Introduction
Due to increased human activities and climate change, infectious diseases have emerged at an increased rate over the last decades. They constitute a major threat to wild animal populations but their impacts are hard to predict (Jones et al. 2008). One fundamental challenge when examining the dynamics of disease outbreaks is understanding how host movements influence the spread of diseases within and among populations (Dougherty et al. 2018), but also reversely, how diseases affect host movements, by changing the behaviour of individuals (Romano et al. 2022; Stockmaier et al. 2021).
In spatially structured populations, individuals living in social groups or highly aggregated colonies are more likely to increase transmission rates compared to solitary individuals (Caillaud et al. 2006; Langwig et al. 2012). Within social groups, individuals with high social status (Höner et al. 2012), good body condition (Dekelaita et al. 2023), fewer parasite load (Gaitan & Millien 2016) and more likely to disperse (Loveridge & Macdonald 2001) can facilitate disease spread. Yet, those individuals are not always the ones which are more likely to die from the disease. For example, dominant female spotted hyenas are more likely to spread the pathogenic bacterium Streptococcus equi ruminatorum to other individuals while yearlings and adult males, which have a lower social status and lower body condition, are more likely to die from the pathogen (Höner et al. 2012). Those individual and social differences in disease exposure induce non-random mortalities within groups which have strong impacts on social group size and structure (Caillaud et al. 2006). It can relax density-dependence mechanisms (Gudelj & White 2004) which can in turn result in demographic changes, such as rearrangements of dispersal flows (Genton et al. 2015; Lachish et al. 2011; Nunn et al. 2008) or an increase of recruitment rates (Muths et al. 2011). At the same time, changes in social behaviour can arise from an active response of individuals to disease exposure: individuals can self-isolate, avoid contacts or exclude infectious individuals from the social groups and this can in turn affect reproductive activities and social cohesion (Stockmaier et al. 2021). For example, silverback male gorillas are more likely to become solitary and females gorillas tend to disperse more from breeding groups towards non-breeding groups during an Ebola outbreak, which loosen social cohesion and change dispersal patterns between groups (Genton et al. 2015).
Non-human primates (hereafter primates), are particularly vulnerable to infectious diseases, because the majority of species exhibit high social interactions, which facilitate disease transmission between individuals and groups (Bermejo et al. 2006; Berthet et al. 2021; Bicca-Marques & de Freitas 2010; Leendertz et al. 2004; Walsh et al. 2003). Additionally, wild primates suffer from the loss and fragmentation of primary forests, their main habitat, and from hunting, as they are used as commercial bushmeat. These threats make them highly endangered worldwide, with 75% of species having declining populations (Estrada et al. 2017). Better understanding and predicting the role of disease on the dynamics of primate populations is therefore urgent for their conservation (Smith et al. 2009). If the interactions between host movements and the spread of directly-transmitted pathogens have been widely studied in primates (Bermejo et al. 2006; Caillaud et al. 2006; Genton et al. 2015; Leendertz et al. 2004; Morrison et al. 2021; Nunn et al. 2008; Walsh et al. 2003), vector-borne diseases have drawn less attention (Stoddard et al. 2009).
Yellow fever is a viral vector-born disease endemic from the tropical regions of Africa and South America. It is transmitted to humans and primates through the bite of infected mosquitoes and represents a major public health threat despite the availability of a safe and effective vaccine for humans (Bicca-Marques & de Freitas 2010; Sacchetto et al. 2020). In South America, yellow fever is mainly restricted to a sylvatic cycle. Arboreal mosquitoes (Haemagogus and Sabethes spp.) circulate the virus through primates which act as reservoirs, so the disease cannot be eradicated (Abreu et al. 2019). In this specific cycle, humans are accidently infected by mosquitoes when they live close to forest edges or in rural areas but sporadic infections can still lead to large episodic outbreaks, as observed in Brazil in 2016-2019 (Dietz et al. 2019; Giovanetti Marta et al. 2019; Possas et al. 2018).
Here, we took advantage of the long-term monitoring survey carried out on a population of golden lion tamarins Leontopithecus rosalia in Brazil to assess the impact of the last yellow fever outbreak—reported from November 2016 to April 2019 (Dietz et al. 2019).—on annual survival and dispersal rates at both local and regional scales. Golden lion tamarins are small, endangered primates endemic from the lowland Atlantic forest in the state of Rio de Janeiro, Brazil. They were on the verge of extinction in the 1970s with less than 400 remaining individuals in the wild. Massive conservation actions were then carried out, notably through reintroductions of captive and zoo-born individuals, translocations of wild individuals from small forest fragments to larger ones and community education for habitat preservation (Kierulff et al. 2012). This successfully led to an increase in the population, reaching more than 3700 individuals in 2014 (Ruiz-Miranda et al. 2019). Nevertheless, 2 years later, the population was supposedly hit by the worst outbreak of sylvatic yellow fever in about a century, which reduced the population size by at least 30% (Dietz et al. 2019). The lack of dead individuals recovered for serological testing has made it difficult to assess the true impact of the outbreak, but surveys of other primate species have documented similar drastic population reductions (Berthet et al. 2021; Possamai et al. 2022; Strier et al. 2019).
Accordingly, we predicted that the yellow fever outbreak would induce a significant decrease in annual adult survival rate. As a result, the local loss of individuals would relax density-dependence and thus competition for reproduction within social groups, which would lead to a decrease in local dispersal and/or a shorter period to settle in a group (Lachish et al. 2011). At the same time, dispersal could constitute an active response of individuals to leave infected groups and avoid further exposure so they would disperse more and/or at a larger spatial scale (Genton et al. 2015; Stockmaier et al. 2021).
