Submitted:
13 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material and Samples Preparation
2.2. Quantity and Quality of Secondary Metabolites in Willow Leaves and Branches
2.2.1. Preparation of Plant Extracts
2.2.2. Phenolic Acids and Flavonoid Assays
2.2.3. Coccid Sporulation Test
2.2.4. Data Analysis
3. Results
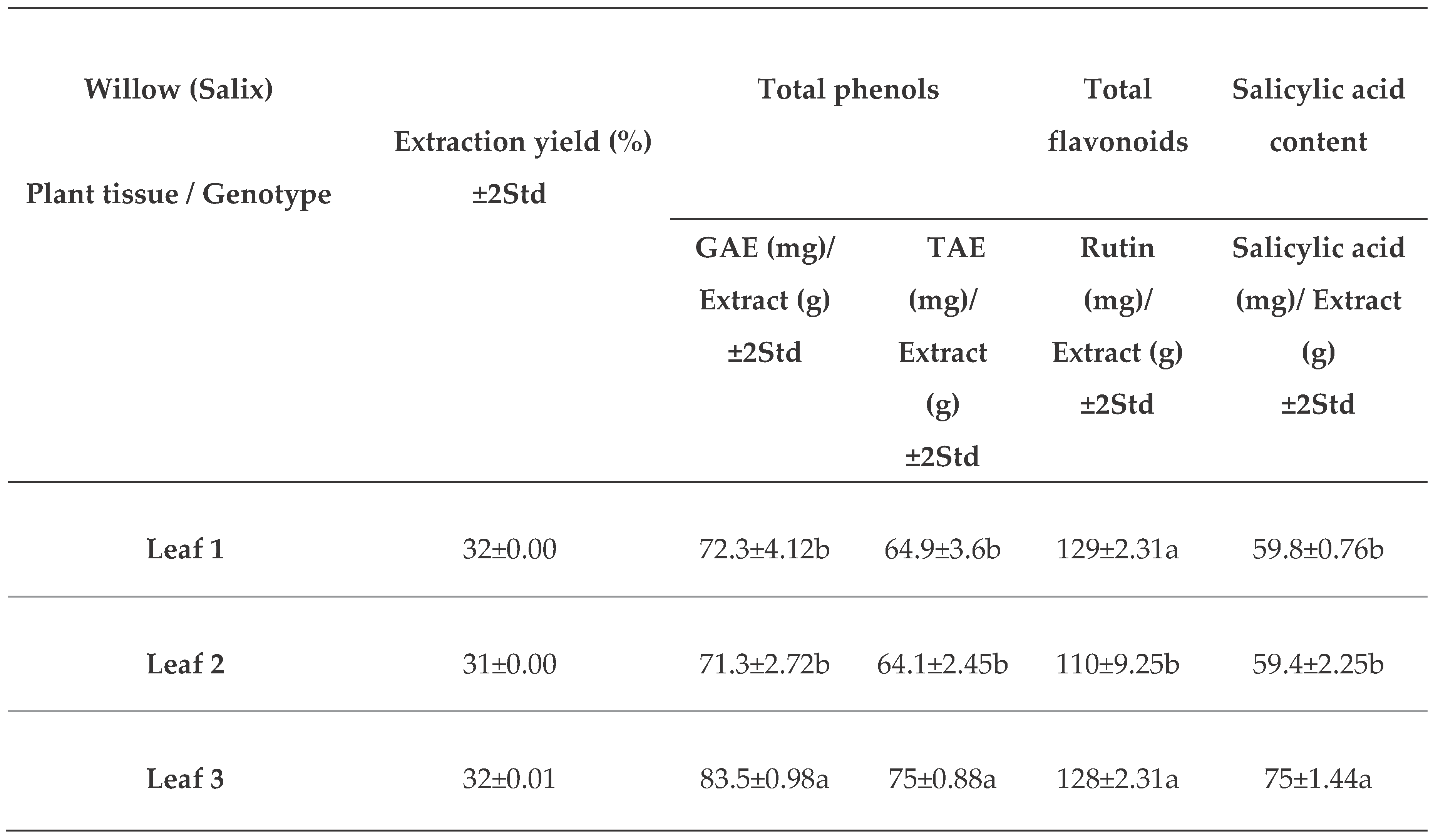
3.1. Yield (%) of Ethanolic Extracts from Willow Leaves and Branches
3.2. Total Phenolic Content of Willow Leaves and Branch Extracts' Obtained from the Different Genotypes
3.3. Total Flavonoid Content of Extracts from Leaves and Branches of the Different Willow Genotypes
3.4. Salicylic Acid Content of Willow Leaves and Branches
3.5. Anticoccidial Activity of Willow Extracts Obtained from Leaves and Branches at Different Concentrations of the Three Genotypes.
