prather, H.; Hayes, M.H.; Clohisy, J.C. Clinical Outcomes Analysis of Conservative and Surgical Treatment of Patients With Clinical Indications of Prearthritic, Intra-articular Hip Disorders. PM&R 2012, 4, 479–487. [Google Scholar] [CrossRef]
- Mahomed NN, Arndt DC, McGrory BJ, Harris WH. The Harris hip score: Comparison of patient self-report with surgeon assessment. J Arthroplasty 2001, 16, 575–580. [CrossRef]
- Edwards, P.K.; Queen, R.M.; Butler, R.J.; Bolognesi, M.P.; Barnes, C.L. Are Range of Motion Measurements Needed When Calculating the Harris Hip Score? J. Arthroplast. 2015, 31, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.B.T.; Chou, A.C.C.; Yeo, W.; Lo, N.N.; Chia, S.-L.; Chin, P.L.; Tay, D.K.J.; Yeo, S.J. Comparison of patient quality of life scores and satisfaction after common orthopedic surgical interventions. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 1007–1012. [Google Scholar] [CrossRef]
- Terkawi, A.S.; Karakitsos, D.; Elbarbary, M.; Blaivas, M.; Durieux, M.E. Ultrasound for the Anesthesiologists: Present and Future. Sci. World J. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Guaraldi, F.; Vannini, F.; Rossi, G.; Timoncini, A.; Buda, R.; Giannini, S. Efficacy of Ultrasound-guided Intra-articular Injections of Platelet-rich Plasma Versus Hyaluronic Acid for Hip Osteoarthritis. Orthopedics 2013, 36, e1501–e1508. [Google Scholar] [CrossRef]
- Conrozier, T.; Bertin, P.; Mathieu, P.; Charlot, J.; Bailleul, F.; Treves, R.; Vignon, E.; Chevalier, X. Intra-articular injections of hylan G-F 20 in patients with symptomatic hip osteoarthritis: an open-label, multicentre, pilot study. . 2003, 21, 605–10. [Google Scholar] [PubMed]
- Eymard, F.; on behalf of the Osteoarthritis Group of the French Society of Rheumatology and of the French Research Group in Interventional Rheumatology; Maillet, B. ; Lellouche, H.; Mellac-Ducamp, S.; Brocq, O.; Loeuille, D.; Chevalier, X.; Conrozier, T. Predictors of response to viscosupplementation in patients with hip osteoarthritis: results of a prospective, observational, multicentre, open-label, pilot study. BMC Musculoskelet. Disord. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Zhu, J.; Lim, A.; McCaskie, A.W.; Khanduja, V. Viscosupplementation is Effective for the Treatment of Osteoarthritis in the Hip. A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2023. [Google Scholar] [CrossRef]
- Clementi, D.; D’ambrosi, R.; Bertocco, P.; Bucci, M.S.; Cardile, C.; Ragni, P.; Giaffreda, G.; Ragone, V. Efficacy of a single intra-articular injection of ultra-high molecular weight hyaluronic acid for hip osteoarthritis: a randomized controlled study. Eur. J. Orthop. Surg. Traumatol. 2017, 28, 915–922. [Google Scholar] [CrossRef]
- Colen, S.; Bekerom, M.P.J.v.D.; Mulier, M.; Haverkamp, D. Hyaluronic Acid in the Treatment of Knee Osteoarthritis. BioDrugs 2012, 26, 257–268. [Google Scholar] [CrossRef]
- Colen S, Van Den Bekerom MPJ, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012, 26, 257–268. [CrossRef]
- Rastogi, A.K.; Davis, K.W.; Ross, A.; Rosas, H.G. Fundamentals of Joint Injection. Am. J. Roentgenol. 2016, 207, 484–494. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.-M.; Liu, Y.-C. Efficacy of intra-articular hyaluronic acid injections in hip osteoarthritis: a meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 86865–86876. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Natov, N.S.; Obadan, I.E.; Price, L.L.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. 2009, 61, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Bella, A.; Bisignani, M.; Calderaro, M.; De Amicis, D.; Logroscino, G.; Mariottini, F.; Moreschini, O.; Massafra, U.; Bizzi, E.; et al. Total hip replacement rate in a cohort of patients affected by symptomatic hip osteoarthritis following intra-articular sodium hyaluronate (MW 1,500–2,000 kDa) ORTOBRIX study. Clin. Rheumatol. 2012, 31, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Cancienne, J.M.; Browne, J.A. The Timing of Total Hip Arthroplasty After Intraarticular Hip Injection Affects Postoperative Infection Risk. J. Arthroplast. 2015, 31, 820–823. [Google Scholar] [CrossRef]
- Saracco, M.; Ciriello, V.; D’angelo, F.; Zagra, L.; Solarino, G.; Logroscino, G. Do prior intra-articular injections impact on the risk of periprosthetic joint infection in patients undergoing total hip arthroplasty? A meta-analysis of the current evidences with a focus on the timing of injection before surgery. EFORT Open Rev. 2023, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Raman, R.; Richette, P.; Bard, H.; Jerosch, J.; Conrozier, T.; Chevalier, X.; Migliore, A. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin. Arthritis Rheum. 2015, 45, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.D. Comparison of Two Injection Techniques for Intra-articular Hip Injections. J. Ultrasound Med. 2016, 35, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
| Tonnis grade | N°of patients |
|---|---|
| 0 | 20 |
| 1 | 32 |
| 2 | 18 |
| 3 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