Methods
Study Site and Population
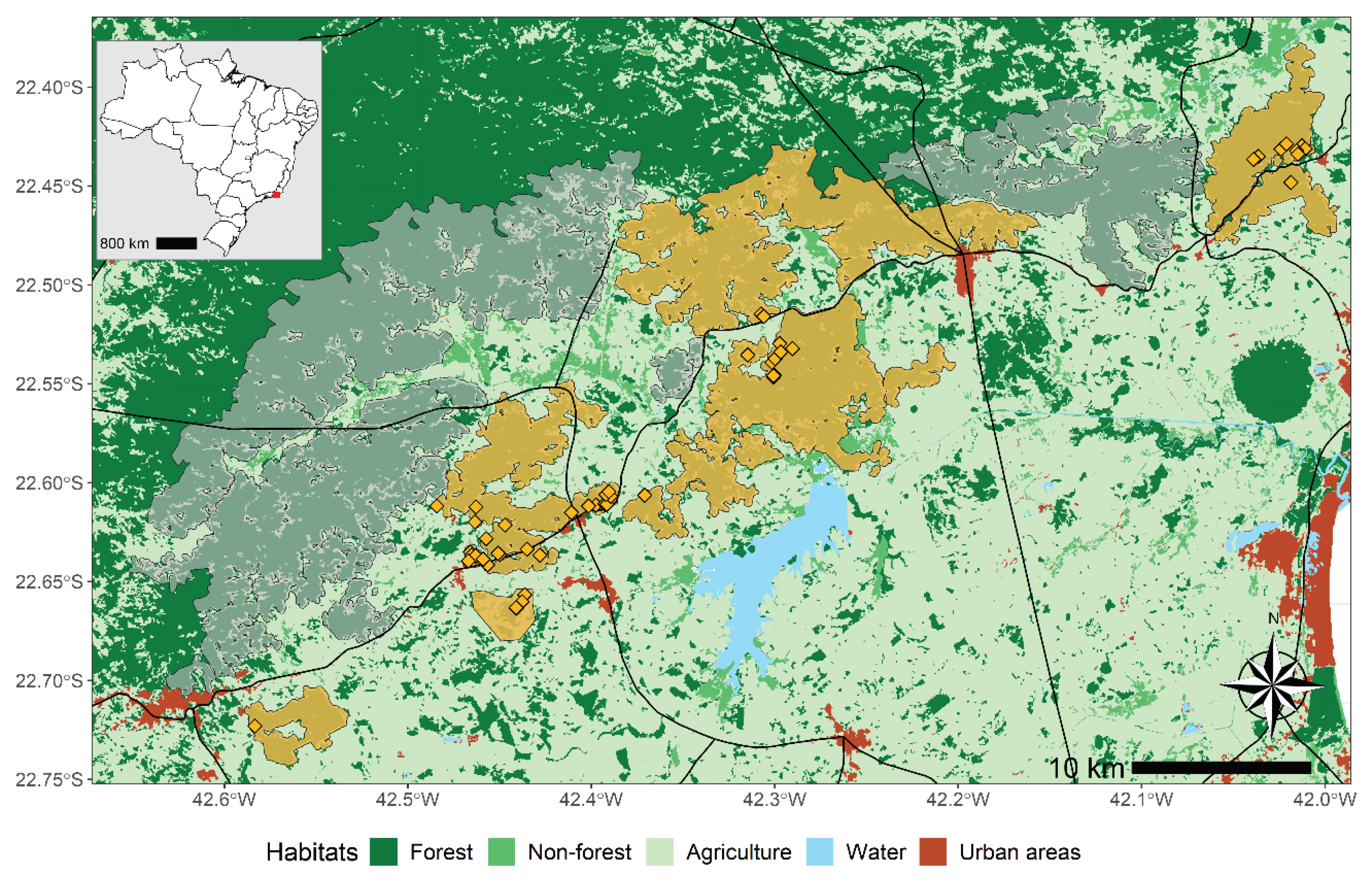
The study population lives in the highly fragmented lowland Atlantic forest in the state of Rio de Janeiro, Brazil. It has been surveyed for more than 30 years by the NGO Associaçao Mico-Leão-Dourado (AMLD) which is responsible for the conservation program of the species (Ruiz-Miranda et al. 2019). Individuals are identified by specific combinations of non-invasive methods: hair-dye when observed in the field and tattoos when they are captured. One member of monitored groups is also equipped with a VHF transmitter so that groups can be located more easily.
Data Selection
Since 1989, between 4 and 109 groups have been monitored annually in different parts of the study region, subdivided in conservation units of relatively continuous forest patches (
Figure 1). The observation effort within and between regions and groups has been highly variable, partly because one initial goal of the survey was to monitor the success of the reintroduction of zoo-born and captive golden lion tamarins which took place between 1981 and 2001 in targeted forest fragments (Kierulff et al. 2012). This created high spatial and temporal heterogeneity in observation data. To correct for this important issue, data were retained only after 2009, when monitoring effort in some defined groups and regions became more regular. Additionally, individual observations were summarized as single yearly occasions to remove within-year temporal heterogeneity. When individuals were observed in different groups during the same year, the group retained corresponded to the group where they were most often seen. If they were seen the same number of times, the group of last observation of the year was retained. Only sexually mature adults (>18 months old; (Romano et al. 2019) with verified sex were included. Observations of 42 translocated individuals were included (see (Kierulff et al. 2012) for translocation details) but the year of translocation, their state was coded 1 as it is for a focal group (see below). The final dataset included 234 males and 172 females living in 71 distinct social groups, representing a total of 1689 observations over 13 yearly occasions, from 2010 to 2022.
All first observation of individuals were coded 1. If individuals dispersed to another group in the same conservation unit compared to the previous occasion, it was coded 2 (local dispersal). If they dispersed to another group in a different conservation unit, it was coded 3 (regional dispersal). If individuals were reobserved in the same group the year following dispersal, the new group became their focal group and their state became 1 again. If they kept changing groups for consecutive years, they remained in state 2 or 3 depending on whether they dispersed to another conservation unit. Note that all dispersing individuals never settle back in their initial group nor conservation unit.
