1. Introduction
Mule, the hybrid between donkey and mare, has been used for several works ranging from military duties, heavy industry work, and recreational and sport activities. Mules were intelligent, not naturally aggressive, and were preferred for military work in the mountain, more than horses and donkeys [
1,
2]. They are infertile but still able to produce sex hormone, which more or less influence on aggressiveness or unwanted behavior [
3,
4]. Therefore, stallion mules usually be castrated before work training. Castration in equids is performed at both scrotal sacs to remove testes and leave the surgical wound un-suturing. Incidence of perioperative and postoperative complications ranged from 10.0-60.0% according to surgical procedure or postoperative management [
5,
6,
7,
8,
9,
10,
11]. Indwelling testis in the abdomen of cryptorchid equids can still produce testosterone [
12], which may contribute to unwanted behavior. Laparotomy or laparoscopic surgery must be performed to remove the testis from cryptorchid animals. This procedure requires several facilities and expensive equipment with a possible high risk for postoperative complications. Any methods to reduce testosterone level in equids, apart from surgery, may provide an alternative choice for the owners. From previous studies, vaccination against gonadotropin-releasing hormone (GnRH) appeared to be the most suitable immunocastration method for clinical uses in horses [
13,
14]. Additionally, the GnRH vaccine was recommended as a balanced method between animal welfare and the reduction of unwanted behaviors due to sex hormone in working animals [
15]. In Thailand, mules are produced mainly for military work around the mountain border. In order to be properly manage a mule herd, it is necessary to castrate intact young mules before maturity. Due to some limitations, there was still a group of intact and cryptorchid mules in the military unit, leading to problems during pasture turn-out. The unit solution was to keep that group of mules either in stall or a small area, which might affect these animals’ mental or overall health. To aid in such situation, our main interest was to evaluate whether the GnRH vaccine could be used as an immunocastration in stallion mules. To the best of our knowledge, immunocastration using the GnRH vaccine has not been previously studied in mules. In Thailand, the only one commercial GnRH vaccine, licensed for male pigs, is available. Nevertheless, this product had been used in several studies in horses [
16,
17,
18,
19] and should be applicable to use for study in intact and cryptorchid mules. The aims of this study were to evaluate the effect of the GnRH vaccine in mules on anti-GnRH antibody production, change in serum testosterone level, change in behavior within the restraint stall when received manipulation from humans, and clinical adverse effects.
2. Materials and Methods
This study was approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Chiang Mai University (R17/2561).
2.1. Animals
Thirteen intact stallion mules, 6 unilateral cryptorchid mules, and 6 castrated mules from a military unit in northern Thailand were included in the study. Two intact stallion mules were randomly selected for Control-intact. The remaining intact and cryptorchid mules were assigned for Treatment, which was further divided into subgroups according to condition (intact or unilateral cryptorchid) and age as followed: (a) Treatment-intact-young (age 5-9 years); (b) Treatment-intact-old (age 10-15 years); (c) Treatment-cryptorchid-young (age 5-9 years); and (d) Treatment-cryptorchid-old (age 10-15 years). Six castrated mules were randomly selected from the same herd as Control-castrated.
2.2. GnRH Vaccination and Clinical Examination for Adverse Effects
The week of the first vaccination was indicated as week 0. Mules in Treatment received 195 µg (1.3 mL) of GnRH vaccine (Improvac, Zoetis, 150 µg/mL) intramuscularly at the neck at week 0, 4 and 8. The first and third injections were on the left side, whereas the second injection was on the right side. Mules in Control-intact and Control-castrated received 1.3 mL of 0.9% NaCl solution using the same method described for Treatment. Clinical examination for adverse effects was performed before vaccination and daily for three days after vaccination, including rectal temperature (℃), injection site swelling (score 0-2), pain at the injection site with hand pressing (yes/no) and neck movement toward or away from the injection site (score 0-2) as described in previous study [
19]. In brief, a score of 0 indicated no swelling, and the neck could bend in normal range. The higher score indicated an increase in the severity of adverse effects. A rectal temperature of more than 39 ℃ indicated having a fever.
2.3. Samples and Data Collection
Blood samples and the video recordings were collected from each mule every two weeks, starting from two weeks (week -2) before the first vaccination until week 24. Ten mL blood sample was collected in a plain tube and kept in an ice box during transportation. Serum was separated after centrifugation and kept at -20 ℃ until analysis. Mule’s behavior within the restraint stall was evaluated from the video recordings in three aspects: (a) response when each mule stood still without human interaction for one minute; (b) response to palpation at the scrotal area; and (c) response when blood collection was performed. One person, unaware of whether mules were in Treatment or Control, evaluated the video recordings. The behavioral score was given as 0, 1, 2 and 3, corresponding with at ease, uneasy, annoyed, and alarmed.
At week 0, 12 and 24, total scrotal width (TSW) was measured 3 times from each intact mule using a stallion scrotal caliper. In the same week, body weight (BW) was measured 3 times from each mule using equine weight measuring tape.