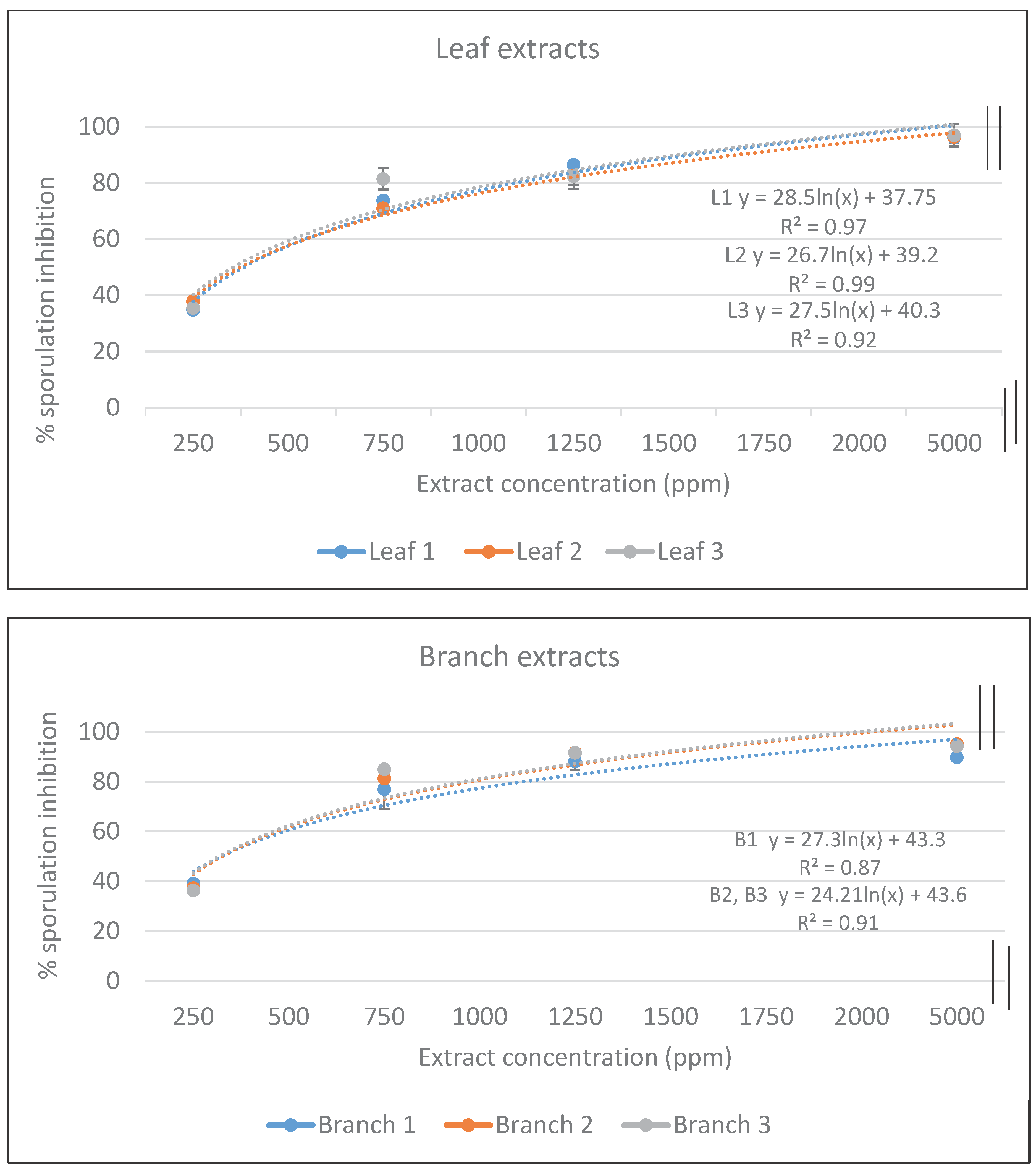
4. Discussion
5. Conclusions
Acknowledgments
Disclosures
References
- Lauron-Moreau, A.; Pitre, F.E.; Argus, G.W.; Labrecque, M.; Brouillet, L. Phylogenetic relationships of American willows (Salix L., Salicaceae). PLoS One 2015, 10, e0121965. [Google Scholar]
- Argus, G.W. Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, north of Mexico. Harv Pap Bot. 2007, 12, 335–369. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Richardson, J. Poplars and willows: trees for society and the environment. Boston, USA: The Food and Agriculture Organization of the United Nations and CABI, 2014, 634.