Capture-Recapture Analysis
To concurrently estimate annual survival and dispersal probabilities, we used a multi-state capture-recapture model (Lagrange et al. 2014). Three states were defined based on the previous and current group and region the individuals were observed and corresponding to the set of observations given above, i.e., state 1: living at focal group, state 2: living in another group within the same conservation unit as previous year, state 3: living in another group in a conservation unit than previous year. All transitions are possible between states.
Goodness-of-fit tests for multistate models were run using U_CARE2.3.2 (Choquet et al. 2009a) to evaluate the existence of overdispersion due to possible heterogeneity in survival and/or resighting probabilities (
Table 1)
. Specifically, three tests were run to check for transience (excess of individuals detected at the time of first capture and never seen again) and one test to check trap-dependence (excess of individuals resighted more than others). The program E-Surge (Choquet et al. 2009b) was used to build and fit multi-state models where transition probabilities were decomposed into three consecutive steps: survival (s), dispersal (Ψ) and resighting (
p). Model selection was performed based on the Quasi-likelihood Akaikes’ information criterion, corrected for small sample size and overdispersion (QAICc, (Burnham & Anderson 2002). The model with the lowest QAICc was considered the best model. The effects of time, state and sex were tested in preliminary models. First results showed that sex was uninformative for all transition probabilities, as models including this effect had similar QAICc as the constant ones (difference < 2). Sex was thus removed from the initial dataset and new GOF tests were run on the pooled dataset (
Table 1). Furthermore, models with fully time-dependent dispersal probabilities performed poorly due to general low dispersal events in the dataset so time effect was replaced by a three periods effect (
Table 2). This three periods effect considered distinct dispersal probabilities before, during and after the outbreak (3T), only outside and during the outbreak (2T) or not (1T). Finally, preliminary models showed that detection probabilities for state 1 and 2 were always equal to 1 so they were grouped together (2f) and fixed to 1 so that the model would only estimate detection probabilities for state 3. Overall, the most complex model included fully time-dependence (t) and 3 cohorts (3c; see results), for survival, 3 periods (3T) for dispersal and two states (2f) for detection probabilities. Full final model selection as well as the different matrices used in E-surge can be found in Supplemental material.
Results
Goodness-of-fit tests run on pooled male and female data detected a significant age effect (
Table 1), for years 2017 and 2018. New individuals of 2016 and 2017 had a different survival than older individuals in years 2017 and 2018. To account for this heterogeneity, we used a cohort effect to separate individuals based on the year of their first capture. Up to 3 cohorts were used for survival (
Table 2), depending on when individuals were first observed (before, during or after the outbreak). Overall, model selection provided the most parsimonious model with fully time-dependent survival probabilities for 2 distinct cohorts for individuals first seen before and during the outbreak (2c.t), two periods for dispersal probabilities (2T) and state-dependent (2f) detection probabilities (
Table 3).
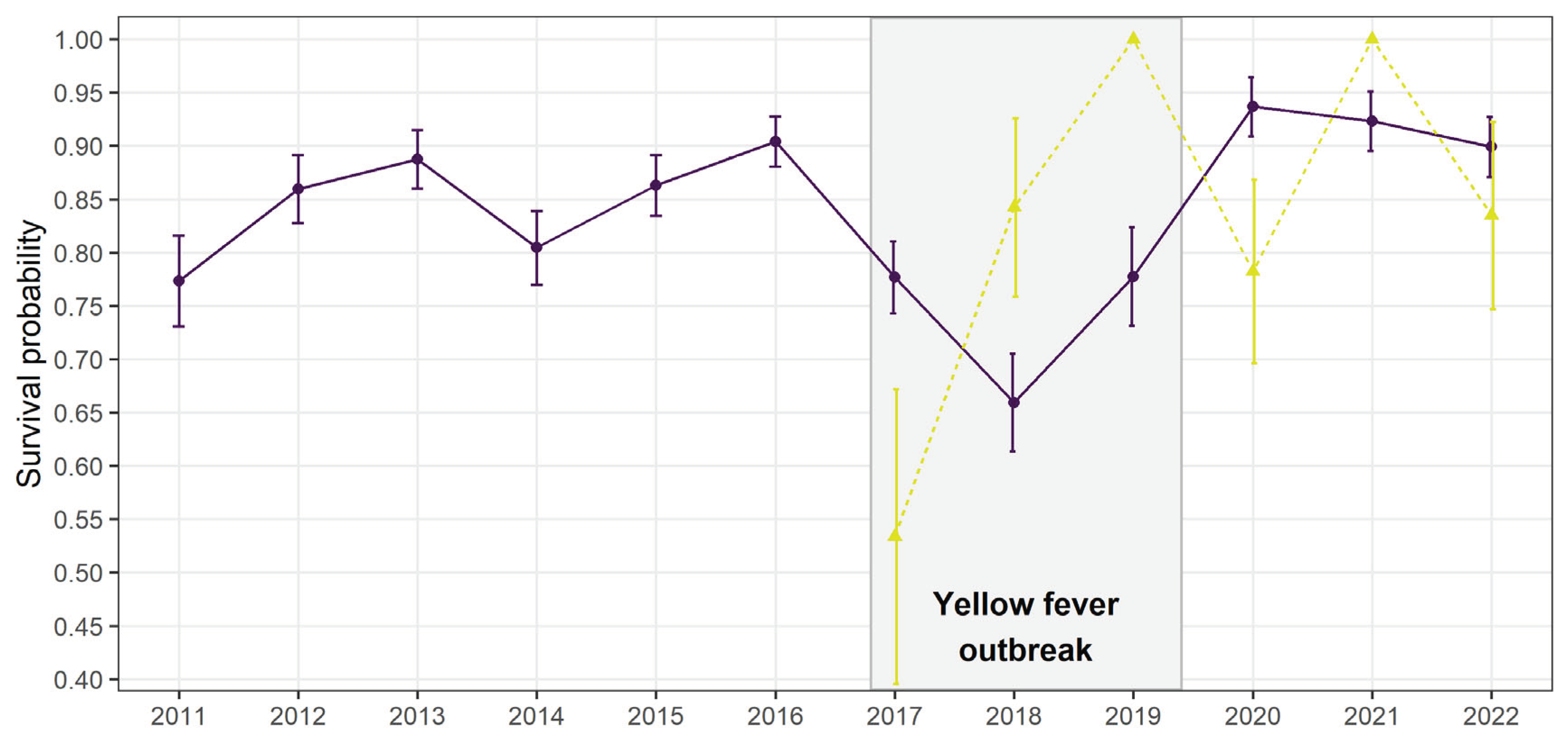
As expected, adult annual survival rate for the first cohort was highly variable over the entire time period but drastically decreased in 2017 and 2018, matching the period of the yellow fever outbreak (
Figure 2). From 2019, which coincides with the reported end of the outbreak, survival bounced back to levels higher than before the outbreak, with survival probabilities higher than 0.9. The second cohort, which individuals were first encountered the year before the outbreak in 2016, had a lower survival rates the first year of the outbreak but higher ones the next 2 years (
Figure 2). Following heterogeneous pics of survival in 2019 and 2021 were potentially due to a lower fieldwork effort and thus, fewer data available.
