Submitted:
13 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics before and after Propensity Score Matching
3.2. Operative Outcomes
3.2. Comparison of Pathologic Results
3.3. Comparison of Survival Outcomes
3.4. Prognostic Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujita, T.; Nakagohri, T.; Gotohda, N.; Takahashi, S.; Konishi, M.; Kojima, M.; Kinoshita, T. Evaluation of the prognostic factors and significance of lymph node status in invasive ductal carcinoma of the body or tail of the pancreas. Pancreas 2010, 39, e48–e54. [Google Scholar] [CrossRef]
- Abe, T.; Ohuchida, K.; Miyasaka, Y.; Ohtsuka, T.; Oda, Y.; Nakamura, M. Comparison of Surgical Outcomes Between Radical Antegrade Modular Pancreatosplenectomy (RAMPS) and Standard Retrograde Pancreatosplenectomy (SPRS) for Left-Sided Pancreatic Cancer. World J Surg 2016, 40, 2267–2275. [Google Scholar] [CrossRef]
- Dai, M.; Zhang, H.; Li, Y.; Xing, C.; Ding, C.; Liao, Q.; Zhang, T.; Guo, J.; Xu, Q.; Han, X.; et al. Radical antegrade modular pancreatosplenectomy (RAMPS) versus conventional distal pancreatosplenectomy (CDPS) for left-sided pancreatic ductal adenocarcinoma. Surgery today 2021. [Google Scholar] [CrossRef]
- Park, H.J.; You, D.D.; Choi, D.W.; Heo, J.S.; Choi, S.H. Role of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. World J Surg 2014, 38, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Saiura, A.; Koga, R.; Seki, M.; Katori, M.; Kato, Y.; Sakamoto, Y.; Kokudo, N.; Yamaguchi, T. Improved Survival of Left-sided Pancreas Cancer after Surgery. Japanese Journal of Clinical Oncology 2010, 40, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Sakamoto, Y.; Sano, T.; Kosuge, T. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery 2006, 139, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Drebin, J.A.; Linehan, D. Radical antegrade modular pancreatosplenectomy. Surgery 2003, 133, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Linehan, D.C.; Hawkins, W.G. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg 2007, 204, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Fengwei, G.; Gong, J.; Xie, Q.; Liu, Y.; Wang, Q.; Lei, Z. Assessement of postoperative long-term survival quality and complications associated with radical antegrade modular pancreatosplenectomy and distal pancreatectomy: a meta-analysis and systematic review. BMC Surg 2019, 19. [Google Scholar] [CrossRef]
- Sham, J.G.; Guo, S.; Ding, D.; Shao, Z.; Wright, M.; Jing, W.; Yin, L.D.; Zhang, Y.; Gage, M.M.; Zhou, Y.; et al. Radical antegrade modular pancreatosplenectomy versus standard distal pancreatosplenectomy for pancreatic cancer, a dual-institutional analysis. Chin Clin Oncol 2020, 9. [Google Scholar] [CrossRef]