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J Saudi Chem Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Knee, M.; Quigley, M.F. Effects of soil compaction and flooding on the growth of 12 willow (Salix L.) species. J Environ. Hort. 2004, 22, 155–160. [Google Scholar] [CrossRef]
- Muklada, H.; Klein, J.; Glasser, T.; Dvash, L.; Azaizeh, H.; Halabi, N.; Davidovich-Rikanati, R.; Lewinsohn, E.; Landau, S.Y. Initial evaluation of willow (Salix acmophylla) irrigated with treated wastewater as a fodder crop for dairy goats. Small Rum Res 2017, 163, 783. [Google Scholar] [CrossRef]
- Larsen, S.U.; Lærke, P.E.; Jørgensen, U. Harvest of green willow biomass for feed - effects of harvest time and frequency on yield, nutrient concentration, silage quality and regrowth. Acta Agri Scand 2020. [Google Scholar] [CrossRef]
- Saller, R.; Melzer, J.; Felder, M. Pain relief with a proprietary extract of Willow bark in rheumatology. An Open Trial Schweiz Zschr GanzheitsMedizin 2008, 20, 156–162. [Google Scholar] [CrossRef]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochem. 2011, 72, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Aboud, A.S. Antimicrobial Activities of Aqueous and Methanolic Extracts from Salvia officinalis and Salix acmophylla Used in the treatment of wound infection isolates. Ibn AL-Haitham J.l for Pure and Appl Sci. 2017, 23, 25–39. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochem. 2003, 64, 3–19. [Google Scholar] [CrossRef]
- El-Shazly, A.; El-Sayed, A.; Fikrey, E. Bioactive secondary metabolites from Salix tetrasperma Roxb. Z Naturforsch 2012, 67, 353–359. [Google Scholar] [CrossRef]
- Gan, R.Y.; Chan, C.L.; Yang, Q.Q.; Li, H.B.; Zhang, D.; Ge, Y.Y.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In: Sprouted Grains, 2019, 91-246. AACC International Press.
- Lindroth, R.L.; Pajutee, M.S. Chemical analysis of phenolic glycosides: art, facts, and artifacts. Oecologia 1987, 74, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Li, F.; Liang, D.; Liu, Z.; Zhang, X.; et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-κB and mitogen-activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm Res. 2013, 62, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Farney, J.K.; Mamedova, L.K.; Coetzee, J.F.; Minton, J.E.; Hollis, L.C.; Bradford, B.J. Sodium salicylate treatment in early lactation increases whole-lactation milk and milk fat yield in mature dairy cows. J Dairy Sci. 2013, 96, 7709–7718. [Google Scholar] [CrossRef] [PubMed]
- Nyman, T.; Julkunen-Tiitto, R. Chemical variation within and among six northern willow species. Phytochem. 2005, 66, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Zhang, X.F.; Wang, L.J.; Zheng, Y.N.; Yuan, C.C.; Sun, G.Z. Isolation and characterization of phenolic compounds from the leaves of Salix matsudana. Molec. 2008, 13, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am J Biochem Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- El-Wakil, E.; El-Sayed, S.; El-Sayed, M.M.; Abdel-Lateef, E. Identification of the chemical composition of the methanolic extract of Salix tetrasperma Roxb. using LC-ESI-MS and evaluation its potential as antioxidant agent. Der Pharma Chem 2015, 7, 168–177. [Google Scholar]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unraveling the conundrum of tannins in animal nutrition and health. J Sci of Food and Agri. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Hernandez, P.M.; Salem, A.Z.M.; Elghandour, M.Y.; Cipriano-Salazar, M.; Cruz-Lagunas, B.; Camacho, L.M. Anthelmintic effects of Salix babylonica L. and Leucaena leucocephala Lam. extracts in growing lambs. Trop Animal Health and Produc. 2014, 46, 173–178. [Google Scholar] [CrossRef]
- Glazer, I.; Salame, L.; Dvash, L.; Muklada, H.; Azaizeh, H.; Mreny, R.; Landau, S. Effects of tannin-rich host plants on the infection and establishment of the entomopathogenic nematode Heterorhabditis bacteriophora. J inver pathol. 2015, 128, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Awwad, S.; Markovics, A.; Halahlih, F.; Yazbak, A.; Haj-Zaroubi, M.; Muklada, H.; Klein, J.D.; Azaizeh, H. Effect of irrigation water source on secondary metabolites in Salix acmophylla and their potential to impair exsheathment of gastro-intestinal nematodes. Ann Agric Crop Sci. 2021, 6, 1079. [Google Scholar] [CrossRef]