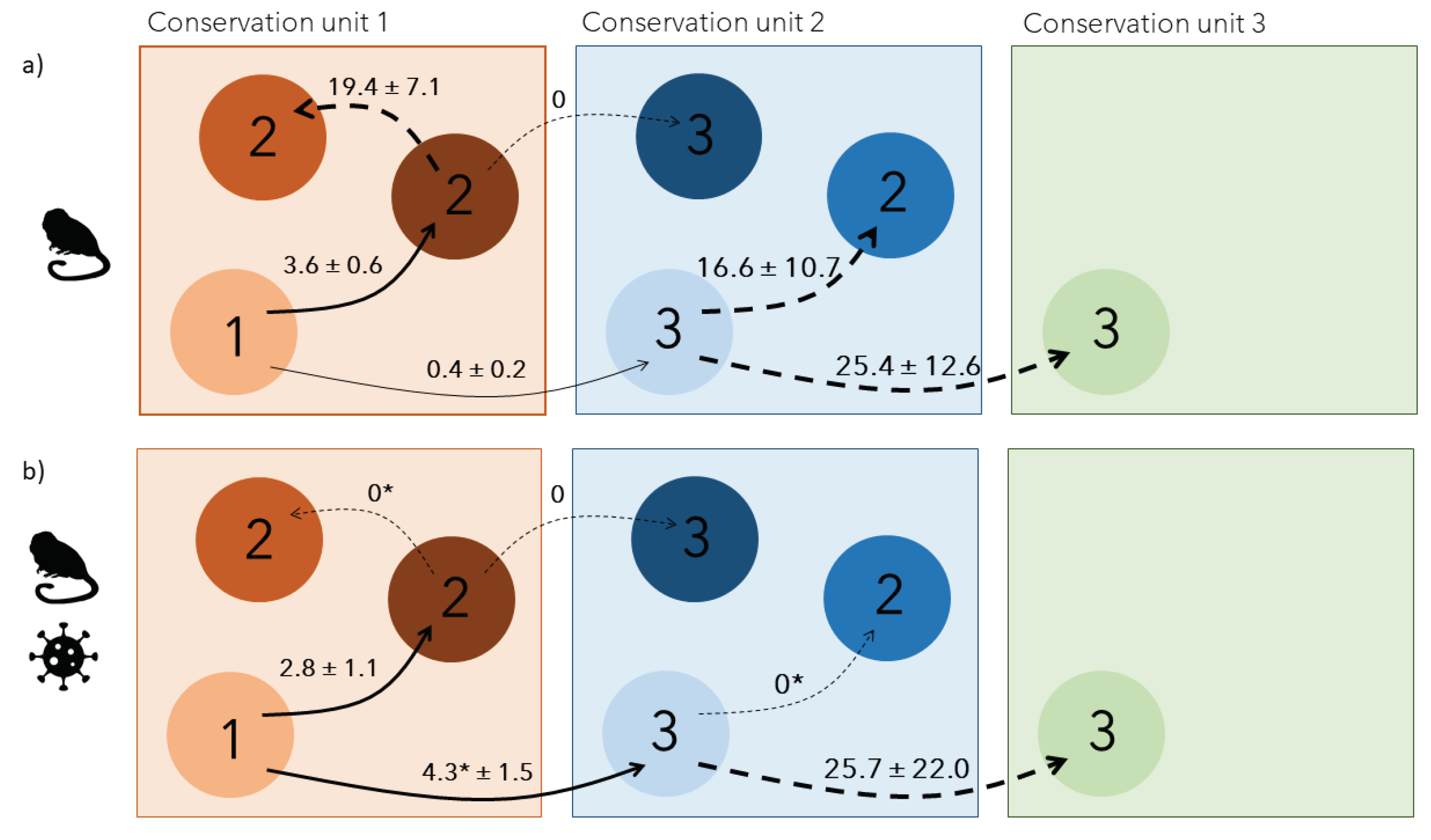
Dispersal was best modelled with 2 sets of distinct dispersal rates (2T;
Table 3): one before/after the outbreak period, in 2012-2016/2019-2022 and one during the outbreak, in 2017-2018. Before the outbreak, dispersal rates were generally low: 3.6 ± 0.6 % of individuals performed a first dispersal event towards groups within the same conservation unit, while less than 0.5% dispersed to groups outside their current conservation unit (
Figure 3a). After first dispersal within the same conservation unit, 19.3 ± 7.1 % of individuals continued dispersing to another group of the same conservation unit the following year, while none continued to another conservation unit. For individuals reaching a new conservation unit at first dispersal, 25.4 ± 12.6 % continued dispersing to another conservation unit the following year while 16.6 ± 10.7% dispersed within the same conservation unit (
Figure 3a).
