1. Introduction
Birth asphyxia and hypoxic-ischemic syndrome (HIS) a very consequential illnesses that are linked to elevated rates of infant mortality and significant neurological consequences [
1]. The syndrome is a topic of contention in scientific literature, with numerous writers attempting to enhance its definition throughout time, resulting in increased precision but also increased complexity. The prevalence of neonatal asphyxia has experienced a significant rise in recent years, including a spectrum of severity from moderate to severe manifestations. Consequently, it has emerged as a fundamental challenge encountered within neonatology units [
2]. The phrase “birth asphyxia” is employed to describe a situation in which a baby exhibits poor Apgar scores, notable metabolic acidosis detected in umbilical cord blood samples, and a modified neurological examination (with or without EEG correspondence) in the absence of any indications of other etiologies of encephalopathy. [
3]
Asphyxia during the perinatal period leads to the formation of hypoxic cerebral lesions, which have a significant impact on the cognitive, neurological, and motor development of neonates. Defining the aetiology of hypoxia is crucial for effectively treating the cause and achieving a positive outcome.
The aetiology of the hypoxic ischemic syndrome at birth is multifactorial, with both intrapartum and antenatal factors. When affected, each link of the pathological chain causes the onset of a mild or more serious form, thereby the need to investigate the most accessible parts of the process. The diagnosis of hypoxic-ischemic encephalopathy is made on clinical criteria (Sarnat classification) as well as on laboratory tests (evidence of metabolic acidosis, multiple organ damage) and brain imaging (EEG, MRI). [
4] Hypoxic-ischemic encephalopathy treatment should be prompt to overcome the onset of permanent neurological sequelae. It has been intensely studied in the last decade leading to the identification of extensive neuroprotective strategies, such as controlled hypothermia or the use of several molecules like erythropoietin and other molecules still under study. [
5]
The identification of pathogenic processes occurring during gestation has been demonstrated by the study of placental pathology. Prompt identification of the hypoxic-ischemic lesion is crucial in the context of encephalopathy to facilitate timely intervention. Despite the extensive research conducted on maternal risk factors and birth events associated with prenatal hypoxia or cerebral palsy, there is a limited number of studies that have established direct associations between placental histological signs and neurological impairment. [
6]
The histological analysis of the placenta is often conducted primarily in instances of challenging births, to identify distinct alterations that are linked to a higher incidence of infant mortality. In recent years, there has been a growing scientific interest in investigating the correlation between the diagnosis of hypoxic-ischemic encephalopathy and abnormalities in the placenta. The Amsterdam Placental Workshop Group Criteria were formulated in 2016 to establish a uniform categorization system for placental lesions. [
7]
Perinatal asphyxia is linked to placental abnormalities that impact the blood flow to the fetus. The identified lesions on the umbilical cord included issues such as damaged velamentous vessels, cord rupture, hypercoiled chords, and cord hematoma. Additionally, there were instances of chorioamnionitis accompanied by fetal vasculitis and fetal thrombotic vasculopathy. [
8,
9]
2. Purpose
We believe that by carefully examining a single “key” component, such as the placenta, we will be able to identify etiological relationships between placental abnormalities and the occurrence of birth asphyxia. To provide a rapid tool for stratifying newborns’ probable future evolution, our goal is to identify and characterize particular macroscopic and microscopic placental damage in hypoxic neonates.
3. Patients, Material and Method
An observational, prospective, non-interventional study was undertaken at “Filantropia” Clinical Hospital in Bucharest, Romania, spanning a duration of three years from 2018 to 2020. The research was conducted with the requisite authorization from the Ethics Council of “Filantropia” Clinical Hospital and according to the privacy protocols established for the participating patients. Before the inclusion of the mother and newborn in the study, the parents or legal guardians provided their signature on an informed consent agreement. The research was carried out following the principles outlined in the Declaration of Helsinki on Human Rights.
Relevant maternal information, including the social, medical, and family history of the mother, was gathered in conjunction with data about the newborn. This data encompassed various aspects such as fetal heartbeat during labour, gestational age, delivery type, birth weight, and gender. Additionally, data from the newborns’ clinical examination, including changes associated with perinatal hypoxia and APGAR score, were also provided. In addition, laboratory findings were documented, including hemograms, metabolic parameters, pH, and acid-base balance parameters, all of which were collected in a dynamic way. However, the database was updated with placental histopathology examination data, including both micro and macroscopic images.