- Kim, E.Y.; You, Y.K.; Kim, D.G.; Hong, T.H. Initial experience with radical antegrade modular pancreatosplenectomy in a single institution. Ann Surg Treat Res 2016, 91, 29–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, B.; Wu, L.; Si, X. A systematic review of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. HPB : the official journal of the International Hepato Pancreato Biliary Association 2017, 19, 10–15. [Google Scholar] [CrossRef]
- Dragomir, M.; Eftimie, M.A. Is Radical Antegrade Modular Pancreatosplenectomy the Solution? A Systematic Literature Review and Meta-Analysis. Chirurgia (Bucur) 2017, 112, 653–663. [Google Scholar] [CrossRef]
- Shirakawa, S.; Matsumoto, I.; Toyama, H.; Shinzeki, M.; Ajiki, T.; Fukumoto, T.; Ku, Y. Pancreatic Volumetric Assessment as a Predictor of New-Onset Diabetes Following Distal Pancreatectomy. Journal of Gastrointestinal Surgery 2012, 16, 2212–2219. [Google Scholar] [CrossRef]
- Thomas, A.S.; Huang, Y.; Kwon, W.; Schrope, B.A.; Sugahara, K.; Chabot, J.A.; Wright, J.D.; Kluger, M.D. Prevalence and Risk Factors for Pancreatic Insufficiency After Partial Pancreatectomy. Journal of Gastrointestinal Surgery 2022. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, S.C.; Shim, I.K.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Park, K.M.; Lee, Y.J. Factors Affecting the Development of Diabetes Mellitus After Pancreatic Resection. Pancreas 2015, 44, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S. Role of Radical Antegrade Modular Pancreatosplenectomy (RAMPS) and Pancreatic Cancer. Ann Surg Oncol 2018, 25, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Li, J.; Li, A.; Li, F. Radical antegrade modular pancreatosplenectomy versus standard procedure in the treatment of left-sided pancreatic cancer: A systemic review and meta-analysis. BMC Surg 2017, 17. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kang, M.J.; Heo, J.S.; Choi, S.H.; Choi, D.W.; Park, S.J.; Han, S.S.; Yoon, D.S.; Yu, H.C.; Kang, K.J.; et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 2014, 259, 656–664. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto, Y.; Sugiura, T.; Okamura, Y.; Ito, T.; Ashida, R.; Ohgi, K.; Uesaka, K. Reconsidering the Optimal Regional Lymph Node Station According to Tumor Location for Pancreatic Cancer. Annals of Surgical Oncology 2021, 28, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakamura, T.; Asano, T.; Nakanishi, Y.; Noji, T.; Tsuchikawa, T.; Okamura, K.; Shichinohe, T.; Hirano, S. Pancreatic body and tail cancer and favorable metastatic lymph node behavior on the left edge of the aorta. Pancreatology 2020, 20, 1451–1457. [Google Scholar] [CrossRef]