During the outbreak, the total dispersal probability remained low but the spatial dispersal patterns changed (
Figure 3b). While individuals dispersing towards groups of the same conservation unit slightly decreased to 2.8 ± 1.1%, individuals dispersing to a new conservation unit increased by 10-fold, reaching 4.3 ± 1.5%. Once individuals had dispersed once, they either settled for good in their new group or continued dispersing towards a new conservation unit only if they had already changed conservation unit (
Figure 3b). After the outbreak, dispersal rates came back to the same levels as before, as the best selected model included two periods, outbreak (2017-2018) and before/after the outbreak (2011-2016/ 2019-2022;
Table 3).
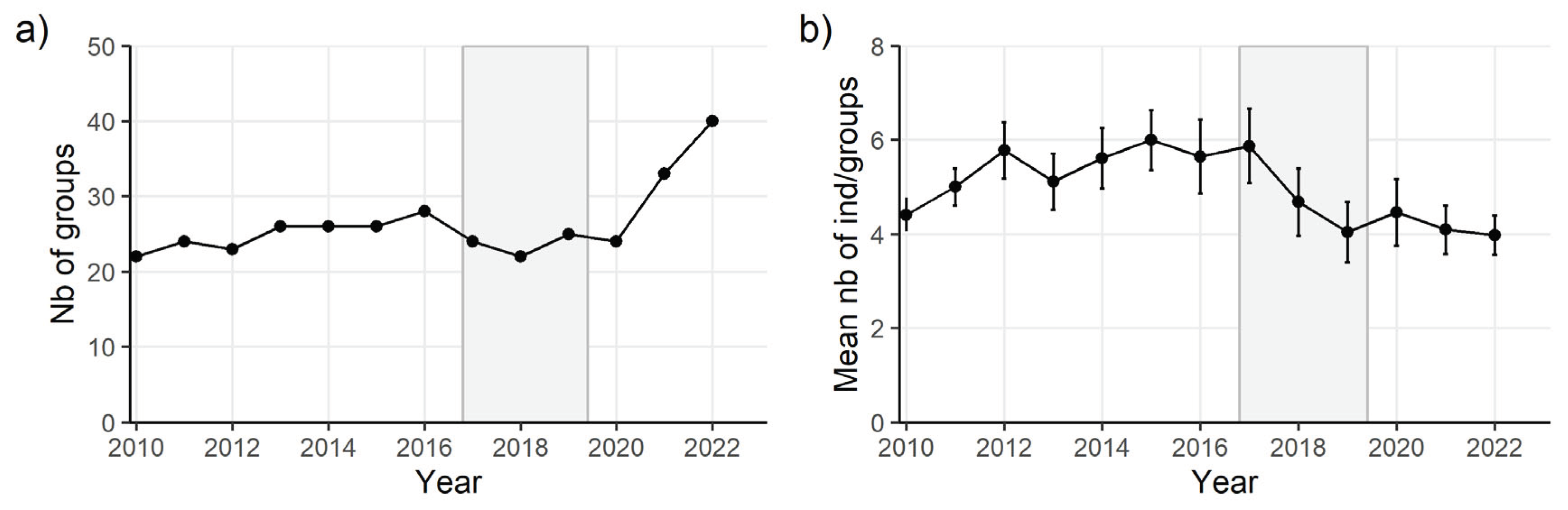
When looking at annual social group dynamics, the number of monitored groups remained relatively stable before and during the outbreak (
Figure 4a) but the number of individuals composing groups significantly decreased during the outbreak (
Figure 4b). After the disease, the number of groups substantially increased while the mean number of individuals in groups remained stable (
Figure 4).
Resighting probabilities depended on individual state: all individuals were always resighted when they remained faithful to their initial conservation area, regardless of local dispersal (p=1). However, after dispersing to a new conservation region, the resighting probability of an individual decreased to 56.5 ± 12.8%.
Discussion
Vector-borne diseases cause major health issues to human and animal populations. Understanding their eco-epidemiological dynamics is key in predicting their emergence but it is hampered by the complexity of ecological interactions between hosts and vectors (Chala & Hamde 2021). Here, we inferred individual host movements within and between forest fragments from long-term capture-recapture data to examine primates’ potential response to a disease outbreak. We revealed that adult annual survival of golden lion tamarins was reduced during an important outbreak of yellow fever. This temporary drop in survival was concomitant to a major temporary change in the dispersal patterns of individuals, both at a local and regional scale. Temporary alteration of host movements have already been demonstrated in previous studies but only for directly-transmitted diseases (Dekelaita et al. 2023; Genton et al. 2015; Lachish et al. 2011).
Dispersal probabilities within the same conservation unit remained relatively stable and low over the entire study period (2.8 ± 1.1% during the outbreak vs. 3.6 ± 0.6 % outside outbreak), as did the total number of groups. Contrastingly, the number of individuals composing those groups significantly decreased during the outbreak. This suggests a non-random disease-induced mortality within social groups which affected group size but not group density. This non-random mortality is supported by the extremely variable annual survival probabilities of a second cohort, mostly assumed to be younger individuals at the time of entering the survey in 2016 and 2017 (
Figure 2). With fewer individuals composing social groups, it might have been easier for local dispersing individuals to integrate other existing groups within the same conservation unit because of lower competition to acquire a new breeding status (Lachish et al. 2011; Muths et al. 2011). Given the simultaneous increase in regional dispersal rate, those locally dispersing individuals may have had higher competitive abilities, forcing other dispersing individuals to find groups at larger spatial scales. As a result, local highly competitive individuals would settle more rapidly after the first dispersal, over shorter distances, leading to a temporary disappearance of secondary local dispersal during the outbreak.
