Submitted:
28 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
The World Health Organization declared the coronavirus disease 2019 (COVID-19) pandemic in 2020, following which a global genetic vaccination program has been rapidly implemented as a fundamental solution. However, it has been reported worldwide that the modified mRNAs encoding spike proteins and lipid nanoparticles, which are used as drug delivery systems, not only cause thrombosis and cardiovascular disorders post vaccination, but might also cause diverse diseases involving all organs and systems, including the nervous system. Furthermore, the toxicity and pathogenicity of spike proteins may necessitate defining these proteins as nonbiological infective material. Based on these reports and the abundant evidence that has come to light in the past few years, this paper aims to draw the attention of medical professionals to the various risks associated with transfusion using blood products derived from long COVID patients or from genetic vaccine recipients, and to make proposals regarding specific inspection items, testing methods, regulations, etc. This paper provides insights to address the post-vaccination syndrome and its consequences following such genetic vaccination programs.
Keywords:
1. Introduction
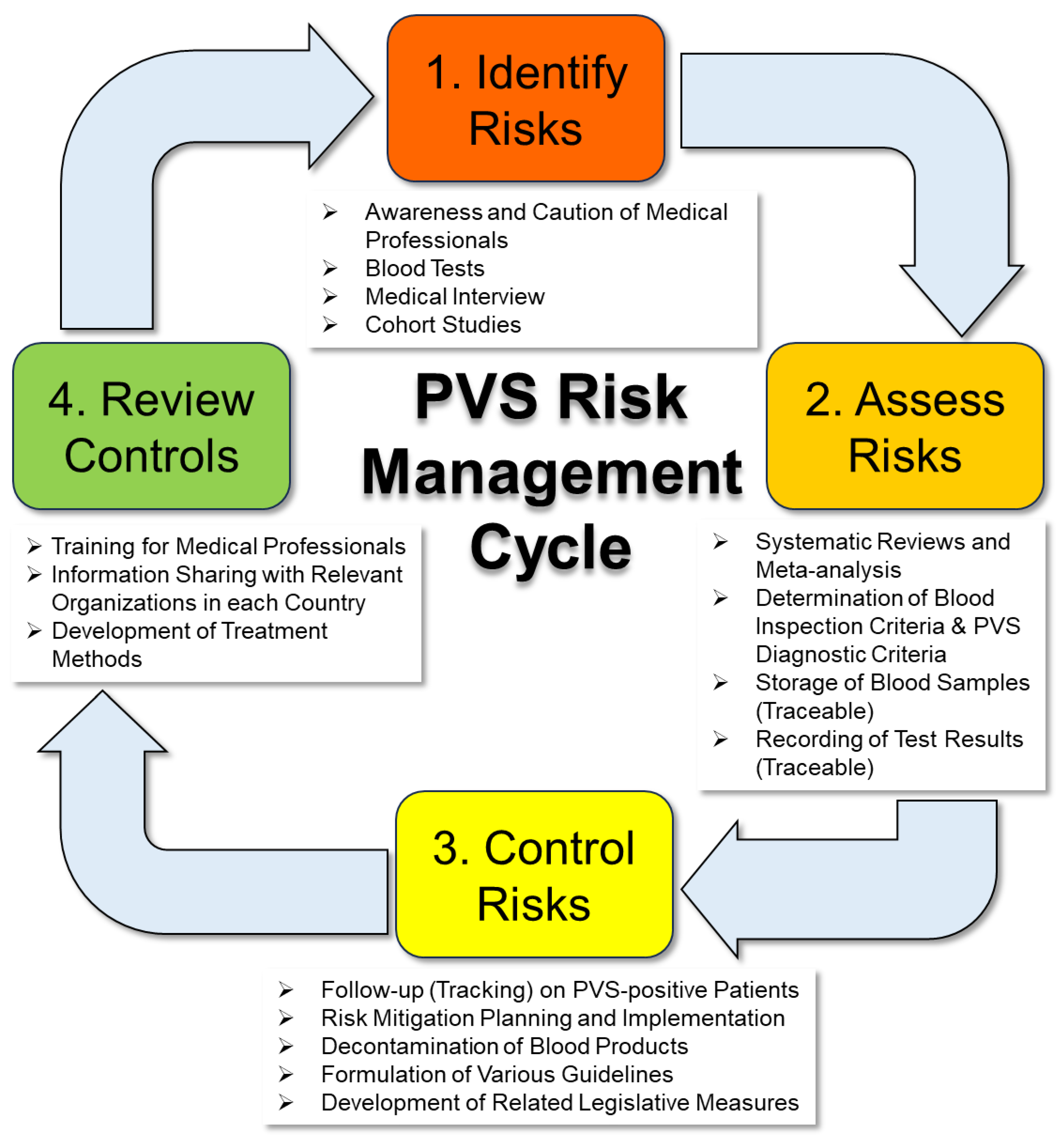
2. Overview of Cases of Blood Abnormalities after Vaccination with Genetic Vaccines
3. Specific Proposals for Blood Sampling and Use of Blood Products from Vaccine Recipients
3.1. Additional Requirements for Blood Collection (Donation)
3.2. Handling of Existing Blood Products