2.4. Serum Anti-GnRH Antibody Analysis
Anti-GnRH antibody was analyzed using direct non-competitive ELISA. The protocol for plate preparation was similar to the previous study [
19]. After plate blocking with protein buffer and one-hour incubation at room temperature (RT), plates were washed five times, loaded with 100 µL each of a control (from an animal vaccinated with Improvac, Zoetis), diluted serum samples (1:50-1:1500), high and low concentration controls, and incubated for two hours at RT. Plates were washed five times, added with 100 µL conjugate (protein G Peroxidase, P8170, Sigma Chemical Co.) at 1:20000 dilution in buffer and incubated for one hour at RT. After washing, 100 µL of 3,3',5,5'- tetramethylbenzidine dihydrochloride (TMB, Sigma Aldrich) dissolved in phosphate-citrate buffer with sodium perborate was added, followed by incubation for 20 minutes at RT. The reaction was stopped with 50 µL stop solution (2M H
2SO
4) and absorbance was measured at 450 nm by a microplate reader (Sunrise, Tecan). The inter-assay coefficient of variation (CV) for the high and low concentration controls were less than 10%. The intra-assay CVs were less than 10%. Data were compared to standard curve to identify anti-GnRH antibody level (unit).
2.5. Serum Testosterone Analysis
Serum testosterone was analyzed using a double-antibody enzyme immunoassay as previously described [
20], with minor modifications. The concentration of two substrates was modified: steroid horseradish peroxidase conjugate (HRP) at 1:20000 and testosterone antibody at 1:110000. Absorbance was measured at 450 nm by a microplate reader (Sunrise, Tecan). The sensitivity of the assay was 0.0714 ng/mL. The inter-assay CV for the high and low concentration controls were less than 10%. The intra-assay CVs were less than 10%. Serum testosterone level was reported as ng/mL.