Males and females had the same dispersal probability, as sex was an uninformative parameter. This aligns with previous findings indicating that both males and females disperse (Romano et al. 2019). Nevertheless, the general primary dispersal rates < 5% are quite low compared to dispersal event counts for the same species, possibly because we only examined adult dispersal with individuals older than 18 months. By doing so, we excluded potential parallel dispersal of parents with younger individuals (Romano et al. 2019). Individuals may also have actively left their social group to self-isolate and become solitary to avoid disease exposure and/or transmission (Genton et al. 2015). If so, this response may have been temporary and shorter than a year so undetected with our data, as all individuals were resighted in a social group at least once a year including during the outbreak (constant detection probability=1 when individuals remain in the same conservation unit).
In normal conditions, dispersal between conservation units was negligible but increased by 10 times to reach 4.3% during the yellow fever outbreak. It even became more important than local dispersal. This increase might be explained by two non-exclusive hypotheses: 1) individuals dispersing regionally may have actively decided to escape from areas highly affected by yellow fever and settle in other potentially safer forest fragments and 2) individuals may not have had sufficient competitive abilities to secure a new social status in surviving local groups so they would have to seek other groups at larger distances and over longer periods, as reflected by the high probability of dispersing again after a first dispersal.
Mortality rates due to yellow fever in primates are relatively high (Possamai et al. 2022) and surviving individuals are likely to present a lower body condition, which may directly reduce their movement ability and thereby, their dispersal ability (Binning et al. 2017; Dekelaita et al. 2023; Lachish et al. 2011). Infected golden lion tamarins are therefore unlikely to vehiculate the disease through dispersal movements, especially at large spatial scales. At a local scale, i.e., within conservation units, the low dispersal probabilities suggest that the potential spread of yellow fever towards other groups through host movements is relatively limited as well.
Because of the irregular monitoring effort within and between years, we had to summarise individual observations to annual ones, missing potential shorter movements towards other groups where individuals could have spread the virus (e.g., (Baker & Dietz 1996). As observed in the raw data, individuals can perform prospecting movements, during which they temporally visit other groups than their current one and gather information which help them make informed dispersal decisions (Ponchon 2024). Those movements have been identified as crucial in an eco-epidemiological context, as they enhance connectivity between social groups and facilitate disease spread (Boulinier et al. 2016). Nevertheless, transmission of vector-borne diseases during host transience mainly depends on the presence of infectious vectors in the environment and the duration of exposure of hosts to vectors (Stoddard et al. 2009). Because prospecting visits are assumed to be relatively short in primates (minutes to hours ; Teichroeb et al. 2011), it is unlikely that such movements may have accelerated the spread of the disease to other groups, especially at a regional scale.
Vector movements are generally acknowledged as the principal mode of propagation of vector-borne diseases, while animal host movements are responsible for introduction to new areas (Sumner et al. 2017). In Brazil, studies have confirmed that yellow fever rapid spread was facilitated by the dispersal of vector mosquitoes, especially when those vectors were transiting in areas with roads adjacent to forests and forest edges going along agricultural areas (Ribeiro Prist et al. 2022). Landscape connectivity is therefore extremely important in vector-host-pathogen interactions: whereas roads and habitat fragmentation could hamper primate host movements between conservation units and thereby negatively affect their population dynamics (Arce-Peña et al. 2019), landscape degradation and fragmentation could facilitate disease spread through vector movements (Ribeiro Prist et al. 2022; Watts et al. 2018). Restoring habitat connectivity is thus key not only for the conservation of primate host species but also to limit the spread of vector-borne diseases over large spatial scales and over short periods (McCallum & Dobson 2002).
Even though the gold lion tamarin population was dramatically hit by yellow fever, annual adult survival rate started increasing again one year before the reported end of the outbreak in April 2019. It reached rates > 0.9 the following years, even higher rates than the ones recorded before the outbreak (
Figure 2). Along with the strong increase in the number of monitored social groups and low dispersal probabilities, this suggests that new individuals (either new adults born locally during/after the outbreak or immigrants from non-monitored area)- entered the population by forming new groups rather than competing to integrate existing groups. This highlights the resilience potential of the golden lion tamarin population after a high mortality event due to a disease outbreak, as already reported in other primate species which recovered relatively quickly after dramatic outbreaks of directly-transmitted pathogens (Caillaud et al. 2006; Genton et al. 2015). Social flexibility has been highlighted as a key adaptive response to unpredictable environments (Schradin et al. 2019). Our study provides new evidence that behavioural changes through dispersal decisions can also be an adaptive response to outbreaks. Detecting those changes will be vital to prevent or respond to outbreaks which may further spill-over to humans.
Ethical note
This study relies on individual data from the Golden Lion Tamarin project which complied with Brazilian, United States, and International laws. The data were collected under permits from the Brazilian National Research Council (CNPq, PO 08/92 and 77/98) and the methods for data collection were approved by the Animal Welfare committee of the Smithsonian’s National Zoological Park (Memorandum of September 23, 1991).
Acknowledgments
We thank the Associação Mico-Leão-Dourado (AMLD) for logistic support and AMLD staff for conducting the long-term survey over all these years. We also thank Romain Monassier for helping check individual sex and life stage in the raw data. AP benefited from a postdoctoral grant from IRD and AOI-Mission au Sud 2023 from IMBE-IRD.
References
- Abreu, F.V.S. de, Ribeiro, I.P., Ferreira-de-Brito, A., Santos, A.A.C. dos, Miranda, R.M. de, Bonelly, I. de S., et al. (2019). Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect., 8, 218–231.
- Arce-Peña, N.P., Arroyo-Rodríguez, V., Dias, P.A.D., Franch-Pardo, I. & Andresen, E. (2019). Linking changes in landscape structure to population changes of an endangered primate. Landsc. Ecol., 34, 2687–2701. 2701.
