Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
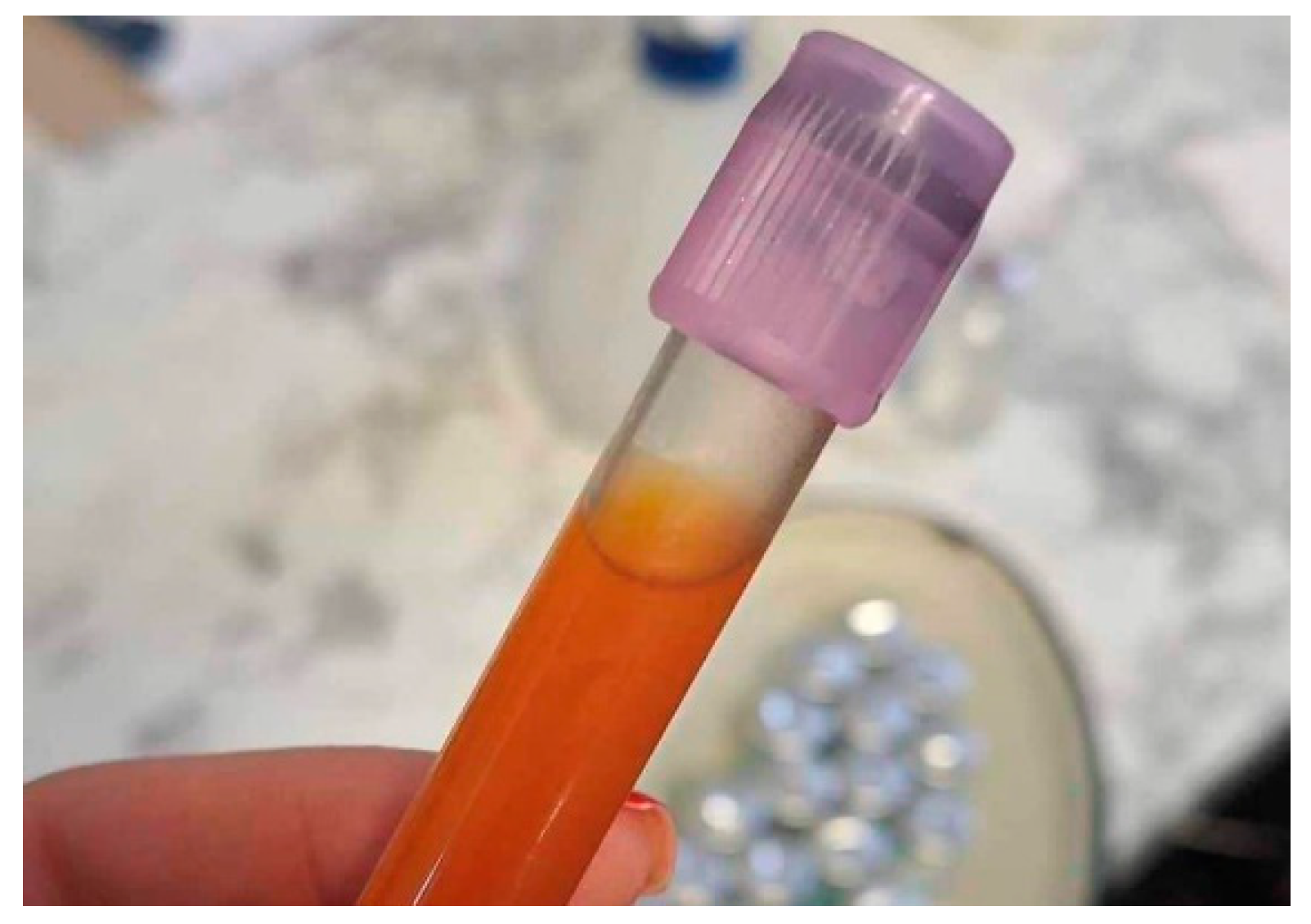
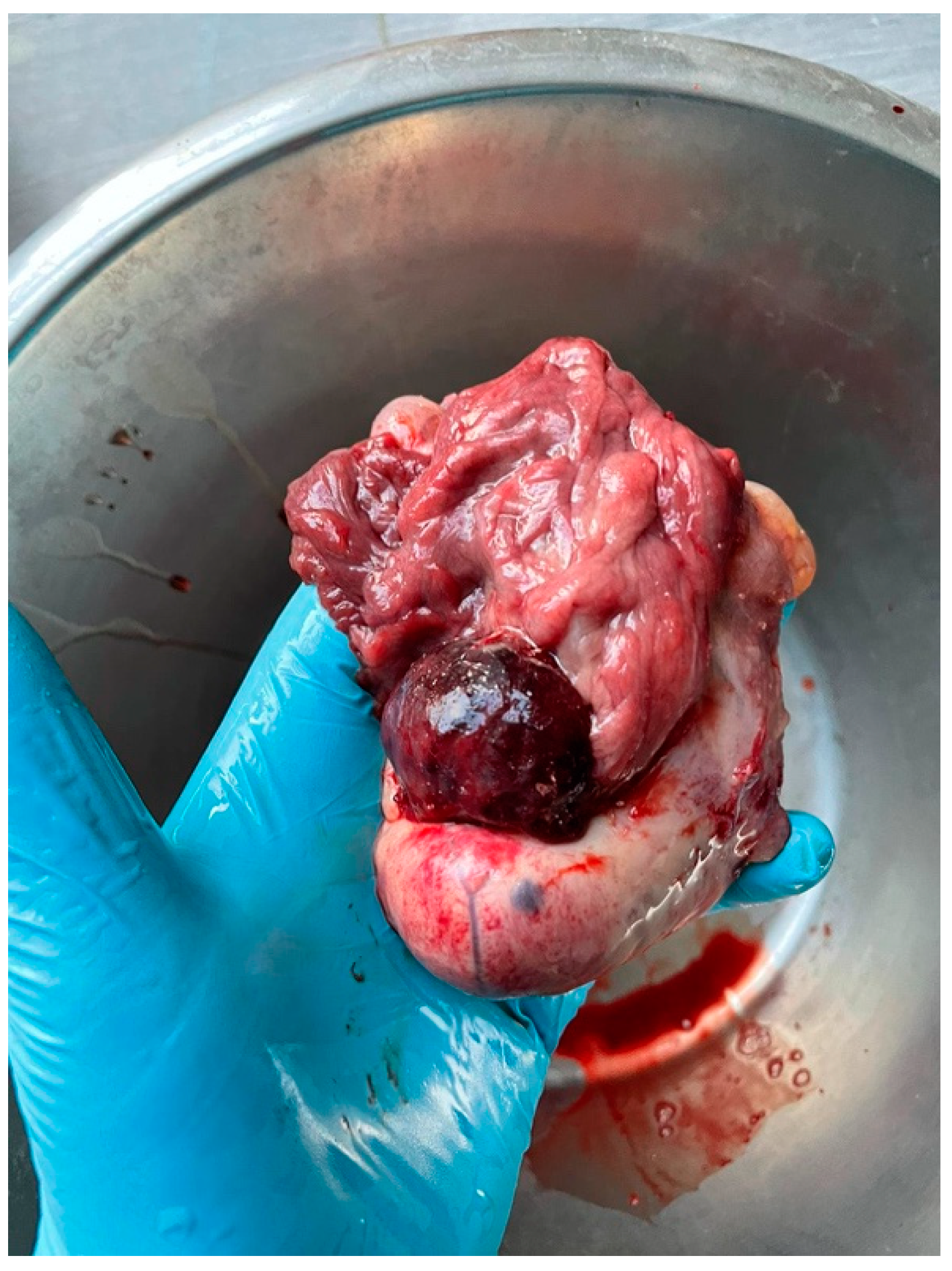
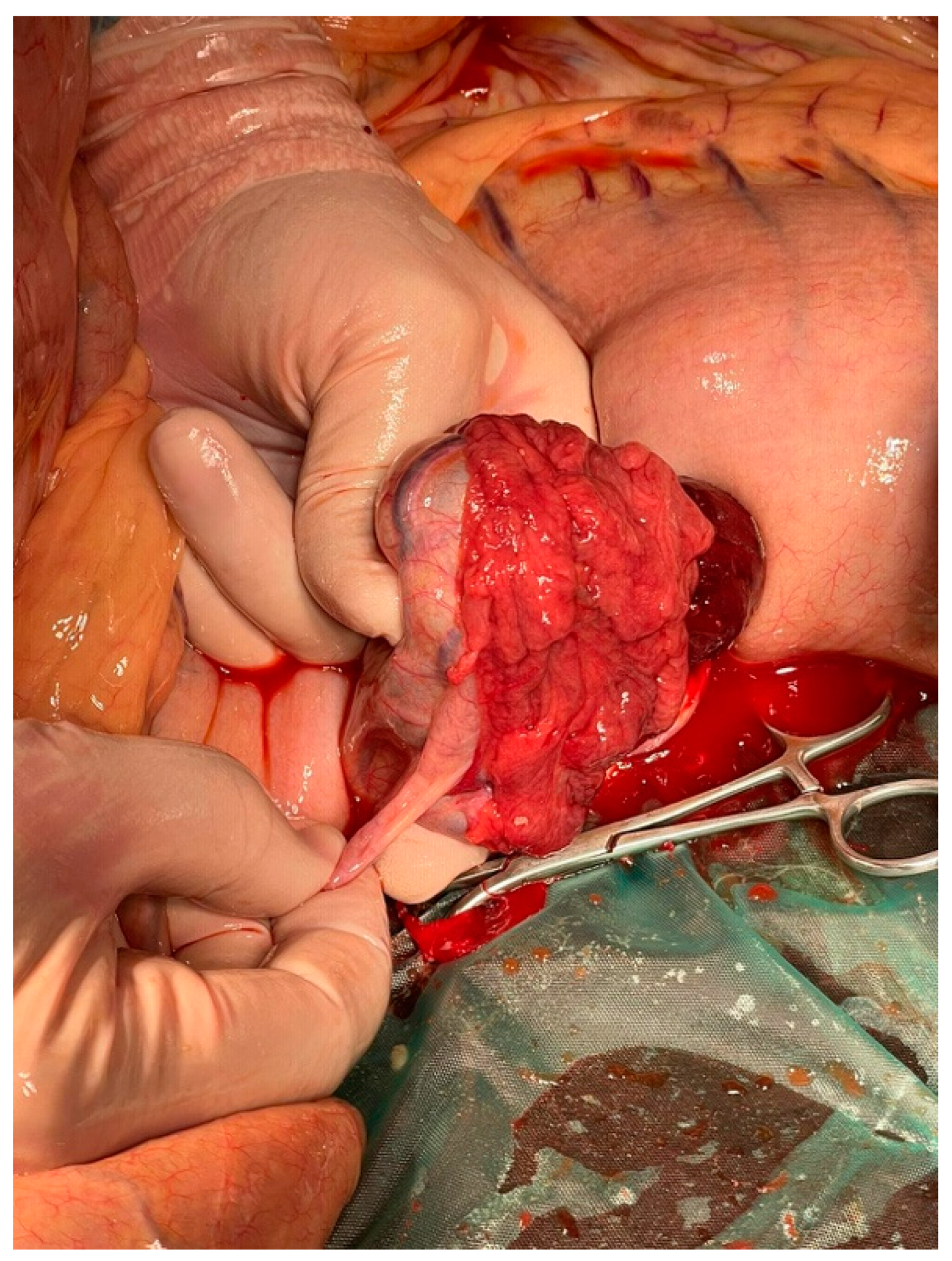
2. Case History
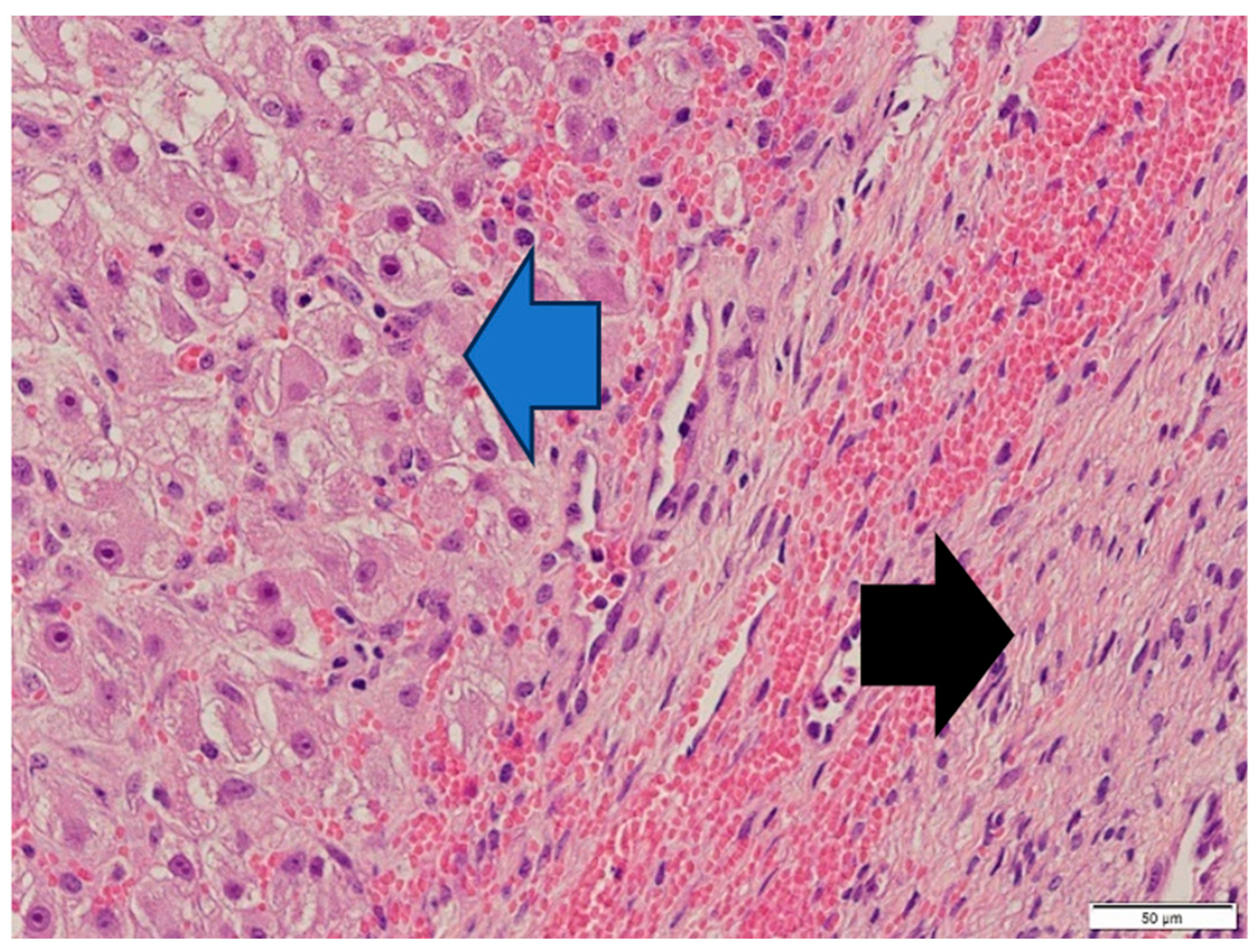
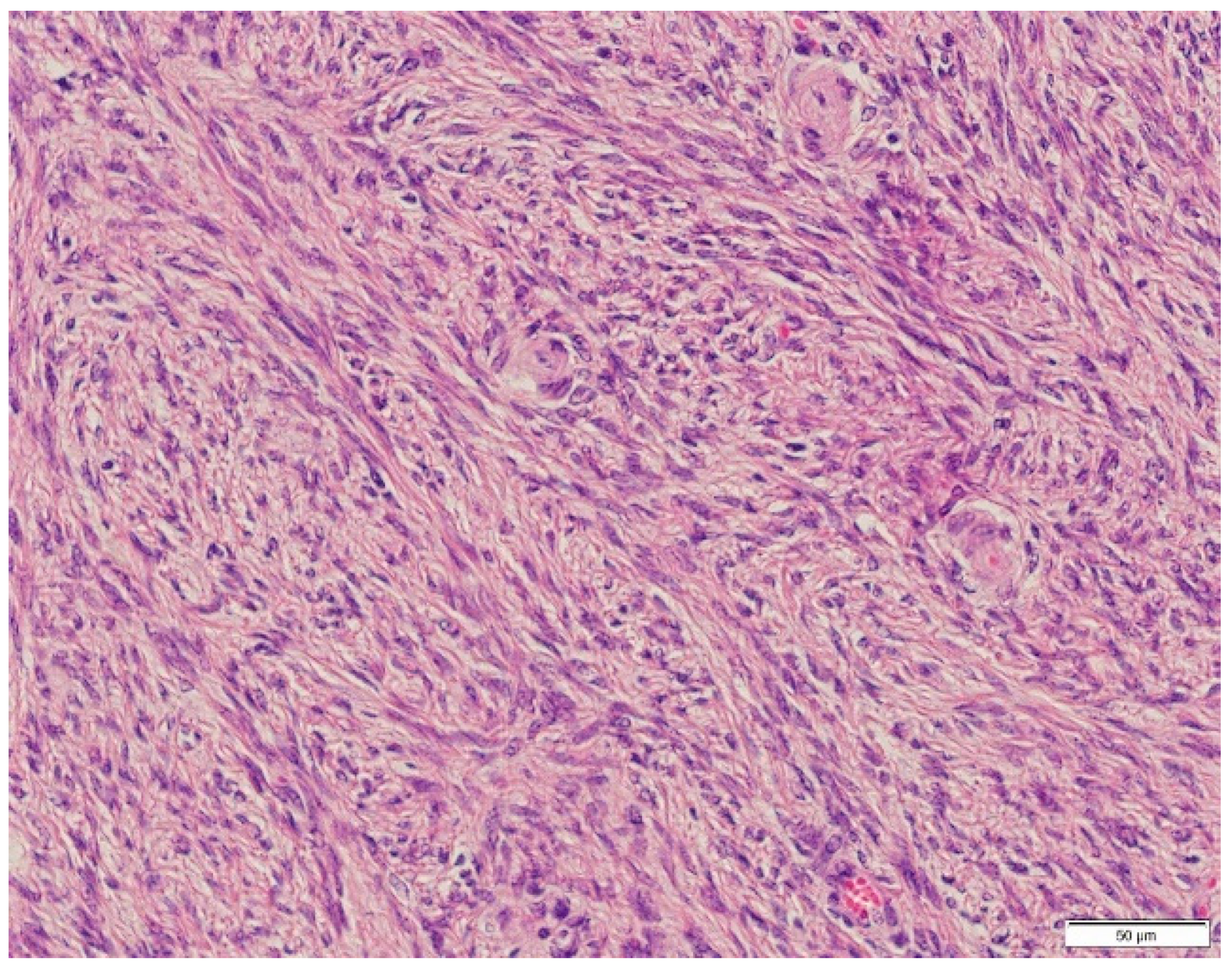
3. Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
References
- Shahrzad Azizi, Mostafa Nourbakhsh, and Reza Kheirandish. Ovarian Fibrothecoma in an Arabian Mare: A Rare Case. J. Equine Vet. Sci. 2014, 34, 314–317. [Google Scholar] [CrossRef]