2.6. Statistical Analysis
Box plots analysis on serum testosterone levels was used to detect outliers, and the data from outliers were excluded from statistical analysis. Serum testosterone levels before vaccination of intact, cryptorchid and castrated mules were analyzed as descriptive analysis using pooled data at week -2 and week 0. An unpaired sample t-test was used to analyze the difference in serum testosterone levels before vaccination between young and old intact, and between intact and unilateral cryptorchid mules. Anti-GnRH antibody, serum testosterone levels, TSW and BW were analyzed by repeated measurement ANOVA (Stata statistical software release 16.1, Stata Corp.). Due to limitation of the video recordings at week 0, the behavioral scores at week -2 were selected as a baseline for comparison with scores from other weeks using repeated measurement ANOVA. Clinical adverse effects were analyzed by descriptive analysis.
3. Results
Five mules were indicated as outliers; therefore, statistical analysis was performed based on 20 mules separating into three groups: (a) Treatment with 4 subgroups (Treatment-intact-young, n = 4; Treatment-intact-old, n = 5; Treatment-cryptorchid-young, n = 4; Treatment-cryptorchid-old, n = 1); (b) Control-intact (10-15 years old, n = 2); and (c) Control-castrated (n = 4). Except for clinical adverse effects which were analyzed from all 17 mules receiving the GnRH vaccine.
3.1. Anti-GnRH Antibody Production
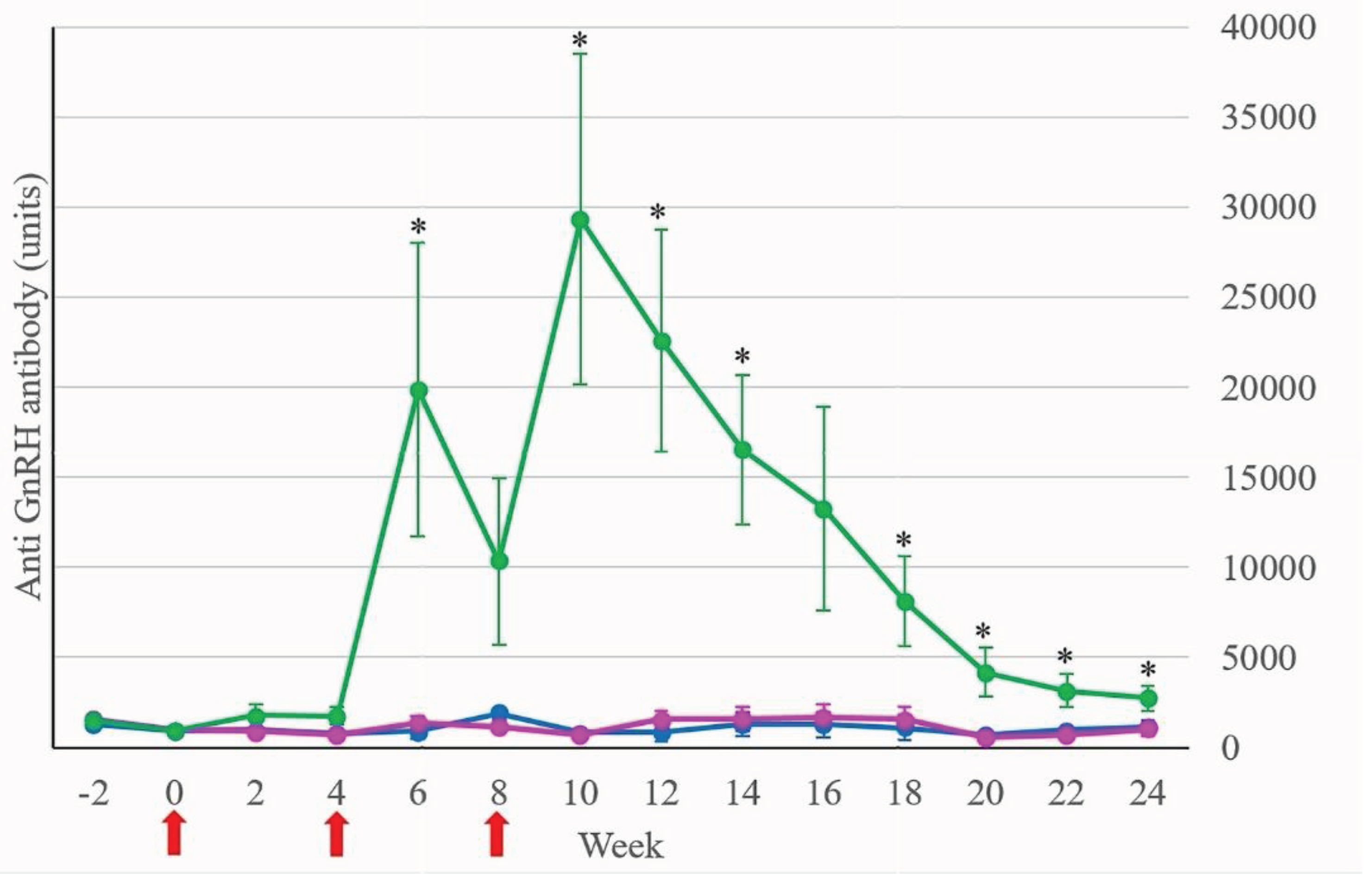
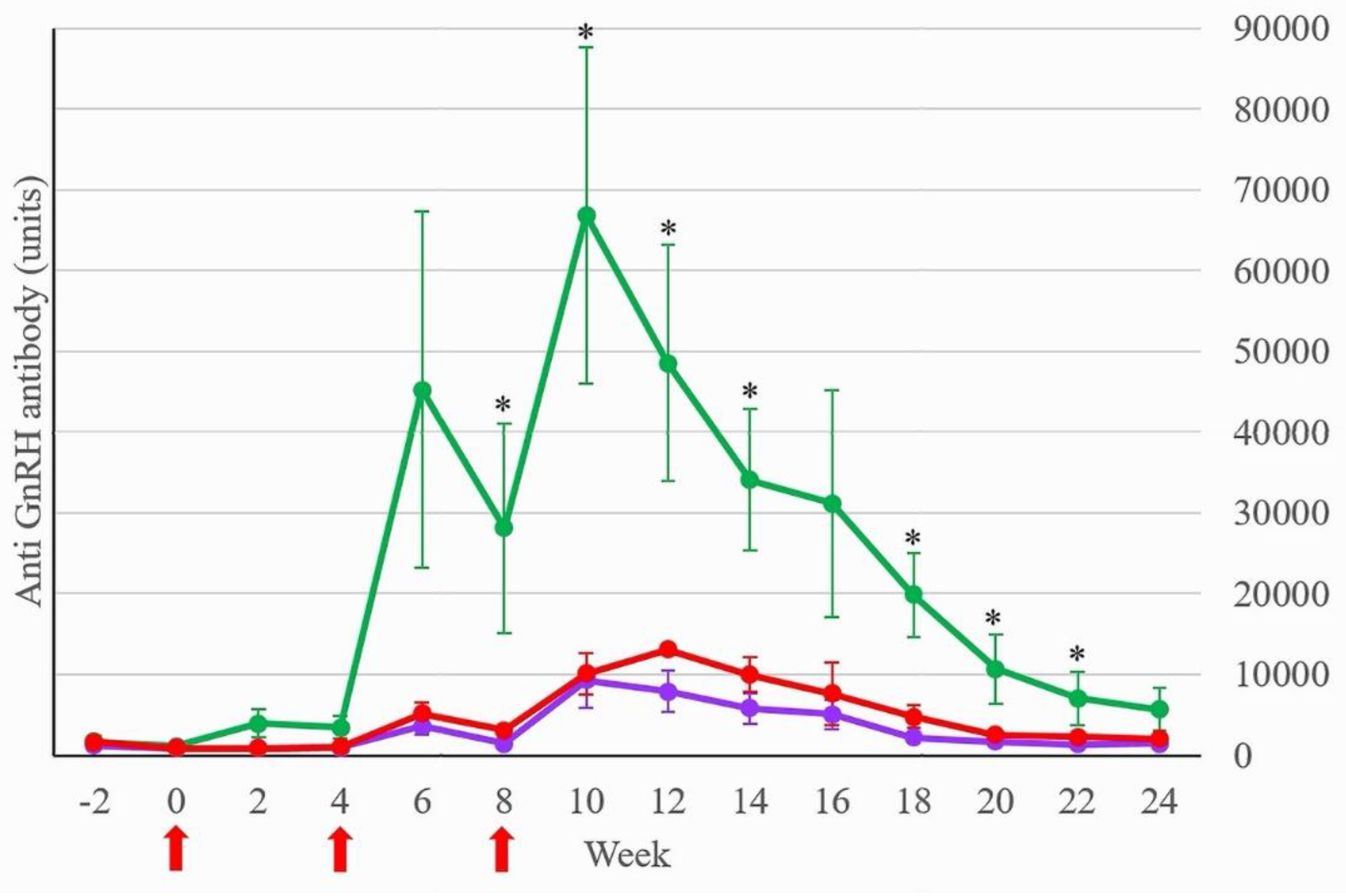
Stallion mules in Treatment respond to the GnRH vaccine, which anti-GnRH antibody level rose 2 weeks after the second and third vaccinations (
Figure 1). When subdivided by age and condition, Treatment-intact-young had the highest anti-GnRH antibody level (66864 ± 20775 units; mean ± SEM) at week 10 as shown in
Figure 2.