- Rottenberg, A.; Zohary, D.; Nevo, E. Patterns of isozyme diversity and vegetative reproduction of willows in Israel. Int J Plant Sci. 1999, 160, 561–566. [Google Scholar] [CrossRef]
- Muklada, H.; Davidovich-Rikanati, R.; Wilkerson, D.G.; Klein, J.D.; Deutch-Traubaum, T.; Zou, J.; Awabdeh, S.; Sweidan, R.; Landau, S.Y.; Schwartz, A.; et al. Genotypic diversity in willow (Salix spp.) is associated with chemical and morphological polymorphism, suggesting human-assisted dissemination in the Eastern Mediterranean. Biochem Syst Ecol 2020, 91, 104081. [Google Scholar] [CrossRef]
- Hussein Muklada, H.; Davidovich-Rikanati, R.; Awabdeh, S.; Weinberg, Z.G.; Hen, Y.; Deutch, T.; Klein, J.D.; Voet, H.; Lewinsohn, E.; Landau, S.Y. Ensiling willow (Salix acmophylla) fodder modifies the contents of plant specialized metabolites, but not nutritional attributes. Animal Feed Sci and Technol. 2021, 278, 115019. [Google Scholar] [CrossRef]
- Keeton, S.T.N.; Navarre, C.B. Coccidiosis in Large and Small Ruminants. Veterinary Clinics of North America: Food Animal Pract. 2018, 34, 201–208. [Google Scholar] [CrossRef]
- Bangoura, B.; Bardsley, K.D. Ruminant Coccidiosis. Veterinary Clinics of North America: Food Animal Pract. 2020, 36, 187–203. [Google Scholar] [CrossRef]
- Mohamaden, W.I.; Sallam, N.H.; Abouelhassan, E.M. Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. Int J Vet Sci and Med. 2018, S2314459918300012. [Google Scholar] [CrossRef]
- Juszczak, M.; Sadowska, N.; Udała, J. Parasites of the digestive tract of sheep and goats from organic farms in Western Pomerania. Poland Ann Parasitol. 2019, 65, 245–250. [Google Scholar] [PubMed]
- Rivero-Perez, N.; Hernández-Alvarado, J.L.; Valladares-Carranza, B.; Delgadillo-Ruiz, L.; Ojeda-Ramírez, D.; Sosa-Gutiérrez, C.G.; Morales-Ubaldo, A.L.; Vega-Sanchez, V.; Zaragoza-Bastida, A. Salix babylonica L. as a Natural Anticoccidial Alternative in Growing Rabbits. Evid Based Complement Altern Med. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Halahleh, F.; Abbas, N.; Markovics, A.; Muklada, H.; Ungar, E.D.; Landau, S.Y. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastro-intestinal nematode larvae. Vet Parasitol. 2013, 191, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. Determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Warrier, R.; Paul, M.; Vineetha, M. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Gen Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Zhang, G.; Li He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Ekawasti, F.; Nurcahyo, W.; Wardhana, A.H.; Shibahara, T.; Tokoro, M.; Sasai, K.; Matsubayashi, M. Molecular characterization of highly pathogenic Eimeria species among beef cattle on Java Island, Indonesia. Parasitol Int. 2019, 72, 101927. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents of Salix: a chemotaxonomic survey of further Finnish species. Phytochem. 1989, 28, 2115–2125. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines; Royal Pharmaceutical Society: London, UK, 2002. [Google Scholar]
- Shah, Z.A.; Hameed, A.; Ahmed, A.; Simjee, S.U.; Jabeen, A.; Ullah, A.; Shaheen, F. Cytotoxic and anti-inflammatory salicin glycosides from leaves of Salix acmophylla. Phytochem Lett. 2016, 17, 107–113. [Google Scholar] [CrossRef]
- Donaldson, J.R.; Lindroth, R.L. Genetics, environment, and their interaction determine efficacy of chemical defense in trembling aspen. Ecol. 2007, 88, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.Z.M.; Elghandour, M.M.Y.; Kholif, A.E. Tree leaves of Salix babylonica extract as a natural anthelmintic for small-ruminant farms in a semiarid region in Mexico. Agrofores Syst. 2016, 91, 111–122. [Google Scholar] [CrossRef]
- Landau, S.Y.; Glasser, T.A.; Zachut, M.; Klein, J.D.; Deutch-Traubman, T.; Voet, H.; Kra, G.; Davidovich-Rikanati, R. Milking performance and plant specialized metabolites in the milk of goats fed silage from willow (Salix acmophylla) irrigated with saline water. Livestock Sci. 2023, 270, 105205. [Google Scholar] [CrossRef]
- Awabdeh, S.; Sweidan, R.; Landau, S.Y. Growth performance and carcass characteristics of fattening Awassi lambs fed willow silage. Small Rum Res. 2022, 215, 106758. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