- Huo, Z.; Zhai, S.; Wang, Y.; Qian, H.; Tang, X.; Shi, Y.; Weng, Y.; Zhao, S.; Deng, X.; Shen, B. Comparison of Radical Antegrade Modular Pancreatosplenectomy with Standard Retrograde Pancreatosplenectomy for Left-Sided Pancreatic Cancer: A Meta-Analysis and Experience of a Single Center. Med Sci Monit 2019, 25, 4590–4601. [Google Scholar] [CrossRef]
- Korrel, M.; Lof, S.; Van Hilst, J.; Alseidi, A.; Boggi, U.; Busch, O.R.; Van Dieren, S.; Edwin, B.; Fuks, D.; Hackert, T.; et al. Predictors for Survival in an International Cohort of Patients Undergoing Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Annals of Surgical Oncology 2021, 28, 1079–1087. [Google Scholar] [CrossRef]
- Latorre, M.; Ziparo, V.; Nigri, G.; Balducci, G.; Cavallini, M.; Ramacciato, G. Standard retrograde pancreatosplenectomy versus radical antegrade modular pancreatosplenectomy for body and tail pancreatic adenocarcinoma. Am Surg 2013, 79, 1154–1158. [Google Scholar] [CrossRef]
- Li, H.J.; Chen, Y.T.; Yuan, S.Q. Proposal of a modified American Joint Committee on Cancer staging scheme for resectable pancreatic ductal adenocarcinoma with a lymph node ratio-based N classification: A retrospective cohort study. Medicine (Baltimore) 2018, 97. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Rahman, A.; Haugk, B.; French, J.J.; Manas, D.M.; Jaques, B.C.; Charnley, R.M.; White, S.A. Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. European Journal of Surgical Oncology (EJSO) 2012, 38, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sivasanker, M.; Desouza, A.; Bhandare, M.; Chaudhari, V.; Goel, M.; Shrikhande, S.V. Radical antegrade modular pancreatosplenectomy for all pancreatic body and tail tumors: rationale and results. Langenbecks Arch Surg 2019, 404, 183–190. [Google Scholar] [CrossRef] [PubMed]
| Pre-PSM | Post-PSM | |||||
| cDPS (n = 130) |
RAMPS (n = 203) |
p-value | cDPS (n = 99) |
RAMPS (n = 99) |
p-value | |
| Age [median (range)] (y) | 66.0 (39-86) | 64.0 (41-84) | 0.503* | 65.0 (39-80) | 66.0 (43-81) | 0.301* |
| Sex [n (%)] | 0.262 | 1.000 | ||||
| Male | 72 (55.4 ) | 125 (61.6) | 53 (53.5) | 53 (53.5) | ||
| Female | 58 (44.6) | 78 (38.4) | 46 (46.5) | 46 (46.5) | ||
| Approach [n (%)] | 0.031† | 0.747† | ||||
| Open | 120 (92.3) | 198 (97.5) | 93 (93.9) | 95 (96.0) | ||
| Lap | 10 (7.7) | 5 (2.5) | 6 (6.1) | 4 (4.0) | ||
| Tumor location [n (%)] | <0.001 | 1.000 | ||||