3.2. Serum Testosterone Level
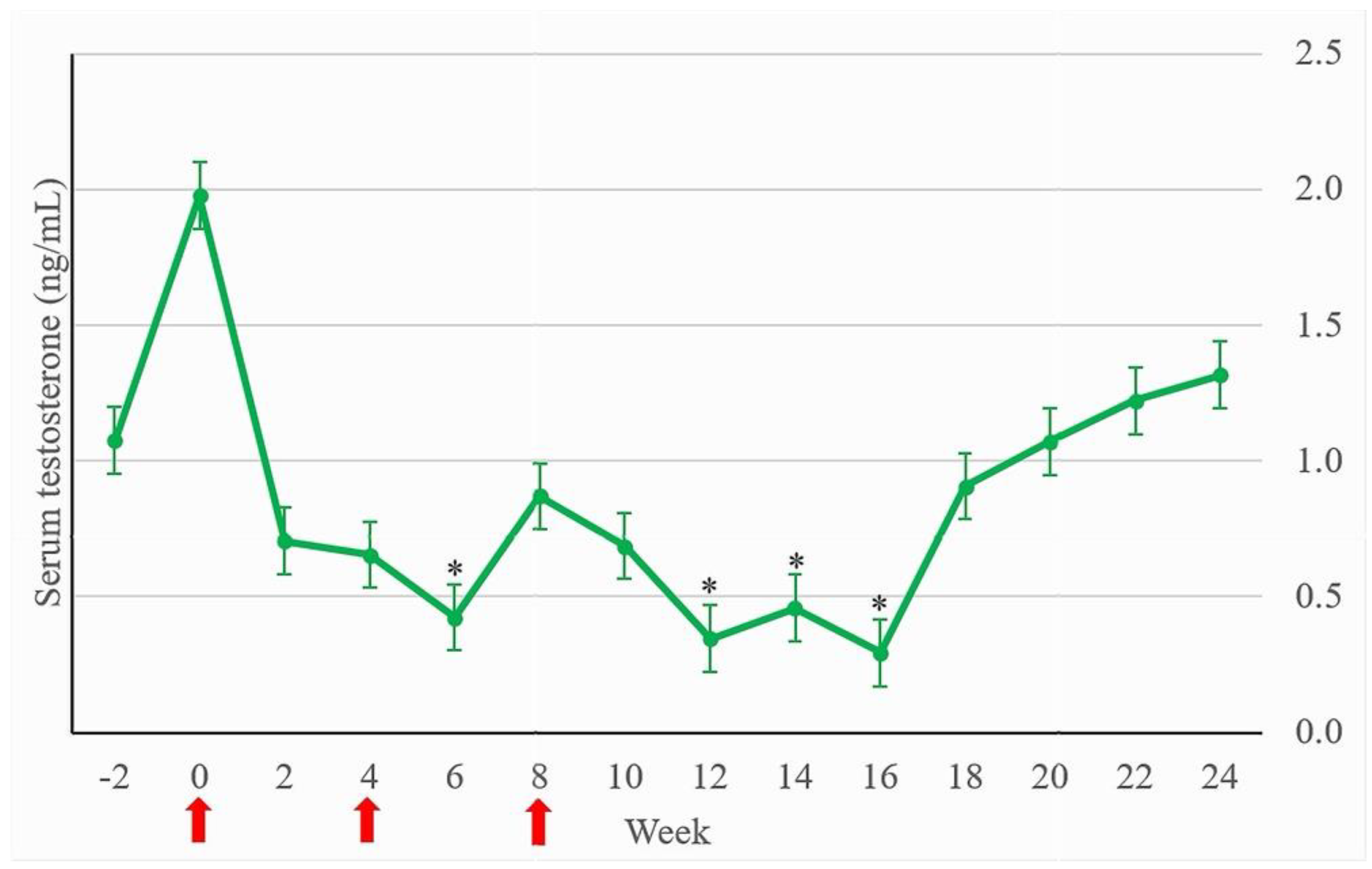
Serum testosterone levels before vaccination; shown as means ± SEM, from all intact stallion mules (n = 11) was 2.55 ± 0.61 ng/mL (range 0.07-11.88 ng/mL). When separated by age, serum testosterone level in young intact stallion mules (n = 4) was 3.08 ± 1.43 ng/mL (range 0.12-11.88 ng/mL), and in old intact stallion mules (n = 7) was2.25 ± 0.56 ng/mL (range 0.07-6.13 ng/mL). For young cryptorchid mules (n = 4), serum testosterone level was 0.35 ± 0.09 ng/mL (range 0.07-0.72 ng/mL). Serum testosterone levels of a 10-year-old cryptorchid mule and Control-castrate were 0.07 ng/mL, and 0.10 ± 0.02 ng/mL (range 0.07-0.23 ng/mL), respectively. Serum testosterone levels between young and old intact stallion mules was not statistically different (p > 0.05). When compared at similar age, serum testosterone levels between young intact stallion and young cryptorchid mules was not statistically different (p > 0.05).