3.3. Need for Regular Checkups and Cohort Studies to Gain a Complete Picture of Blood Components
3.4. Need for Expedited Development of Clinical Practice Guidelines and Diagnostic Criteria for COVID-19 PVS
4. Effects of Blood Transfusion from Genetic Vaccine Recipients and the Need for Traceability of Blood Products for Transfusion
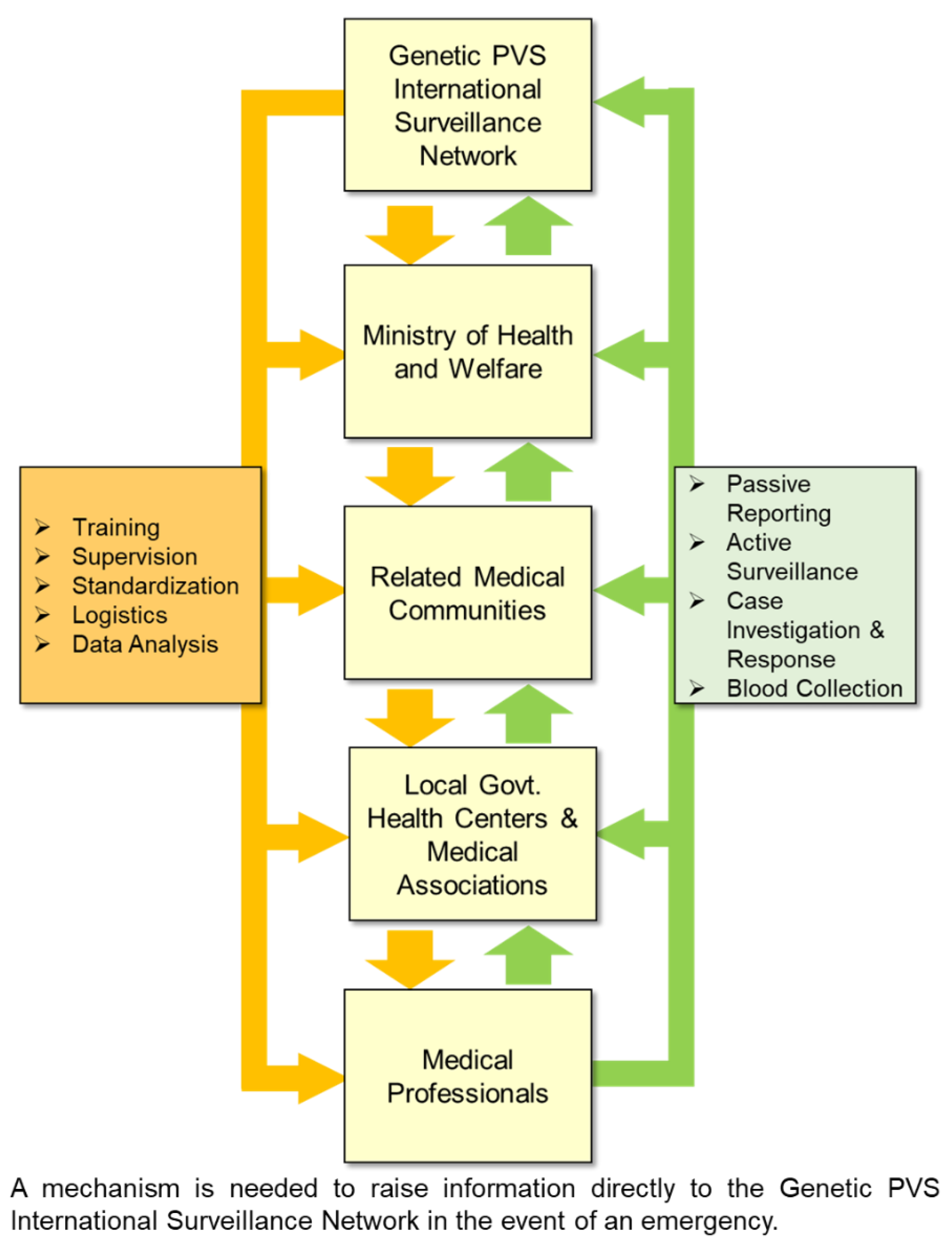
5. Need for the Development of Relevant Legislation
6. Further Considerations
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O'Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. , World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). International Journal of Surgery 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Francis, A. I.; Ghany, S.; Gilkes, T.; Umakanthan, S. , Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgraduate Medical Journal 2022, 98, 389–94. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V. S.; Kahar, P.; Khanna, D. , A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Human Vaccines & Immunotherapeutics 2022, 18.
- Harrison, A. G.; Lin, T.; Wang, P. , Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends in Immunology 2020, 41, 1100–15. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M. M.; Haagmans, B. L. , SARS-CoV-2 pathogenesis. Nature Reviews Microbiology 2022, 20, 270–84. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; Wang, X. , Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–20. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; Zhang, S.; Fan, Z.; Dong, J.; Yuan, Z.; Ding, Z.; Zhang, Y.; Hu, L. , SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of Hematology & Oncology 2020, 13.