| confined to neck/body | 58 (44.6) | 159 (78.3) | 56 (56.6) | 56 (56.6) | ||
| body to tail | 19 (14.6) | 11 (5.4) | 10 (10.1) | 10 (10.1) | ||
| confined to tail | 53 (40.8) | 33 (16.3) | 33 (33.3) | 33 (33.3) | ||
| BMI (mean ± SD) | 23.70 ± 3.288 | 23.29 ± 2.937 | 0.234 | 23.71 ± 3.459 | 23.41 ± 2.781 | 0.513 |
| ASA score [n (%)] | 0.503 | 0.571 | ||||
| I | 14 (10.8) | 31 (15.3) | 12 (12.1) | 14 (14.1) | ||
| II | 102 (78.5) | 151 (74.4) | 78 (78.8) | 72 (72.7) | ||
| III | 14 (10.8) | 21 (10.3) | 9 (9.1) | 13 (13.1) | ||
| CEA, ng/ml [median (range)] | 2.52 (0.20-165.10) | 2.20 (0.44-56.92) | 0.126* | 2.49 (0.20-165.10) | 2.51 (0.44-56.92) | 0.734* |
| CA 19-9, U/ml [median (range)] | 59.93 (1.00-11387.00) |
75.01 (1.20-10028.49) |
0.436* | 168.13 (1.80-11387.00) |
244.32 (2.00-6808.37) |
0.101* |
| Tumor size, cm [median (range)] |
3.0 (0.5-9.5) |
2.5 (0.3-10.0) |
0.282* | 3.0 (1.0-8.0) |
3.2 (1.2-8.5) |
0.132* |
| Neoadjuvant treatment [n (%)] | 0.606† | 0.246† | ||||
| No | 125 (96.2) | 192 (94.6) | 96 (97.0) | 99 (100) | ||
| Yes | 5 (3.8) | 11 (5.4) | 3 (3.0) | 0 (0.0) | ||
| Adjuvant treatment [n (%)] | 0.164 | 0.66 | ||||
| No | 54 (41.5) | 69 (34.0) | 36 (36.4) | 39 (39.4) | ||
| Yes | 76 (58.5) | 134 (66.0) | 63 (63.6) | 60 (60.6) | ||
| Pre-PSM | Post-PSM | |||||
| cDPS (n = 130) |
RAMPS (n = 203) |
p-value | cDPS (n = 99) |
RAMPS (n = 99) |
p-value | |
| LOS [median (range)](days) | 10.0 (5–52) | 9.0 (6–152) | 0.065* | 10.0 (5–52) | 10.0 (7–35) | 0.806* |
| Approach [n (%)] | 0.031 | 0.747 | ||||
| Open | 120 (92.3) | 198 (97.5) | 93 (93.9) | 95 (96.0) | ||
| Laparoscopic | 10 (7.7) | 5 (2.5) | 6 (6.1) | 4 (4.0) | ||
| Op. time [median (range)] (minutes) | 195 (93–420) | 204 (117–494) | 0.045* | 195 (98–420) | 210 (118–458) | 0.305* |
| EBL [median (range)] (ml) | 250 (30–1600) | 300 (50–3000) | 0.488* | 250 (50–1600) | 250 (50–3000) | 0.934* |
| Retrieved LN count [median (range)] | 10 (0–39) | 15 (4–51) | <0.001* | 10.0 (0–36) | 15.0 (5–51) | 0.001* |
| R0 resection [n (%)] | 120/130 (92.3) | 198/203 (97.5) | 0.031 | 93/99 (93.9) | 94/99 (94.9) | 0.756 |
| Transfusion [n (%)] | 0.692 | >0.99 | ||||
| No | 123 (94.6) | 194 (95.6) | 94 (94.9) | 94 (94.9) | ||
| YES | 7 (5.4) | 9 (4.4) | 5 (5.1) | 5 (5.1) | ||
| POPF [n (%)] | 0.045† | 0.165† | ||||
| No or BCL | 110 (84.6) | 187 (92.1) | 85 (85.9) | 92 (92.9) | ||
| CR-POPF | 20 (15.4) | 16 (7.9) | 14 (14.1) | 7 (7.1) | ||
| DGE [n (%)] | 0.262 | 0.261 | ||||
| No | 127 (97.7) | 199 (98.0) | 96 (97.0) | 96 (97.0) | ||
| Grade A | 0 (0.0) | 3 (1.5) | 0 (0.0) | 2 (2.0) | ||