A macroscopic examination was conducted as part of the investigation. The aforementioned components encompassed the presence of the umbilical cord, the membranes, and the placenta. The weight of the placenta was measured within the initial hour following delivery, after the removal of the membranes and umbilical cord. The assessment of the umbilical cord encompassed various aspects, such as its length, the location of its insertion about the centre or borders of the placenta, the existence or absence of a hypercoiled or hypocoiled look, and the identification of a single umbilical artery. The membranes were described as having an opaque look and colour and were inserted in a circumvallate or circummarginal manner.
An experienced pathologist conducted the placental examination. On fresh placenta sections, the microscopic examination was performed before they were fixed and stained with hematoxylin-eosin. Five samples were obtained for microscopic examination: one from the membranes, one from the umbilical cord, and three from the placental parenchyma. The classification included the primary six pre-established classifications of injuries, according to the Amsterdam Criteria: maternal vascular malperfusion, fetal vascular malperfusion, chronic villitis of unknown aetiology, delayed villous maturation, chorioamnionitis and abruption. The following abnormalities were associated with maternal vascular malperfusion (MVM): placental hypoplasia (weight below the 10th percentile for gestational age), villi abnormalities (hypoplasia, increased fibrin deposits at villous levels, necrosis), and placental infarction.
Fetal vascular malperfusion (FVM) includes both segmental and generalized alterations, which can appear as thrombosis with or without occlusive thrombi, avascular villi, intramural fibrin deposits in the major veins, and stromal-vascular karyorrhexis. Chronic villitis of unknown etiology that associates vascular obstruction, and avascular villi as diagnosed as the presence of inflammation that affects over 30% of the distal villi. Delayed villous maturation was highlighted by the monotonous villous population. The defining characteristic of chorioamnionitis is the presence of inflammatory cells inside the layers of chorion membranes. This condition is sometimes accompanied by necrosis and acute vasculitis at certain levels of the umbilical cord and chorion. The clinical manifestation of abruptio placentae was characterized by the observation of retroplacental haemorrhage. Placentas with distinct lesions across the six categories were excluded from the analysis. The Zeiss Axioscope 5 microscope was used to analyze all placental samples.
The study group included newborns who had perinatal asphyxia, with a gestational age above 36 weeks and a diagnosis of hypoxia at birth (mild, moderate, and severe). The control group consisted of newborns with a gestational age exceeding 36 weeks who required neonatal intensive care for a minimum of three days. Placental examinations were conducted on these patients. The diagnosis of birth asphyxia in newborns was established with a comprehensive neurological clinical examination and subsequently categorized using the Sarnat score, which ranged from 1 (mild) to 3 (severe). The blood collected from the clamped umbilical cord immediately after birth had a pH value under 7.20. A complete acid-base study is conducted on the collected blood samples, including measurements of pH, pCO2, pO2, and base deficit. Patients who were excluded from the study did not match the specified inclusion criteria. These patients included those who had viral intrauterine infections, congenital abnormalities, chromosomal anomalies or were unable to undergo placental investigation.
SPSS 25.0 for Windows was used to statistically analyze the recorded data. The Chi-square or t-test was employed for independent samples, whereas Mann-Whitney tests were conducted for nonparametric variables. The determination of statistical significance was based on a P-value of less than .05.
4. Results
131 patients who met the inclusion criteria were enrolled, 5 were excluded. Also, 73 (55.3%) of them were males. The incidence of cesarean section was found to be 57.2%, with a total of 75 neonates undergoing emergency caesarian section. Additionally, a significant proportion of the cases 92 (70.2%) were attributed to complex pregnancies, with 25 cases (19.0%) including pregnancy-induced hypertension, 34 cases (25.9%) involving gestational diabetes, 11 cases (8.3%) involving preeclampsia, and 22 cases (16.7%) involving pregnancy cholestasis. The features of the patients are presented in
Table 1.
Additionally, it is important to note that the control group consisted of neonates who had been diagnosed with transient tachypnea of the newborn 27 (38 %), early neonatal sepsis 15 (23 %), hypoglycemia 14 (20 %), pneumonia 9 (14 %), and meconium aspiration syndrome 4 (5 %).