Before vaccination, the serum testosterone level of Treatment-intact was similar to Control-intact (
p > 0.05), while the level in Treatment-cryptorchid-young was lower than the level in Control-intact (
Table 1). During 2-6 weeks and 12-16 weeks after the first vaccination, serum testosterone levels in all Treatment subgroups were lower than in Control-intact (
p < 0.05). When compared within Treatment, serum testosterone levels at week 6, 12, 14 and 16 were significantly lower when compared with week 0 (
p < 0.05) (
Figure 3).
3.3. Behavior Response, Changes in Total Scrotal Width and Body Weight
A comparison of the behavioral scores at week 6, 12, 18, 22 and 24 with the score at week -2 was reported in
Table 2. The behavioral scores of Control-intact were not significantly different throughout the study period (
p > 0.05), while the score of response with human manipulation from Treatment significantly decreased at week 12, 18 and 22 (
p < 0.05). When compared with week 0, the TSW of Treatment-intact-young significantly decreased (
p < 0.05) at week 12 (mean difference -1.3 cm) and week 24 (mean difference -1.5 cm), whereas the TSW of Treatment-intact-old was not different (
p > 0.05, range 5.9-6.2 cm). Body weights of Control-intact significantly increased (
p < 0.05) at week 12 (mean difference 5.3 kg) and at week 24 (mean difference 4.7 kg) when compared with week 0, whereas BW of Treatment and Control-castrated was not significantly different (
p > 0.05). Mean ± SD of BW at week 24 of Control-intact, Control-castrated, and Treatment were 223 ± 21, 286 ± 32, and 251 ± 32 kg, respectively.
3.4. Clinical Adverse Effests
The occurrence of clinical adverse effects in Treatment was shown in
Table 3. Affected mules developed fever on the next day after vaccination, which was subsided on the following day after intravenous phenylbutazone administration. Swelling at the injection site was pronounced after booster vaccination. Large subcutaneous edema, considered as score 2, occurred around or beneath the injection site on day 3 after the second or third vaccination. There was no heat or pain response to palpation. Edema subsided after the application of topical anti-inflammatory gel for a few days. From a total of three vaccinations, clinical adverse effects occurred in 10 out of 11 intact stallion mules. Minor clinical adverse effects occurred in 4 out of 6 cryptorchid mules. Large subcutaneous edema occurred only in intact stallion mules, 100% in the young group and 71.43% in the old group.
4. Discussion
The GnRH vaccine has been evaluated for immunocastration in stallion horses [
14,
21,
22,
23,
24] and stallion donkeys [
25]. To our knowledge, this study was the first to report information on GnRH vaccination in stallion mules. Mules responded to the GnRH vaccine in a similar pattern as that of the stallion horses [
21]. Anti-GnRH antibody levels in Treatment-intact-young were higher than that in Treatment-intact-old. This finding might relate to immunosenescence, the decrease in immune system ability that occurs in older animals [
14,
26]. Treatment-intact-young, which had the highest anti-GnRH antibody level, had a decrease in serum testosterone level two weeks after the first vaccination. A similar period of testosterone depletion, 15 days after surgical castration, was reported in 3-4 years old horses, although most of the horses still be able to ejaculate at day 15 [
27]. This period might state the lag period between hormone reduction and diminished sexual behavior. Treatment-intact-young, the group with highest anti-GnRH antibody level, also had the largest decrease in serum testosterone level and significant reduction of total scrotal width at week 12 and 24. These findings might indicate the functional impairment of the hypothalamic-pituitary-testicular axis due to GnRH reduction. A similar finding was observed in stallion horses treated with the GnRH vaccine [
22,
23]. Reduction of seminiferous tubular tissue relative to interstitial tissue resulted in 70% testicular volume reduction at 90 days after vaccination [
22]. In another study, a reduction of scrotum size by around 37% was observed three months after the first GnRH vaccination [
23].
Serum testosterone levels in Treatment began to decline two weeks after the first vaccination. When compared within Treatment, testosterone level at week 6, two weeks after the second vaccination, was lower than before vaccination. Stallion horses receiving 200 µg GnRH vaccine twice had similar results, in which serum testosterone decreased within 1-2 months after the first vaccination [
24]. However, the period of testosterone suppression in that study was more extended (5-6 months) than 16 weeks in this study. In stallion donkeys receiving the different brand of GnRH vaccine at 400 µg twice, serum testosterone decreased at day 60 after the first vaccination, and remained at a low level until the end of the study at day 120 [
25], similar to the duration of testosterone suppression of stallion mules in this study. It was unknown whether the serum testosterone of stallion donkeys beyond 120 days would be maintained at a low level or rise in the similar pattern as mules in this study. Variation in duration of testosterone suppression among species might be due to physiological differences in each type of equids since inter-individual variation against the GnRH vaccine occurred even in the same species [
21,
23].