- Baker, A.J.; Dietz, J.M. Immigration in wild groups of golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 1996, 38, 47–56. [Google Scholar] [CrossRef]
- Bermejo, M., Rodríguez-Teijeiro, J.D., Illera, G., Barroso, A., Vilà, C. & Walsh, P.D. Ebola Outbreak Killed 5000 Gorillas. Science 2006, 314, 1564–1564. [Google Scholar] [CrossRef]
- Berthet, M., Mesbahi, G., Duvot, G., Zuberbühler, K., Cäsar, C. & Bicca-Marques, J.C. Dramatic decline in a titi monkey population after the 2016–2018 sylvatic yellow fever outbreak in Brazil. Am. J. Primatol. 2021, 83, e23335. [Google Scholar] [CrossRef] [PubMed]
- Bicca-Marques, J.C. & de Freitas, D.S. The Role of Monkeys, Mosquitoes, and Humans in the Occurrence of a Yellow Fever Outbreak in a Fragmented Landscape in South Brazil: Protecting Howler Monkeys is a Matter of Public Health. Trop. Conserv. Sci. 2010, 3, 78–89. [Google Scholar] [CrossRef]
- Binning, S.A., Shaw, A.K. & Roche, D.G. Parasites and Host Performance: Incorporating Infection into Our Understanding of Animal Movement. Integr. Comp. Biol. 2017, 57, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Boulinier, T., Kada, S., Ponchon, A., Dupraz, M., Dietrich, M., Gamble, A., et al. Migration, Prospecting, Dispersal? What Host Movement Matters for Infectious Agent Circulation? Integr. Comp. Biol. 2016, 56, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P. & Anderson, D.R. Model selection and multimodel inference: a practical information-theoretic approach, 2nd ed.; Springer: New York, 2002. [Google Scholar]
- Caillaud, D., Levréro, F., Cristescu, R., Gatti, S., Dewas, M., Douadi, M., et al. Gorilla susceptibility to Ebola virus: the cost of sociality. Curr Biol 2006, 16, R489-91. [Google Scholar]
- Chala, B. & Hamde, F. Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9. [Google Scholar]
- Choquet, R., Lebreton, J.-D., Gimenez, O., Reboulet, A.-M. & Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating CApture–REcapture data. Ecography 2009a, 32, 1071–1074. [Google Scholar] [CrossRef]
- Choquet, R., Rouan, L. & Pradel, R. (2009b). Program E-Surge: software application for fitting multievent models. In: Modeling demographic processes in marked populations, Environmental and Ecological Statistics (eds. Thomson, D., Cooch, E. & Conroy, M.). Springer US, pp. 845–865. 845–865.
- Dekelaita, D.J., Epps, C.W., German, D.W., Powers, J.G., Gonzales, B.J., Abella-Vu, R.K., et al. Animal movement and associated infectious disease risk in a metapopulation. R. Soc. Open Sci. 2023, 10, 220390. [Google Scholar] [CrossRef]
- Dietz, J.M; Dietz, J.M., Hankerson, S.J., Alexandre, B.R., Henry, M.D., Martins, A.F., Ferraz, L.P., et al. Yellow fever in Brazil threatens successful recovery of endangered golden lion tamarins. Sci. Rep. 2019, 9, 12926. [Google Scholar] [CrossRef]
- Dougherty, E.R., Seidel, D.P., Carlson, C.J., Spiegel, O. & Getz, W.M. Going through the motions: incorporating movement analyses into disease research. Ecol. Lett. 2018, 21, 588–604. [Google Scholar] [CrossRef]
- Estrada, A., Garber, P.A., Rylands, A.B., Roos, C., Fernandez-Duque, E., Di Fiore, A., et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv 2017, 3, e1600946. [Google Scholar] [CrossRef] [PubMed]
- Gaitan, J. & Millien, V. Stress level, parasite load, and movement pattern in a small-mammal reservoir host for Lyme disease. Can. J. Zool. 2016, 94, 565–573. [Google Scholar] [CrossRef]
- Genton, C., Pierre, A., Cristescu, R., Lévréro, F., Gatti, S., Pierre, J.-S., et al. How Ebola impacts social dynamics in gorillas: a multistate modelling approach. J. Anim. Ecol. 2015, 84, 166–176. [Google Scholar] [CrossRef]
- Giovanetti Marta, de Mendonça Marcos Cesar Lima, Fonseca Vagner, Mares-Guia Maria Angélica, Fabri Allison, Xavier Joilson, et al. Yellow Fever Virus Reemergence and Spread in Southeast Brazil, 2016–2019. J. Virol. 2019, 94. [Google Scholar] [CrossRef]
- Gudelj, I. & White, K.A.J. Spatial heterogeneity, social structure and disease dynamics of animal populations. Theor. Popul. Biol. 2004, 66, 139–149. [Google Scholar] [CrossRef]
- Höner, O.P., Wachter, B., Goller, K.V., Hofer, H., Runyoro, V., Thierer, D., et al. The impact of a pathogenic bacterium on a social carnivore population. J. Anim. Ecol. 2012, 81, 36–46. [Google Scholar] [CrossRef]
- Jones, K.E., Patel, N.G., Levy, M.A., Storeygard, A., Balk, D., Gittleman, J.L., et al. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Kierulff, M.C.M., Ruiz-Miranda, C.R., de Oliveira, P.P., Beck, B.B., Martins, A., Dietz, J.M., et al. The Golden lion tamarin Leontopithecus rosalia: a conservation success story. Int. Zoo Yearb. 2012, 46, 36–45. [Google Scholar] [CrossRef]
- Lachish, S., Miller, K.J., Storfer, A., Goldizen, A.W. & Jones, M.E. Evidence that disease-induced population decline changes genetic structure and alters dispersal patterns in the Tasmanian devil. Heredity 2011, 106, 172–182. [Google Scholar] [CrossRef]
- Lagrange, P., Pradel, R., Bélisle, M. & Gimenez, O. Estimating dispersal among numerous sites using capture–recapture data. Ecology 2014, 95, 2316–2323. [Google Scholar] [CrossRef]