Newborns with perinatal asphyxia showed lower umbilical cord pH, measuring 7.05 (p =.00). Additionally, they had a smaller umbilical cord base deficit of -13.4 mmol/L (p =.01), a lower umbilical cord pCO2 of 64.2 mmHg (p =.00), and greater median ventilation days (p =.01). It is important to note that the duration of hospital stay was significantly longer in individuals with asphyxia compared to the control group, with a p-value of.03 (
Table 1).
The macroscopic examination records of the placenta contained many indicators, such as placental weight, membrane aspect, appearance, and implantation of the umbilical cord. The birth asphyxia group showed a significantly increased placental weight compared to the control group (p =.01). Furthermore, 57 neonates (92.0%) with hypoxia revealed umbilical cord anomalies, including insertion, vessels, hypercoiled, and hypocoiled. Furthermore, the occurrence of perinatal hypoxia was found to be linked with alterations in the umbilical cord, namely the presence of knots (p =.01) and the existence of a distinct umbilical artery (p =.01). The data that has been gathered is succinctly presented in
Table 2.
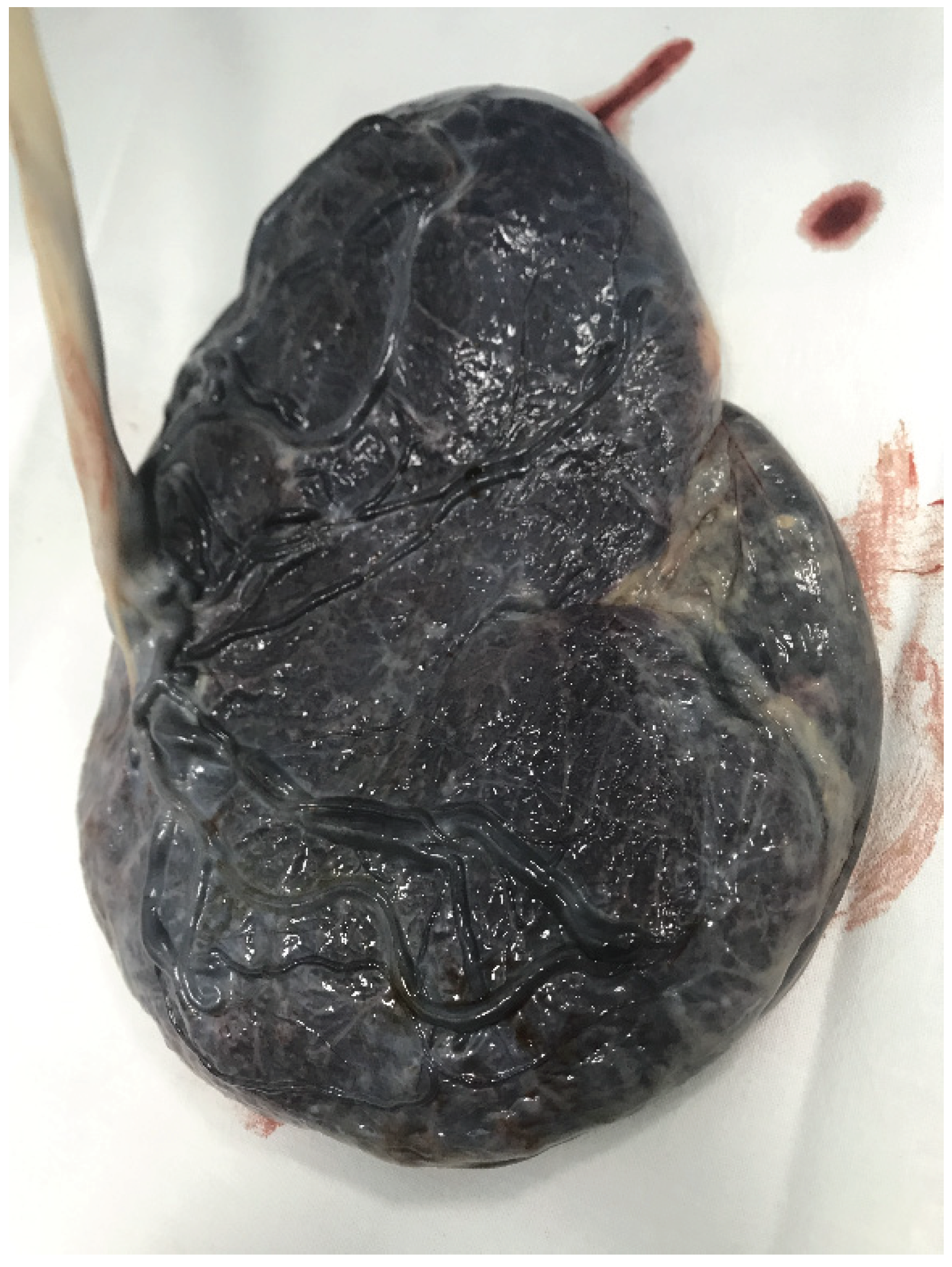
Figure 1.