- Berzuini, A.; Bianco, C.; Migliorini, A. C.; Maggioni, M.; Valenti, L.; Prati, D. , Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus 2021, 19, 34–36. [Google Scholar] [PubMed]
- Melkumyants, A.; Buryachkovskaya, L.; Lomakin, N.; Antonova, O.; Serebruany, V. , Mild COVID-19 and Impaired Blood Cell–Endothelial Crosstalk: Considering Long-Term Use of Antithrombotics? Thrombosis and Haemostasis 2021, 122, 123–30. [Google Scholar] [CrossRef]
- Boschi, C.; Scheim, D. E.; Bancod, A.; Militello, M.; Bideau, M. L.; Colson, P.; Fantini, J.; Scola, B. L. , SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- Scheim, D. E. , A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- McFadyen, J. D.; Stevens, H.; Peter, K. , The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circulation Research 2020, 127, 571–87. [Google Scholar] [CrossRef]
- 13. Grobbelaar, Lize M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, Gert J.; Lourens, Petrus J.; Steenkamp, J.; Kell, Douglas B.; Pretorius, E., SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Bioscience Reports 2021, 41.
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G. F.; Uzzo, M. L.; Argo, A.; Zerbo, S. , COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front Immunol 2021, 12, 729251. [Google Scholar] [CrossRef] [PubMed]
- Garg, R. K.; Paliwal, V. K. , Spectrum of neurological complications following COVID-19 vaccination. Neurological Sciences 2021, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R. E.; Pötzsch, B.; Greinacher, A. , Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hämostaseologie 2021, 41, (03), 184-89.