- Langwig, K.E., Frick, W.F., Bried, J.T., Hicks, A.C., Kunz, T.H. & Marm Kilpatrick, A. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol. Lett. 2012, 15, 1050–1057. [Google Scholar] [CrossRef]
- Leendertz, F.H., Ellerbrok, H., Boesch, C., Couacy-Hymann, E., Mätz-Rensing, K., Hakenbeck, R., et al. Anthrax kills wild chimpanzees in a tropical rainforest. Nature 2004, 430, 451–452. [Google Scholar] [CrossRef]
- Loveridge, A.J. & Macdonald, D.W. Seasonality in spatial organization and dispersal of sympatric jackals (Canis mesomelas and C. adustus): implications for rabies management. J. Zool. 2001, 253, 101–111. [Google Scholar] [CrossRef]
- McCallum, H. & Dobson, A. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 2041–2049. [Google Scholar] [CrossRef]
- Morrison, R.E., Mushimiyimana, Y., Stoinski, T.S. & Eckardt, W. Rapid transmission of respiratory infections within but not between mountain gorilla groups. Sci. Rep. 2021, 11, 19622. [Google Scholar] [CrossRef]
- Muths, E., Scherer, R.D. & Pilliod, D.S. Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J. Appl. Ecol. 2011, 48, 873–879. [Google Scholar] [CrossRef]
- Nunn, C.L., Thrall, P.H., Stewart, K. & Harcourt, A.H. Emerging infectious diseases and animal social systems. Evol. Ecol. 2008, 22, 519–543. [Google Scholar] [CrossRef]
- Ponchon, A. (2024). Prospecting for informed dispersal: reappraisal of a widespread but overlooked ecological process. Preprints.
- Possamai, C.B., Rodrigues de Melo, F., Mendes, S.L. & Strier, K.B. Demographic changes in an Atlantic Forest primate community following a yellow fever outbreak. Am. J. Primatol. 2022, 84, e23425. [Google Scholar] [CrossRef]
- Possas, C., Lourenço-de-Oliveira, R., Tauil, P.L., Pinheiro, F. de P., Pissinatti, A., Cunha, R.V. da, et al. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 113. [Google Scholar]
- Ribeiro Prist, P., Reverberi Tambosi, L., Filipe Mucci, L., Pinter, A., Pereira de Souza, R., de Lara Muylaert, R., et al. Roads and forest edges facilitate yellow fever virus dispersion. J. Appl. Ecol. 2022, 59, 4–17. [Google Scholar] [CrossRef]
- Romano, V., Lussiana, A., Monteith, K.M., MacIntosh, A.J.J. & Vale, P.F. Host genetics and pathogen species modulate infection-induced changes in social aggregation behaviour. Biol. Lett. 2022, 18, 20220233. [Google Scholar] [CrossRef] [PubMed]
- Romano, V., Martins, A.F. & Ruiz-Miranda, C.R. Unraveling the dispersal patterns and the social drivers of natal emigration of a cooperative breeding mammal, the golden lion tamarin. Am. J. Primatol. 2019, 81, e22959. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Miranda, C.R., de Morais, M.M., Jr., Dietz, L.A., Rocha Alexandre, B., Martins, A.F., Ferraz, L.P., et al. Estimating population sizes to evaluate progress in conservation of endangered golden lion tamarins (Leontopithecus rosalia). PLOS ONE 2019, 14, e0216664. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, L., Drumond, B.P., Han, B.A., Nogueira, M.L. & Vasilakis, N. Re-emergence of yellow fever in the neotropics — quo vadis? Emerg. Top. Life Sci. 2020, 4, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Schradin, C., Pillay, N. & Bertelsmeier, C. Social flexibility and environmental unpredictability in African striped mice. Behav. Ecol. Sociobiol. 2019, 73, 94. [Google Scholar] [CrossRef]
- Smith, K.F., Acevedo-Whitehouse, K. & Pedersen, A.B. The role of infectious diseases in biological conservation. Anim. Conserv. 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Stockmaier, S., Stroeymeyt, N., Shattuck, E.C., Hawley, D.M., Meyers, L.A. & Bolnick, D.I. Infectious diseases and social distancing in nature. Science 2021, 371, eabc8881. [Google Scholar] [CrossRef]
- Stoddard, S.T., Morrison, A.C., Vazquez-Prokopec, G.M., Paz Soldan, V., Kochel, T.J., Kitron, U., et al. The Role of Human Movement in the Transmission of Vector-Borne Pathogens. PLoS Negl. Trop. Dis. 2009, 3, e481. [Google Scholar] [CrossRef]
- Strier, K.B., Tabacow, F.P., de Possamai, C.B., Ferreira, A.I.G., Nery, M.S., de Melo, F.R., et al. Status of the northern muriqui (Brachyteles hypoxanthus) in the time of yellow fever. Primates 2019, 60, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sumner, T. Sumner, T., Orton, R.J., Green, D.M., Kao, R.R. & Gubbins, S. (2017). Quantifying the roles of host movement and vector dispersal in the transmission of vector-borne diseases of livestock. PLOS Comput. Biol.
- Teichroeb, J.A., Wikberg, E.C. & Sicotte, P. (2011). Dispersal in male ursine colobus monkeys (Colobus vellerosus): influence of age, rank and contact with other groups on dispersal decisions. Behaviour, 148, 765–793.
- Walsh, P.D. Walsh, P.D., Abernethy, K.A., Bermejo, M., Beyers, R., De Wachter, P., Akou, M.E., et al. (2003). Catastrophic ape decline in western equatorial Africa. Nature.
- Watts, A.G. Watts, A.G., Saura, S., Jardine, C., Leighton, P., Werden, L. & Fortin, M.-J. (2018). Host functional connectivity and the spread potential of Lyme disease. Landsc. Ecol. 1938. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).