Marginal insertion of Umbilical Cord.
Figure 1.
Marginal insertion of Umbilical Cord.
The placental microscopical changes were highlighted in 81 (61.8%) of cases, while 50 (38.2%) were normal. The most commonly identified lesions in birth asphyxia were chronic from the maternal vascular malperfusion 15 ( 24.2%) and fetal vascular malperfusion 11 (17.7%) class, meanwhile in the control group the normal aspect predominate. Furthermore, the group with perinatal asphyxia exhibited a higher prevalence of chronic villitis of unknown cause, with 10 cases (16.2%) out of 62, compared to the control group with 3 cases (4.2%) out of 69. In instances of delayed villous maturation, similar findings were observed, with 12 cases (19.4%) out of 62 and 4 cases (5.6%) out of 69. These results highlight a statistical correlation between these lesions and the occurrence of hypoxic lesions at birth, chronic villitis of unknown cause (p=.00), and delayed villous maturation (p=.01).
Out of the total 7 cases of abruption, 6 (9.6%) cases were assigned to the study group, while 1 (1.4%) case was assigned to the control group. Due to its small number, the data did not demonstrate a strong significant statistical correlation between the two variables (p= .03). Also, there were no differences in the two groups regarding chorioamnionitis, (p= .NS). Further data are presented in