Before GnRH vaccination, the serum testosterone level of intact mules in this study (2.55 ± 0.61 ng/mL, mean ± SEM) was close to the levels in the previous study [
28]. In that study, the serum testosterone levels of twelve 5-6 years old stallion mules, separated into 4 groups, were 2.12 ± 0.05, 2.05 ± 0.05, 2.07 ± 0.04, and 2.05 ± 0.01 ng/mL (mean ± SEM) [
28]. Serum testosterone increases with age but becomes stable after puberty at six years old in stallion horses [
29]. Ages of young stallion mules in this study ranged from 5-9 years old. This might explain the similar serum testosterone level between young intact and old intact stallion mules in this study. Comparing between unilateral cryptorchid and intact mules at young ages, serum testosterone levels were not statistically significantly different. A similar finding was indicated in horses, in which unilateral cryptorchid horses, both with and without scrotal testis on the other side, had similar testosterone levels as in intact stallions [
30]. This finding might relate to the activity of Leydig cells of retained abdominal testes in young stallion horses, which still actively responded to human chorionic gonadotropin stimulation [
31] and subsequence of an increase in serum testosterone level after stimulation. Older cryptorchid horses, especially those more than nine years old, had significantly low serum testosterone level [
32]. Chronic exposure to high temperature of indwelling testis might affect Leydig cell function, resulting in a decrease in testosterone synthesis and an increase in conversion of androgens into estrogens [
33]. This might explain the low serum testosterone level in a 10-year-old cryptorchid mule in this study, which was comparable to the level in castrated mules.
Change in the behavioral score toward at ease with human manipulative work began at week 6, concurrent with a significant decrease in the serum testosterone level of vaccinated mules compared to before vaccination. A similar finding was found in stallion horses, in which aggressive behavior diminished in all seven stallions receiving 200 µg GnRH vaccine at week 0, 4 and 12 [
16]. Nonetheless, that study did not state the onset and duration of a decrease in aggressiveness. Change in the behavioral score of vaccinated mules last until week 22, even after returning of serum testosterone level to baseline at week 18. The reason for this lag period was unclear. Mules might accommodate with the study protocol until their serum testosterone level was high enough to overcome their attitude. The behavioral score when each mule stood still without human interaction was not statistically affected by the GnRH vaccine. This finding might relate to inherent behavior and not to testosterone. Some mules in Control-castrated were graded as uneasy when stood still by themselves in the restraint stall, as well as statistical significant changes in the behavioral score toward uneasy at week 18, 22 and 24 for blood collection in this group.
In this study, 11 out of 17 mules developed a fever after vaccination, with only 2 mules developed a fever after vaccine booster. In a study using a licensed GnRH vaccine for horses, mares and stallions neither developed a fever after receiving 200 µg nor any clinical adverse effects after the second vaccination [
21,
34]. In our study, 13 mules developed the injection site swelling after vaccination. Mares receiving high dose of the GnRH vaccine (Improvac) at 400 µg had no injecting site swelling after primary vaccination but 10.9% developed visible swelling, and 3.6% developed visible swelling accompanied by lameness after the second vaccination [
35]. Donkeys did not develop any clinical adverse effects even receiving high dose of the GnRH vaccine at 400 µg twice [
25], although the brand of vaccine in that study was different from the one using in our study. Other composition of chemicals and adjuvants within different brands of vaccine might contribute to the occurrence of clinical adverse effects. Mules developed large subcutaneous edema around or beneath the injection site, approximately 2-3 days after booster vaccination. This might relate to hypersensitivity type III or Arthus reaction [
36]. Immune complex formation within or around blood vessels at the injection site triggered the inflammatory process, resulting in local swelling and pain. The reaction of mules in this study might relate to the alteration in vascular permeability, resulting in fluid leakage from capillaries to subcutaneous space around the injection site. The reason why this reaction occurred only in intact mules and not in cryptorchid mules was unclear. It might relate to the level of anti-GnRH antibody and the amount of antigen-antibody complex formation. Treatment-intact-young had the highest level of anti-GnRH antibody, concurrent with 100% occurrence of subcutaneous edema in this group. Although this swelling seemed like a blemish, the painful sensation was minimal to none. In addition, the swelling subsided within three days with only topical anti-inflammatory gel.
5. Conclusions
Stallion and cryptorchid mules responded to the GnRH vaccine, resulting in measurable anti-GnRH antibodies in serum. Antibody level raised within two weeks after each booster vaccination. Young intact stallion mules had the highest antibody level, resulting in a reduction of serum testosterone level of this subgroup during week 2-16 after the first vaccination. The total scrotal width in this subgroup also decreased compared to before vaccination. Subcutaneous edema without pain was developed in intact stallion mules with high anti-GnRH antibody level. Response of vaccinated mules to human manipulative works in the restraint stall changed toward at ease and less rejection approximately six weeks and lasted for 22 weeks after the first vaccination.