- Shahrzad Azizi, Mostafa Nourbakhsh, and Reza Kheirandish. Ovarian Fibrothecoma in an Arabian Mare: A Rare Case. J. Equine Vet. Sci. 2014, 34, 314–317. [Google Scholar] [CrossRef]
- Lm Cellio and Da Degner. Testosterone-producing thecoma in a female cat. J. Am. Anim. Hosp. Assoc. 2000, 36, 323–325. [Google Scholar] [CrossRef]
- Massimo Conte, Lorenzo Guariglia, Pierluigi Benedetti Panici, Giovanni Scambia, Carla Rabitti, Arnaldo Capelli, and Salvatore Mancuso. Ovarian Fibrothecoma: Sonographic and Histologic Findings. Gynecol. Obstet. Invest. 1991, 32, 51–54. [Google Scholar] [CrossRef]
- Massimo Conte, Lorenzo Guariglia, Pierluigi Benedetti Panici, Giovanni Scambia, Carla Rabitti, Arnaldo Capelli, and Salvatore Mancuso. Ovarian Fibrothecoma: Sonographic and Histologic Findings. Gynecol. Obstet. Invest. 1991, 32, 51–54. [Google Scholar] [CrossRef]
- K. T. T. Corley, L. L. Donaldson, and M. O. Furr. Arterial lactate concentration, hospital survival, sepsis and SIRS in critically ill neonatal foals. Equine Vet. J. 2005, 37, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Aurelia Dolin, Peter Schweiger, Martin Waselau, Monika Egerbacher, and Ingrid Walter. Immunohistochemical markers for equine granulosa cell tumors: A pilot study. J. Equine