Table 3.
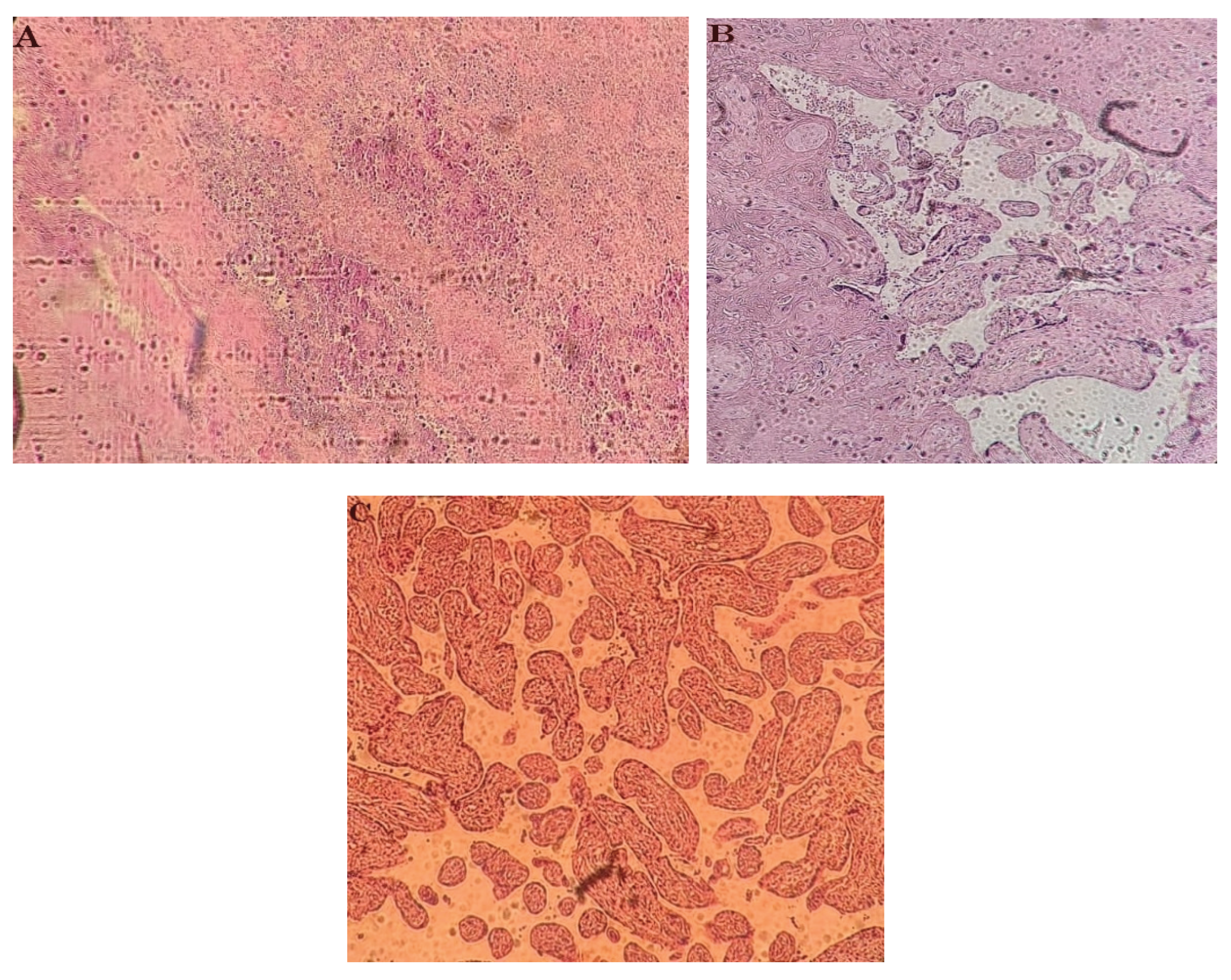
Figure 2.
A: Chorioamnionitis B: Villous fibrinoid deposition and necrosis C: Villous hypoplasia.
Figure 2.
A: Chorioamnionitis B: Villous fibrinoid deposition and necrosis C: Villous hypoplasia.
A few other lesions were excluded during the placental histopathological examination since they could not be classified based on the Amsterdam criteria, and the placental histopathological examination (0.7%) did not reveal any chirangiosis). Moreover, in the instance of the presence of multiple simultaneous lesions ( 3 cases (2.2%)), they were classified according to the extent of the most extensive lesions ( more than 30%).
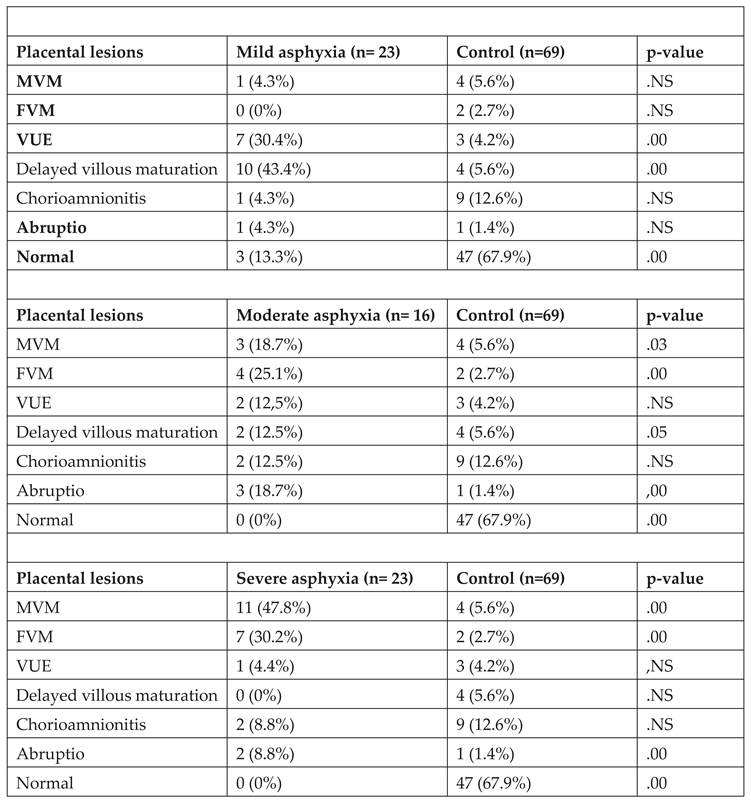
The analysis of placental changes in relation to the severity of hypoxia revealed significant associations between maternal (p =.00) or fetal (p =.00) vascular malperfusion type lesions and severe perinatal asphyxia. In cases of moderate asphyxia, both maternal (p= .03) and fetal vascular malperfusion (p=.00) lesions, as well as abruptio -type (p= .05) lesions, were observed. Additionally, the presence of chronic villitis of unknown etiology (p =.00) and delayed villous maturation (p =.00) type lesions were associated with mild perinatal asphyxia.