Author Contributions
Conceptualization, S.K. and C.S.; methodology, S.K. and C.S.; validation, P.T. and C.S.; formal analysis, S.T.; investigation, S.K.,P.T. and C.S.; writing-original draft preparation, S.K.; writing-review and editing, S.K.,S.T. and C.S.; visualization, S.K.,S.T.,P.T. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Faculty of Veterinary Medicine, Chiang Mai University, grant number CMUMIS : R000020435.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Chiang Mai University (Ref.No. R17/2561, approved on 18 January 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to appreciate the Pack Squadron Unit, Veterinary & Remount Department for contribution on animals, facilities and supporting staff during data collection in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McLean, A.; Varnum, A.; Ali, A.; Heleski, C.; González, F.J.N. Comparing and Contrasting Knowledge on Mules and Hinnies as a Tool to Comprehend Their Behavior and Improve Their Welfare. Animals 2019, 9, 488. [Google Scholar] [CrossRef]
- Lagos, J.; Rojas, M.; Rodrigues, J.B.; Tadich, T. Perceptions and Attitudes towards Mules in a Group of Soldiers. Animals 2021, 11, 1009. [Google Scholar] [CrossRef]
- Heaton, K.; Ragle, C.; Godderidge, M.T.; Farrell, A.; Tibary, A. Estrous behavior in mules—an owner's perspective. J. Equine Vet. Sci. 2018, 60, 109–112. [Google Scholar] [CrossRef]
- Desta, T.T.; Teklemariam, H.; Mulugeta, T. The insights of smallholder farmers on special attributes of the genetically robust mule. Biodiversitas J. Biol. Divers. 2022, 23. [Google Scholar] [CrossRef]
- Mason, B.J.; Newton, J.R.; Payne, R.J.; Pilsworth, R.C. Costs and complications of equine castration: a UK practice-based study comparing 'standing nonsutured' and 'recumbent sutured' techniques. . 2005, 37, 468–72. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, I.; Watson, J.L.; Kass, P.H.; Spier, S.J. Incidence, management, and outcome of complications of castration in equids: 324 cases (1998–2008). J. Am. Vet. Med. Assoc. 2013, 242, 820–825. [Google Scholar] [CrossRef]
- Robert, M.P.; Chapuis, R.J.J.; De Fourmestraux, C.; Geffroy, O.J. Complications and risk factors of castration with primary wound closure: Retrospective study in 159 horses. . 2017, 58, 466–471. [Google Scholar] [PubMed]
- Owens, C.; Hughes, K.; Hilbert, B.; Heller, J.; Nielsen, S.; Trope, G. Survey of equine castration techniques, preferences and outcomes among Australian veterinarians. Aust. Veter- J. 2017, 96, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rosanowski, S.M.; MacEoin, F.; Graham, R.J.T.Y.; Riggs, C.M. Open standing castration in Thoroughbred racehorses in Hong Kong: Prevalence and severity of complications 30 days post-castration. Equine Veter- J. 2017, 50, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.B.; Sinclair, M.; Sorge, U.S. Comparison of the use of a braided multifilament transfixation suture for field castration with other castration techniques. Equine Veter- Educ. 2017, 31, 427–431. [Google Scholar] [CrossRef]
- Baldwin, C.M. A review of prevention and management of castration complications. Equine Veter- Educ. 2023, 36, 97–106. [Google Scholar] [CrossRef]
- Vilar, J.M.; Batista, M.; Carrillo, J.M.; Rubio, M.; Sopena, J.; Álamo, D. Histological, cytogenetic and endocrine evaluation in twenty-five unilateral cryptorchid horses. J. Appl. Anim. Res. 2017, 46, 441–444. [Google Scholar] [CrossRef]
- Stout, T.; Colenbrander, B. Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Anim. Reprod. Sci. 2004, 82-83, 633–643. [Google Scholar] [CrossRef]
- Stout, T. Modulating reproductive activity in stallions: A review. Anim. Reprod. Sci. 2005, 89, 93–103. [Google Scholar] [CrossRef]
- Palmer, C.; Pedersen, H.G.; Sandøe, P. Beyond castration and culling: Should we use non-surgical, pharmacological methods to control the sexual behavior and reproduction of animals? J. Agric. Environ. Ethics. 2018, 31, 197–218. [Google Scholar] [CrossRef]
- Burger, D.; Janett, F.; Vidament, M.; Stump, R.; Fortier, D.; Imboden, I.; Thun, R. Immunization against GnRH in adult stallions: effects on semen characteristics, behaviour, and shedding of equine arteritis virus. Anim. Reprod. Sci. 2006, 94, 107–111. [Google Scholar] [CrossRef]
- Schulman, M.L.; Botha, A.E.; Muenscher, S.B.; Annandale, C.H.; Guthrie, A.J.; Bertschinger, H.J. Reversibility of the effects of GnRH -vaccination used to suppress reproductive function in mares. Equine Vet. J. 2013, 45, 111–3. [Google Scholar] [CrossRef]