Sci. 2023, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- R. Ebert. [Prognostic parameters in equine colic]. Tierarztl. Prax. 1994, 22, 256–263.
- Fernanda Carlini Cunha dos Santos, Fernanda Aquino Franco, Thaisi Piazza, Ricardo Zanella, Carlos Bondan, and Leonardo Porto Alves. 2023. Thecoma in an Elderly Crioulo Mare. 866 5, Acta Scientiae Veterinariae, (April 2023). [CrossRef]
- İbrahim Firat, Kıvılcım Sönmez, and Marija Pajić. Tek Tarafl ı Ovariektomi Yapılan Bir İngiliz Atında Fibrotekoma: Olgu Sunumu. Kafkas Univ. Vet. Fak. Derg. 2009. [CrossRef]
- Reproductive performance following unilateral ovariectomy for treatment of ovarian tumors in 7 mares. Reproductive performance following unilateral ovariectomy for treatment of ovarian tumors in 7 mares. Turk. J. Vet. Anim. Sci. 2010. [CrossRef]
- D. M. Hassel, A.E. Hill, and R.A. Rorabeck. Association between Hyperglycemia and Survival in 228 Horses with Acute Gastrointestinal Disease. J. Vet. Intern. Med. 2009, 23, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- S. F. Henderson. Diagnostic and prognostic use of L -lactate measurement in equine practice. Equine Vet. Educ. 2013, 25, 468–475. [Google Scholar] [CrossRef]
- Katrin Hinrichs and P., R. Hunt. Ultrasound as an aid to diagnosis of granulosa cell tumour in the mare. Equine Vet. J. 1990, 22, 99–103. [Google Scholar] [CrossRef]
- R. K. Hjortkjaer and C. K. Svendsen. Simulated small intestinal volvulus in the anesthetized horse. Nord. Vet. Med. 1979, 31, 466–483. [Google Scholar]
- İbrahim FIRAT and Kıvılcım SÖNMEZ. Fibrothecoma in A Trough Bred Mare with Unilateral Ovariectomy: A Case Report. Researchgate 2011, 4, 329–332. [Google Scholar]
- Jubb KVK and Kennedy PC. 1985. Pathology of domestic animals.Second edition. New York.