Table 4 provides a summary of all the statistical correlations that have been found between the identified placental lesions and the degree of prenatal hypoxia.
5. Discussions
Our study emphasized that a careful examination of both the umbilical cord and the placenta is critical to a better understanding of the multifactorial aetiology of perinatal asphyxia. Based on the findings in this study, the presence of both placental changes and the presence of hypoxic lesions at birth were associated with the presence of placental changes as well as the umbilical cord.
Our results highlight the importance of placental examination in birth asphyxia as other studies revealed as Nielsen et al. mentioned.[
10] Also previous studies revealed, that both macroscopic and microscopic exams are associated with hypoxic brain lesions.[
11,
12,
13] In our study, the most common placental microscopic changes in perinatal asphyxia were from the maternal and fetal vascular malperfusion categories, unlike the control group where most placentas were normal. According to the classification of placental lesions, we can conclude that those that determined the appearance of hypoxic lesions were chronic, these results being similar to Bingham et al. [
14].
According to the results, we can support the statement that hypoxic-ischemic lesions are associated with both macro and microscopic placental lesions and changes in the umbilical cord. As Wintermark et al. mention, the following pathological lesions of the umbilical cord were highlighted in our study: circumvallated membranes, single umbilical artery, hypo/hypercoiled and abnormal insertions. [
9] These lesions from the umbilical cord and membranes have been associated with fetal death or perinatal death in previous studies. [
15,
16,
17] In our study they were associated with newborns with birth asphyxia (p = .01).
The macroscopic examination of the placentas, as well as in other scientific studies, did not present significant changes.[
18] Lachapelle et al. have demonstrated that the weight of the placenta and the ratio between birth weight and placenta has proved to be a valuable factor [
19]. As in our study, placental weight tends to be greater in newborns who have developing brain lesions, especially in those with an extended lesion that causes hypoxic-ischemic encephalopathy (p = .01). The underlying mechanisms responsible for the augmentation of placental weight remain unknown. Placental hypertrophy during pregnancy may potentially serve as a protective mechanism for the fetus against stressful events in utero, which raises the risk of perinatal depression following bad events during labour and birth. This predisposition is primarily associated with the incidence of significant brain damage.
Other studies have observed associations between velamentous and marginal insertion of the umbilical cord into the placenta with the presence of hypoxic-ischemic encephalopathy [
20]. These changes in the umbilical cord can cause acute injuries during labour or chronic circulatory obstruction, both of which can lead to birth asphyxia. Nomiyama et al. demonstrated the utility of Doppler imaging in the context of normal second-trimester sonography to identify cord insertion, particularly velamentous insertion [
21]. The rapid examination, characterized by a high level of precision, aids in the identification of pregnancies that are at risk. In our study, the results were strongly associated with a single umbilical artery of the umbilical cord and birth asphyxia due to compromised blood flow through the umbilical vessels (p=.01).
Redline et al., pointed out that placental lesions are the only explanation for asphyxia in some cases. They identified the presence of placental pathological lesions in over 90% of infants who subsequently developed cerebral palsy [
22]. In our study we have identified a statistically significant association between the presence of placental changes and the diagnosis of birth asphyxia (p = .00). They also demonstrated the association between placental vasculopathy and umbilical arterial pH under 7.10, which causes thrombotic events and death. [
22]
Due to the extensive lesions encountered in the histopathological examination, in our study, the Amsterdam standardized classification was used to make the changes associated with HIE as specific as possible. Other classifying systems of placental injuries are used by other authors in their publications. For example, Chang et al. [
23] classified the lesions into inflammatory, vascular and other, while others have made more detailed classifications with 10 to 16 subgroups of histopathological lesions [
20]. In the present study, only 6 main groups were chosen, the other lesions that could not be classified in them being very few and insignificant. Each lesion was made according to the Amsterdam Criteria [
7] and included 6 large categories: maternal vascular malperfusion, fetal vascular malperfusion, chorioamnionitis, chronic villitis of unknown aetiology, delayed villous maturation and abruption placenta. In the analyzed placentas, although several concomitant lesions were present, the division was performed after the dominant lesion from the pre-specific Amsterdam group.