| Grade B | 1 (0.8) | 1 (0.5) | 1 (1.0) | 1 (1.0) | ||
| Grade C | 2 (1.5) | 0 (0.0) | 2 (2.0) | 0 (0.0) | ||
| PPH [n (%)] | 0.22 | 0.384 | ||||
| No | 125 (96.2) | 201 (99.0) | 95 (96.0) | 98 (99.0) | ||
| Grade A | 1 (0.8) | 0 (0.0) | 1 (1.0) | 0 (0.0) | ||
| Grade B | 3 (2.3) | 0 (0.0) | 2 (2.0) | 0 (0.0) | ||
| Grade C | 1 (0.8) | 2 (1.0) | 1 (1.0) | 1 (1.05) | ||
| Chyle leak [n (%)] | 0.300 | 0.251 | ||||
| No | 126 (96.9) | 191 (94.1) | 95 (96.0) | 90 (90.9) | ||
| Yes | 4 (3.1) | 12 (5.9) | 4 (4.0) | 9 (9.1) | ||
| SSI [n (%)] | 0.966 | 0.884 | ||||
| No | 128 (98.5) | 199 (98.0) | 97 (98.0) | 96 (97.0) | ||
| Superficial | 1 (0.8) | 3 (1.5) | 1 (1.0) | 2 (2.0) | ||
| Organ/space | 1 (0.8) | 1 (0.5) | 1 (1.0) | 1 (1.0) | ||
| Severe complication [n (%)] | 0.998 | 0.663 | ||||
| No | 114 (87.7) | 178 (87.7) | 86 (86.9) | 88 (88.9) | ||
| Yes | 16 (12.3) | 25 (12.3) | 13 (13.1) | 11 (11.1) | ||
| Recurrence [n (%)] | 0.251 | >0.99 | ||||
| No | 37 (28.5) | 70 (34.5) | 24 (24.2) | 24 (24.2) | ||
| Yes | 93 (71.5) | 133 (65.5) | 75 (75.8) | 75 (75.8) | ||
| Recurrence pattern [n (%)] | 0.177 | 0.507 | ||||
| No | 37 (28.5) | 70 (34.5) | 24 (24.2) | 24 (24.2) | ||
| Locoregional | 20 (15.4) | 17 (8.4) | 16 (16.2) | 11 (11.1) | ||
| Systemic | 61 (46.9) | 92 (45.3) | 49 (49.5) | 51 (51.5) | ||
| Both | 12 (9.2) | 24 (11.8) | 10 (10.1) | 13 (13.1) | ||
| Pre-PSM | Post-PSM | |||||
| cDPS (n = 130) |
RAMPS (n = 203) |
p-value | cDPS (n = 99) |
RAMPS (n = 99) |
p-value | |
| Differentiation [n (%)] | 0.472 | 0.677 | ||||
| Well | 18 (13.8) | 20 (9.9) | 13 (13.4) | 12 (12.1) | ||
| Moderate | 82 (63.1) | 129 (63.5) | 61 (62.9) | 68 (68.7) | ||
| Poorly/undifferentiated | 30 (23.1) | 54 (26.6) | 23 (23.7) | 19 (19.2) | ||
| T-stage [n (%)] | 0.208 | 0.216 | ||||
| T1 | 30 (23.1) | 65 (32.0) | 23 (23.2) | 19 (45.2) | ||
| T2 | 72 (55.4) | 98 (48.3) | 54 (54.5) | 47 (47.5) | ||
| T3 | 28 (21.5) | 40 (19.7) | 22 (22.2) | 33 (33.3) | ||
| Involved LN count [median (range)] | 0.0 (0-15) | 1.0 (0–22) | 0.028 | 1.0 (0–15) | 1.0 (0–17) | 0.006 |
| LNR [median (range)] | 0.048 (0.0-1.00) | 0.057 (0–0.71) | 0.383 | 0.042(0–1.0) | 0.090 (0–0.71) | 0.059 |
| N-stage [n (%)] | 0.083* | 0.024 | ||||
| N0 | 62 (47.7) | 83 (40.9) | 44 (44.4) | 31 (41.3) | ||
| N1 | 48 (36.9) | 84 (41.4) | 38 (38.4) | 42 (42.4) | ||
| N2 | 12 (9.2) | 36 (17.7) | 12 (12.1) | 26 (26.3) | ||
| Nx | 8 (6.2) | 0 (0.0) | 5 (5.1) | 0 (0.0) | ||
| Perineural invasion [n (%)] | 0.360 | 0.290 | ||||
| PNI- | 17 (13.1) | 21 (10.3) | 13 (13.5) | 8 (8.2) | ||
| PNI+ | 106 (81.5) | 180 (88.7) | 83 (86.5) | 90 (91.8) | ||