- Thatiane Kievitsbosch, Nereu Carlos Prestes, Viciany Erique Fabris, Luíz Fernando Scagion Salgado, and Marco Antônio Alvarenga. Ovarian Fibrothecoma in Mare – Case Report. J. Equine Vet. Sci. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Vesna Kadunc Kos, Petra Kramaric, and Maja Brloznik. Packed cell volume and heart rate to predict medical and surgical cases and their short-term survival in horses with gastrointestinal-induced colic. Can. Vet. J. Rev. Veterinaire Can. 2022, 63, 365–372. [Google Scholar]
- Leonardo Porto Alves, Carlos Bondan, Ricardo Zanella, Thaisi Piazza, Fernanda Aquino Franco, and Fernanda Carlini Cunha dos Santos. Thecoma in an Elderly Crioulo Mare. Case Rep. 2023, 5, 1–5. [Google Scholar] [CrossRef]
- Patrick, M. McCue, Janet F. Roser, Coralie J. Munro, Irwin K.M. Liu, and Bill L. Lasley. Granulosa Cell Tumors of the Equine Ovary. Vet. Clin. North Am. Equine Pract. 2006, 22, 799–817. [Google Scholar] [CrossRef]
- Donald J. Meuten (Ed.). 2016. Tumors in Domestic Animals (1st ed.). Wiley. [CrossRef]
- Ikuko Okada, Shoko Nakagawa, Yuriko Takemura, Takashi Takenobu, Tatsunari Matsuoka, Hiroyuki Mandai, and Tadashi Takemura. . Ovarian thecoma associated in the first trimester of pregnancy. J. Obstet. Gynaecol. Res. 2004, 30, 368–371. [Google Scholar] [CrossRef] [PubMed]
- J. A. Orsini, A. H. Elser, D. T. Galligan, W. J. Donawick, and D. S. Kronfeld. Prognostic index for acute abdominal crisis (colic) in horses. Am. J. Vet. Res. 1988, 49, 1969–1971. [Google Scholar]
- B. W. Parry, G. A. Anderson, and C. C. Gay. Prognosis in equine colic: A study of individual variables used in case assessment. Equine Vet. J. 1983, 15, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Fet Pauwels, Sj Wigley, Js Munday, and Wd Roe. Bilateral ovarian adenocarcinoma in a mare causing haemoperitoneum and colic. N. Z. Vet. J. 2012, 60, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Nereu Carlos Prestes, Carolina Nogueira De Moraes, Leandro Maia, Isabelle Rayanne Sousa De Oliveira, Viciany Erique Fabris, and Marco Antonio Alvarenga. Ovarian Tumor in a Mare—Thecoma—Case Report. J. Equine Vet. Sci. 2013, 33, 196–200. [Google Scholar] [CrossRef]
- Rolfe, M. Radcliffe, Sharon Y. Liu, Vanessa L. Cook, Samuel D.A. Hurcombe, and Thomas J. Divers. Interpreting abdominal fluid in colic horses: Understanding and applying peritoneal fluid evidence. J. Vet. Emerg. Crit. Care 2022, 32, 81–96. [Google Scholar] [CrossRef]
- Afshin Raoofi, Seyed Hossein Mardjanmehr, Majid Masoudifard, Farajollah Adibhashemi, and Peyman Asadian. Thecoma in a mare. J. Equine Vet. Sci. 2006, 26, 588–591. [Google Scholar] [CrossRef]
- Debra, C. Sellon. Thrombocytopenia in horses. Equine Vet. Educ. 1998, 10, 133–139. [Google Scholar] [CrossRef]
- E. R. Singer and M. A. Smith. Examination of the horse with colic: Is it medical or surgical? Equine Vet. Educ. 2002, 14, 87–96. [Google Scholar] [CrossRef]
- E. Târcoveanu, D. Niculescu, C. Bradea, G. Dimofte, A. Vasilescu, D. Ferariu, and Felicia Crumpei. [Incidence and management of the ovarian fibroma and thecoma. Experience of The First Surgical Clinic Iaşi]. Chir. Buchar. Rom. 2006, 101, 325–330. [Google Scholar]
- E. J. Van Der Zaag, A.B.M. Rijkenhuizen, H.C. Kalsbeek, and N.H.M.T. Peperkamp. A mare with colic caused by an ovarian tumour. Vet. Q. 1996, 18, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Raquel M. Walton, Rick L. Cowell, and Amy C. Valenciano (Eds.). 2021. Equine Hematology, Cytology, and Clinical Chemistry (1st ed.). Wiley. [CrossRef]
- M. D. Whitacre, S. D. Van Camp, N. J. Maclachlan, and J. A. Umstead. 1988. Premature lactation in a heifer with a sex cord-stromal tumor. J. Am. Vet. Med. Assoc. 1988, 193, 946–948. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