- Dordas-Perpinya, M.; Gorréguès, M.; Gervasoni, M.A.; Berder, C.; Thorin, C.; Jaillardon, L.; Bruyas, J.F. What is the effect of anti- GnRH immunization on plasmatic levels of anti mullerian hormone? J. Equine Vet. Sci. 2018, 66, 26–28. [Google Scholar] [CrossRef]
- Khumsap, S.; Thitaram, C.; Somgird, C. GnRH vaccine could suppress serum progesterone level in Thai pony mares; A preliminary study. Vet. Integr. Sci. 2020, 18, 43–51. [Google Scholar]
- Khonmee, J.; Brown, J.L.; Li, M.-Y.; Somgird, C.; Boonprasert, K.; Norkaew, T.; Punyapornwithaya, V.; Lee, W.-M.; Thitaram, C. Effect of time and temperature on stability of progestagens, testosterone and cortisol in Asian elephant blood stored with and without anticoagulant. Conserv. Physiol. 2019, 7, coz031. [Google Scholar] [CrossRef] [PubMed]
- Janett, F.; Stump, R.; Burger, D.; Thun, R. Suppression of testicular function and sexual behavior by vaccination against GnRH (Equity™) in the adult stallion. Anim. Reprod. Sci. 2008, 115, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.E; Birrell, J.; Schulman, M.L.; Du Plessis, L.; Laver, P.N.; Soley, J.T.; Bertschinger, H.J. Effects of the GnRH vaccine Improvac® on testicular tissue of young stallions. Anim. Reprod. Sci. 2016, 169, 97. [Google Scholar] [CrossRef]
- Bailly-Chouriberry, L.; Loup, B.; Popot, M.-A.; Dreau, M.-L.; Garcia, P.; Bruyas, J.-F.; Bonnaire, Y. Two complementary methods to control gonadotropin-releasing hormone vaccination (Improvac®) misuse in horseracing: Enzyme-linked immunosorbent assay test in plasma and steroidomics in urine. Drug Test. Anal. 2017, 9, 1432–1440. [Google Scholar] [CrossRef]
- Miszczak, F.; Burger, D.; Ferry, B.; Legrand, L.; Fortier, G.; Laine, A.-L.; Vabret, A.; Pronost, S.; Vidament, M. Anti- GnRH vaccination of stallions shedding equine arteritis virus in their semen: A field study. Vet. Arh. 2020, 90, 543–556. [Google Scholar] [CrossRef]
- Rocha, J.M.; Ferreira-Silva, J.C.; Neto, H.F.V.; Moura, M.T.; Ferreira, H.N.; Júnior, V.A.S.; Filho, H.C.M.; Oliveira, M.A.L. Immunocastration in donkeys: clinical and physiological aspects. Pferdeheilkunde Equine Med. 2018, 34, 12–16. [Google Scholar] [CrossRef]
- Horohov, D.; Adams, A.; Chambers, T. Immunosenescence of the Equine Immune System. J. Comp. Pathol. 2010, 142, S78–S84. [Google Scholar] [CrossRef]
- Guasti, P.; Papa, P.; Schmith, R.; Camargo, L.; Andrade, L.; Silva, L.; Freitas-DellAqua, C.; Souza, F.; Papa, F. Serum Testosterone Levels and Seminal Plasma Proteins in Castrated Stallions. J. Equine Veter- Sci. 2018, 66, 64–65. [Google Scholar] [CrossRef]
- Akhtar, R.W.; Shah, S.A.H.; Qureshi, I.Z. Effect of kisspeptin-10, LH and hCG on serum testosterone concentrations in stallions, donkeys and mules. Theriogenology 2017, 102, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.; Jawad, M.; Weld, J.; Kaufman, W.; Witherspoon, D.; Wilson, E.; Douglas, R. Effects of season, age and increased photoperiod on reproductive hormone concentrations and testicular diameters in thoroughbred stallions. J. Equine Veter- Sci. 1984, 4, 202–208. [Google Scholar] [CrossRef]
- Coryn, M.; De Morr, A.; Bouters, R.; Vandeplassche, M. Clinical, morphological and endocrinological aspects of cryptorchidism in the horse. Theriogenology 1981, 16, 489–496. [Google Scholar] [CrossRef]
- Cox, J. Testosterone concentrations in normal and cryptorchid horses. Response to human chorionic gonadotrophin. Anim. Reprod. Sci. 1989, 18, 43–50. [Google Scholar] [CrossRef]
- Claes, A.; Ball, B.A.; Corbin, C.J.; Conley, A.J.; Dvm, D.A.C.; Dvm, B.A.B.; Bs, C.J.C.; Bvsc, M.A.J.C. Age and season affect serum testosterone concentrations in cryptorchid stallions. Veter- Rec. 2013, 173, 168–168. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilińska, B. The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. . 2008, 59, 112–8. [Google Scholar]
- Elhay, M.; Newbold, A.; Britton, A.; Turley, P.; Dowsett, K.; Walker, J. Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Aust. Veter- J. 2007, 85, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.E.; Schulman, M.L.; Bertschinger, H.J.; Guthrie, A.J.; Annandale, C.H.; Hughes, S.B. The use of a GnRH vaccine to suppress mare ovarian activity in a large group of mares under field conditions. Wildl. Res. 2008, 35, 548–554. [Google Scholar] [CrossRef]
- Gershwin, L.J. Adverse reactions to vaccination: From anaphylaxis to autoimmunity. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 279–290. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).