| Unknown | 7 (5.4) | 2 (1.0) | 3 (3.0) | 1 (1.0) | ||
| Lymphovascular invasion [n (%)] | 0.376 | 0.281 | ||||
| LVI- | 64 (49.2) | 95 (46.8) | 44 (44.4) | 37 (37.4) | ||
| LVI+ | 46 (35.4) | 85 (41.9) | 39 (39.4) | 50 (50.5) | ||
| unknown | 20 (15.4) | 23 (11.3) | 16 (16.2) | 12 (42.9) | ||
| Univariate | Multivariate | ||||||
| n | 2YSR (%) | 5YSR (%) | MST(months) | p-value | HR (95% CI) | p-value | |
| Age (years) | 0.008 | ||||||
| <65 | 95 | 75.5 | 56.1 | 85.62 | 1 (Reference) | ||
| 65–75 | 75 | 66.1 | 34.3 | 51.77 | 1.663 (0.899–3.079) | 0.105 | |
| >75 | 28 | 47.5 | 34.6 | 46.71 | 1.328 (0.511–3.453) | 0.561 | |
| Sex | 0.060 | ||||||
| Male | 106 | 64.2 | 33.7 | 61.72 | |||
| Female | 92 | 73.4 | 55.5 | 80.35 | |||
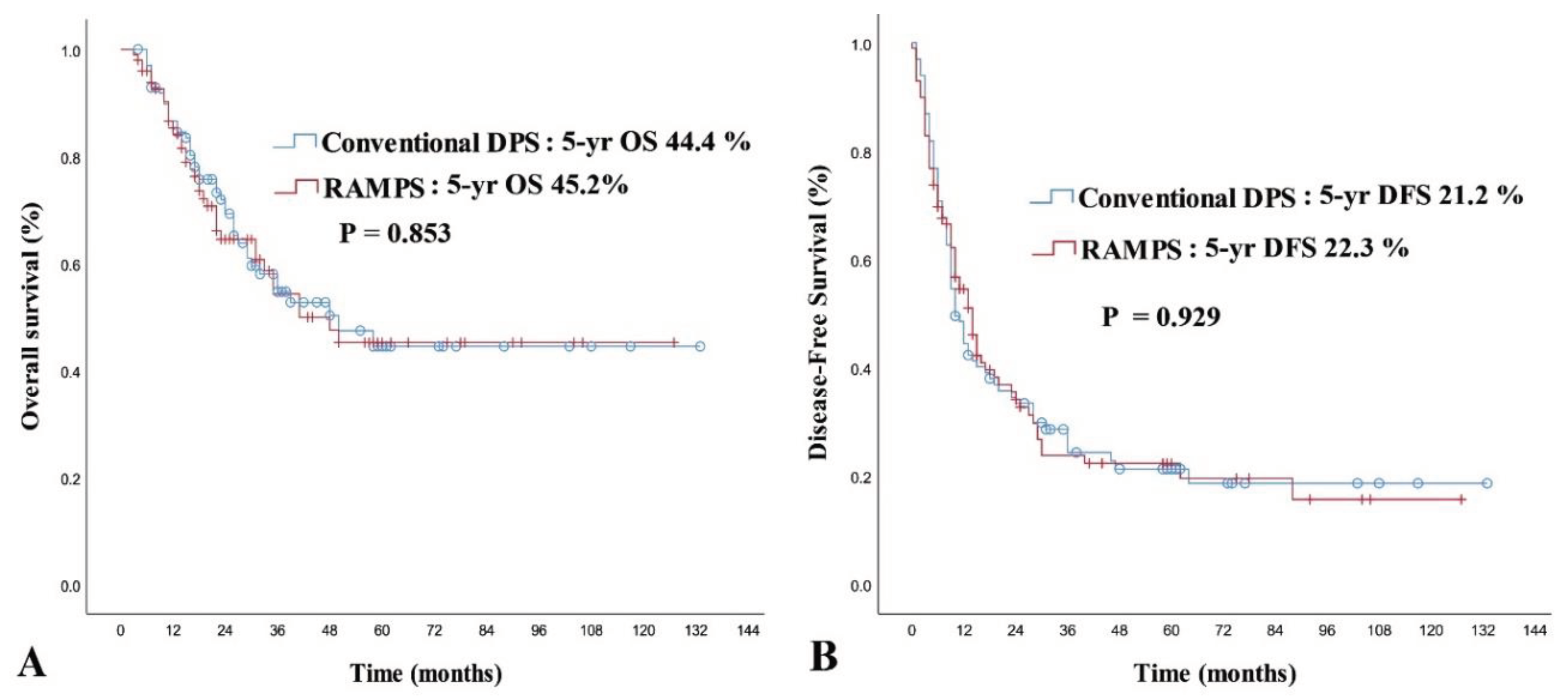
| Operation type | 0.853 | ||||||
| Conventional DPS | 99 | 71.9 | 44.4 | 72.90 | 1 (Reference) | ||
| RAMPS | 99 | 64.5 | 45.2 | 69.82 | 1.014 (0.568–1.811) | 0.962 | |
| ASA physical status | 0.047 | ||||||
| 1 | 26 | 87.6 | 74.9 | 105.04 | 1 (Reference) | ||
| 2 | 150 | 66.0 | 41.9 | 66.87 | 4.553 (1.081–19.172) | 0.039 | |
| 3 | 22 | 63.6 | 31.9 | 51.69 | 5.494 (1.148–26.298) | 0.033 | |
| CEA | 0.079 | ||||||
| ≤6 ng/ml | 147 | 69.0 | 45.9 | 74.40 | |||
| >6 ng/ml | 24 | 65.4 | 29.3 | 36.86 | |||
| CA 19-9 | 0.039 | ||||||
| ≤37 U/ml | 49 | 75.9 | 57.9 | 84.98 | 1 (Reference) | ||
| >37 U/ml | 149 | 65.9 | 39.6 | 66.59 | 2.155 (1.112–4.174) | 0.023 | |
| Differentiation | <0.001 | ||||||
| Well/moderate | 25 | 74.0 | 49.8 | 79.24 | 1 (Reference) | ||
| Poorly/undifferentiated | 42 | 46.8 | 25.3 | 40.58 | 2.299(1.290–4.096) | 0.005 | |
| T-stage | 0.143 | ||||||
| T1 | 42 | 74.4 | 58.8 | 79.15 | |||
| T2 | 101 | 68.0 | 37.0 | 64.39 | |||
| T3 | 55 | 64.7 | 42.9 | 59.61 | |||
| N-stage (Nx exluded) | 0.254 | ||||||
| N0 | 75 | 74.4 | 53.0 | 72.89 | |||
| N1 | 80 | 65.0 | 37.6 | 65.97 | |||
| N2 | 38 | 58.9 | 26.9 | 45.57 | |||
| Nx | 5 | 80.0 | 80.0 | 45.40 | |||
| Lymph node ratio | 0.016 | ||||||
| <0.2 | 143 | 72.1 | 49.6 | 78.24 | 1 (Reference) | ||
| ≥0.2 | 50 | 55.8 | 26.0 | 44.81 | 1.758 (0.718–4.305) | 0.217 | |
| Margin status | <0.001 | ||||||
| R0 | 187 | 70.9 | 47.5 | 75.93 | 1 (Reference) | ||
| R1 | 11 | 31.8 | 0.0 | 21.06 | 4.583 (2.034–10.325) | <0.001 | |
| Adjuvant treatment | 0.005 | ||||||
| Yes | 118 | 74.8 | 50.2 | 79.90 | 1 (Reference) | ||
| No | 80 | 56.6 | 35.3 | 54.20 | 1.915 (1.112–3.298) | 0.019 | |
| Perineural invasion | 0.032 | ||||||
| No | 21 | 80.7 | 74.9 | 99.32 | 1 (Reference) | ||
| Yes | 173 | 65.9 | 39.4 | 67.08 | 3.423 (0.813–14.415) | 0.093 | |