Also, in our study the most common microscopic placental lesions associated with the hypoxic-ischemic syndrome were in the category of maternal and fetal placental malperfusion, being labelled as chronic lesions predominant with the damage of villi (infarction, fibrin depositions and necrosis). Badawi et al. [
24], emphasized that fetal vascular malperfusion lesions present in newborns can cause both acute neonatal lesions and long-term damage through cord compression. Such lesions emphasize the multitude of ways of the aetiology of hypoxic lesions. The results of our study showed an association between the presence of fetal vascular malperfusion-type lesions and the presence of perinatal asphyxia (p=.00).
In addition, the present study identified a strong correlation between the presence of placental infarction and the occurrence of severe asphyxia, as in other literature [
25]. The connection between placental vascular changes and the aetiology of hypoxia at birth was a large research subject and has been correlated with an increased risk of cerebral palsy [
26]. Our results revealed a strong statistical association between maternal vascular malperfusion lesions and birth asphyxia (p=.00), both moderate and severe.
The presence of vascular lesions increases the vulnerability of the fetus during labour, leading to hypoxic lesions at birth. Furthermore, these results underscore the presence of a persistent thrombotic mechanism occurring inside the fetal and placental blood vessels, which demonstrate a close interconnection. There is a positive correlation between hypoxia and the occurrence of clots inside the fetoplacental circulation. The findings of our study align with those of Redline, who emphasized a statistically significant correlation between prenatal vascular malperfusion and hypoxic-ischemic encephalopathy [
20].
The correlation between villous destruction, caused by chronic villitis of unknown aetiology (p= .00) or delayed villous maturation (p= .00), and mild forms of the disease is not unexpected. This is because these mechanisms contribute to the gradual development of lesions by activating stress-adaptive mechanisms in the fetus before birth. Consequently, the infant presents a reduced level of hypoxia and a better response at birth.
In contrast to Wintermark et al., who reported an association between chorioamnionitis and brain injury [
9], our results did not show any such association. This can be explained by the good protective function of the brain in acute injuries. Additionally, Stunk et al. found that the risk of late-onset sepsis for infants exposed to histological chorioamnionitis decreased [
27]. Although this study included newborns with gestational ages under 32 weeks, it is notable that our neonates were at term.
Previous research has established a connection between placental abruption and cerebral palsy [
28]. Although the diagnosis of placental abruption is primarily clinical, histopathological examination has revealed the presence of retroplacental hematoma. Following the findings of Bingham et al. [
14], the appearance of retroplacental hematoma did not demonstrate a statistically significant correlation with the occurrence of ischemic hypoxic syndrome at birth. Also, our research results did not show a significant relationship between the two factors.
Our study stands out from the vast literature because, contrary to the numerous studies that have been published in the past, the particularity of our study lies in the clear diagnostic criteria for perinatal asphyxia and a standardized classification of placental lesions. A significant strength of our study is that we were able to identify placental lesions in a group of patients who had a clear diagnosis of birth asphyxia, as well as that we were able to categorize placental lesions according to Amsterdam criteria and that we identified a large number of lesions on the examined placenta. The limitations of the study are linked to the relatively limited number of patients included, the unblinded nature of the single pathologist responsible for the placental examination, and the absence of brain imaging for newborns with hypoxic-ischemic syndrome.
6. Conclusions
In conclusion, we can say that birth asphyxia is associated with micro and macroscopic placental lesions, as well as abnormalities of the umbilical cord. In addition, the weight of the placenta tends to be greater in newborns with hypoxia at birth. Also, the placental histopathological examination showing changes in both maternal and fetal vascular malperfusion is associated with the presence of hypoxic syndrome at birth.
Moreover, the categorization of patients based on the manifestation of the disease enabled us to emphasize that the less severe versions are linked to the existence of persistent villous inflammation and the postponement of delayed villous maturation. Furthermore, it has been observed that the presence of maternal and fetal vascular malperfusion-type lesions is related to moderate and severe types of hypoxic lesions.
Conflicts of Interests
The authors declare that they have no conflict of interests.
References
- Ambalavanan N, Carlo WA, Shankaran S, et al. Predicting outcomes of neonates diagnosed with hypoxic-ischemic encephalopathy. Pediatrics 2006, 118, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Volpe JJ: Neurology of the newborn, ed 5, Philadelphia, 2008, Saunders.
- Fanaroff, Avroy A: Care of the high-risk neonate, ed 6, Philadelphia, 2013, Saunders.