| Lymphovascular invasion | 0.003 | ||||||
| No | 81 | 80.5 | 55.8 | 83.83 | 1 (Reference) | ||
| Yes | 89 | 63.6 | 31.7 | 50.78 | 2.054 (1.196–3.528) | 0.009 | |
| Univariate | Multivariate | ||||||
| n | 2YSR (%) | 5YSR (%) | MST(months) | p-value | HR (95% CI) | p-value | |
| Age (years) | 0.290 | ||||||
| <65 | 95 | 38.5 | 28.1 | 40.71 | |||
| 65–75 | 75 | 32.1 | 14.5 | 26.58 | |||
| >75 | 28 | 29.7 | 14.8 | 25.32 | |||
| Sex | 0.341 | ||||||
| Male | 106 | 32.7 | 17.1 | 33.14 | |||
| Female | 92 | 37.7 | 26.7 | 38.46 | |||
| Operation type | 0.929 | ||||||
| Conventional DPS | 99 | 34.5 | 21.2 | 36.25 | |||
| RAMPS | 99 | 35.4 | 22.3 | 34.19 | |||
| ASA physical status | 0.665 | ||||||
| 1 | 26 | 38.1 | 29.6 | 43.63 | |||
| 2 | 150 | 34.8 | 20.4 | 33.09 | |||
| 3 | 22 | 33.8 | 21.1 | 32.15 | |||
| CEA | 0.580 | ||||||
| ≤6 ng/ml | 147 | 37.0 | 22.3 | 37.56 | |||
| >6 ng/ml | 24 | 32.5 | 17.3 | 23.77 | |||
| CA 19-9 | 0.003 | ||||||
| ≤37 U/ml | 49 | 48.8 | 28.4 | 49.56 | 1 (Reference) | ||
| >37 U/ml | 149 | 30.6 | 20.0 | 30.65 | 1.808 (1.182–2.767) | 0.006 | |
| Differentiation | 0.002 | ||||||
| Well/moderate | 25 | 39.9 | 25.0 | 39.62 | 1 (Reference) | ||
| Poorly/undifferentiated | 42 | 16.0 | 0.0 | 18.48 | 1.729 (1.122–2.664) | 0.013 | |
| T-stage | <0.001 | ||||||
| T1 | 42 | 57.9 | 36.5 | 48.88 | 1 (Reference) | ||
| T2 | 101 | 34.9 | 20.6 | 35.52 | 1.627 (0.975–2.718) | 0.063 | |
| T3 | 55 | 18.0 | 12.8 | 20.52 | 2.611 (1.504–4.532) | 0.001 | |
| N-stage (Nx exluded) | 0.039 | ||||||
| N0 | 75 | 41.8 | 29.2 | 41.74 | 1 (Reference) | ||
| N1 | 80 | 33.1 | 16.6 | 30.07 | 1.030 (0.621–1.630) | 0.899 | |
| N2 | 38 | 21.3 | 15.9 | 21.59 | 1.255 (0.599–2.627) | 0.548 | |
| Nx | 5 | 60.0 | 0.0 | 24.40 | 1.905 (0.565–6.420) | 0.299 | |
| Lymph node ratio | 0.012 | ||||||
| <0.2 | 143 | 38.4 | 23.9 | 40.35 | 1 (Reference) | ||
| ≥0.2 | 50 | 24.9 | 15.6 | 21.18 | 0.963 (0.629–1.474) | 0.864 | |
| Margin status | 0.642 | ||||||
| R0 | 187 | 34.9 | 22.1 | 35.88 | |||
| R1 | 11 | 36.4 | 18.2 | 21.18 | |||
| Adjuvant treatment | 0.018 | ||||||
| Yes | 114 | 36.7 | 23.1 | 37.20 | 1 (Reference) | ||
| No | 75 | 27.9 | 17.6 | 28.40 | 1.848 (1.257–2.717) | 0.002 | |
| Perineural invasion | 0.013 | ||||||
| No | 21 | 52.4 | 46.6 | 58.40 | 1 (Reference) | ||
| Yes | 173 | 31.8 | 17.0 | 30.80 | 1.740 (0.831–3.647) | 0.142 | |
| Lymphovascular invasion | 0.001 | ||||||
| No | 81 | 46.2 | 26.8 | 42.50 | 1(Reference) | ||
| Yes | 89 | 22.6 | 13.4 | 22.20 | 1.846 (1.256–2.714) | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