- Higgins RD, Raju TN, Perlman J, et al. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr 2006, 148, 170–5. [Google Scholar] [CrossRef] [PubMed]
- Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014, 123, 896–901. [CrossRef] [PubMed]
- Redline, RW. Placental pathology: a systematic approach with clinical correlations. Placenta 2008, 29, S86–S91. [Google Scholar] [CrossRef]
- Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016 Jul;140(7):698-713. Epub 2016 May 25. PMID: 27223167. [CrossRef]
- de Laat MW, Franx A, Bots ML, Visser GH, Nikkels PG. Umbilical coiling index in normal and complicated pregnancies. Obstet Gynecol 2006, 107, 1049–1055. [Google Scholar] [CrossRef]
- Wintermark P, Boyd T, Gregas MC, et al. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am J Obstet Gynecol 2010, 203, 579.e1–9. [Google Scholar] [CrossRef]
- Nielsen LF, Schendel D, Grove J, et al. Asphyxia-related risk factors and their timing in spastic cerebral palsy. BJOG 2008, 115, 1518–1528. [Google Scholar] [CrossRef]
- Fox A, Doyle E, Geary M, Hayes B. Placental pathology and neonatal encephalopathy. Int J Gynaecol Obstet. 2023, 160, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Redline, RW. Disorders of placental circulation and the fetal brain. Clin Perinatol. 2009, 36, 549–559. [Google Scholar] [CrossRef]
- Chang, KT. Examination of the placenta: medico-legal implications. Semin Fetal Neonatal Med. 2014, 19, 279–284. [Google Scholar] [CrossRef]
- Bingham A, Gundogan F, Rand K, Laptook AR, Placental findings among newborns with hypoxic ischemic encephalopathy. Journal of Perinatology. Springer, 2019. [CrossRef]
- Parast MM, Crum CP, Boyd TK. Placental histologic criteria for umbilical blood flow restriction in unexplained stillbirth. Hum Pathol 2008, 39, 948–53. [Google Scholar] [CrossRef]
- Tantbirojn P, Saleemuddin A, Sirois K, et al. Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta 2009, 30, 1083–8. [Google Scholar] [CrossRef]
- Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014, 134, e444–53. [Google Scholar] [CrossRef]
- de Regnier RA. Placental windows into neonatal encephalopathy. J Pediatr. 2018, 202, 3. [Google Scholar] [CrossRef]
- J. Lachapelle, M. Chen, M. Oskoui, N. Ali, R. Brown, P. Wintermark. Placental pathology in asphyxiated newborns treated with therapeutic hypothermia. Journal of Neonatal-Perinatal Medicine 2015, 8, 33–40. [Google Scholar] [CrossRef]
- Nasiell J, Papadogiannakis N, Lof E, Elofsson F, Hallberg B. Hypoxic ischemic encephalopathy in newborns linked to placental and umbilical cord abnormalities. J Matern Fetal Neonatal Med. 2016, 29, 721–6. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama M, Toyota Y, Kawano H. Antenatal diagnosis of velamentous umbilical cord insertion and vasa previa with color Doppler imaging. Ultrasound Obstet Gynecol 1998, 12, 426–9. [Google Scholar] [CrossRef] [PubMed]
- Redline, RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstetr Gynecol 2005, 192, 452–57. [Google Scholar] [CrossRef] [PubMed]
- Chang T, Reyes C, Teng J, Placette J, Massaro AN, Nelson KB. Neonatal encephalopathy, sentinel events, and the placenta. J Neonatal-Perinat Med. 2012, 5, 41–8. [Google Scholar] [CrossRef]
- Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O’Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998, 317, 1554–8. [Google Scholar] [CrossRef] [PubMed]
- McDonald DG, Kelehan P, McMenamin JB, Gorman WA, Madden D, Tobbia IN, et al. Placental fetal thrombotic vasculopathy is associated with neonatal encephalopathy. Hum Pathol. 2004, 35, 875–80. [Google Scholar] [CrossRef] [PubMed]
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol, 2015; 213, 849 e841–7. [Google Scholar]
- Strunk T, Doherty D, Jacques A, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 2012, 129, e134–41. [Google Scholar] [CrossRef] [PubMed]
- Thorngren-Jerneck K, Herbst A. Perinatal factors associated with cerebral palsy in children born in Sweden. Obstet Gynecol. 2006; 108, 108–505. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).