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. , Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci 2021, 428, 117607. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Yeomans, A.; Shakir, S. , Reports of myocarditis and pericarditis following mRNA COVID-19 vaccination: a systematic review of spontaneously reported data from the UK, Europe and the USA and of the scientific literature. BMJ Open 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Oster, M. E.; Shay, D. K.; Su, J. R.; Gee, J.; Creech, C. B.; Broder, K. R.; Edwards, K.; Soslow, J. H.; Dendy, J. M.; Schlaudecker, E.; Lang, S. M.; Barnett, E. D.; Ruberg, F. L.; Smith, M. J.; Campbell, M. J.; Lopes, R. D.; Sperling, L. S.; Baumblatt, J. A.; Thompson, D. L.; Marquez, P. L.; Strid, P.; Woo, J.; Pugsley, R.; Reagan-Steiner, S.; DeStefano, F.; Shimabukuro, T. T. , Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. Jama 2022, 327. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A. R.; Asghar, M. S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; Fadelallah Eljack, M. M.; Tahir, M. J.; Waqar, F. , Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun Inflamm Dis 2023, 11, e807. [Google Scholar] [CrossRef] [PubMed]
- Konishi, N.; Hirai, Y.; Hikota, H.; Miyahara, S.; Fujisawa, A.; Motohashi, H.; Ueda, J.; Inoue, M.; Fukushima, M. , Quantifying side effects of COVID-19 vaccines: A PubMed survey of papers on diseases as side effects presented at academic conferences in Japan. Rinsho Hyoka (Clinical Evaluation) 2024; 51. [Google Scholar]
- Parry, P. I.; Lefringhausen, A.; Turni, C.; Neil, C. J.; Cosford, R.; Hudson, N. J.; Gillespie, J. , ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B. Z. , The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24. [Google Scholar]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; Lindquist, J.; Kjellman, T.; Mårtensson, I.-L.; Jin, T.; Sunnerhagen, P.; Östman, S.; Lindfors, L.; Valadi, H. , Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nature Communications 2019, 10. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R. M.; Mohanakumar, T. , Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. The Journal of Immunology 2021, 207, 2405–10. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A. M.; McCullough, P. A. , Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol 2022, 164, 113008. [Google Scholar] [CrossRef]
- Polykretis, P.; Donzelli, A.; Lindsay, J. C.; Wiseman, D.; Kyriakopoulos, A. M.; Mörz, M.; Bellavite, P.; Fukushima, M.; Seneff, S.; McCullough, P. A. , Autoimmune inflammatory reactions triggered by the COVID-19 genetic vaccines in terminally differentiated tissues. Autoimmunity 2023, 56. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. , Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Appl 2023, 17, e2300048. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L. M.; Swank, Z.; Bartsch, Y. C.; Burns, M. D.; Kane, A.; Boribong, B. P.; Davis, J. P.; Loiselle, M.; Novak, T.; Senussi, Y.; Cheng, C. A.; Burgess, E.; Edlow, A. G.; Chou, J.; Dionne, A.; Balaguru, D.; Lahoud-Rahme, M.; Arditi, M.; Julg, B.; Randolph, A. G.; Alter, G.; Fasano, A.; Walt, D. R. , Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–76. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X. M.; Shuai, Z. W.; Ye, D. Q.; Pan, H. F. , New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Polykretis, P.; McCullough, P. A. , Rational harm-benefit assessments by age group are required for continued COVID-19 vaccination. Scandinavian Journal of Immunology 2022, 98. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R. M.; Doshi, P. , Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–805. [Google Scholar] [CrossRef] [PubMed]
- Bardosh, K.; Krug, A.; Jamrozik, E.; Lemmens, T.; Keshavjee, S.; Prasad, V.; Makary, M. A.; Baral, S.; Høeg, T. B. , COVID-19 vaccine boosters for young adults: a risk benefit assessment and ethical analysis of mandate policies at universities. Journal of Medical Ethics 2024, 50, 126–38. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, S. J.; New, H. V.; Apelseth, T. O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; Shehata, N.; So-Osman, C.; Thachil, J. , Effects of the COVID-19 pandemic on supply and use of blood for transfusion. The Lancet Haematology 2020, 7, e756–e64. [Google Scholar] [CrossRef]
- Chang, L.; Yan, Y.; Wang, L. , Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfusion Medicine Reviews 2020, 34, 75–80. [Google Scholar] [CrossRef]
- Bouhou, S.; Lahjouji, K.; Masrar, A. , Blood donor eligibility after COVID-19 vaccination: the current state of recommendations. Pan Afr Med J 2021, 40, 207. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J. W.; Bibb, L. A.; Savani, B. N.; Booth, G. S. , Refusing blood transfusions from COVID-19-vaccinated donors: are we repeating history? British Journal of Haematology 2021, 196, 585–88. [Google Scholar] [CrossRef] [PubMed]
- Hunain, R.; Uday, U.; Rackimuthu, S.; Nawaz, F. A.; Narain, K.; Essar, M. Y.; Rehman, M. U.; Ahmad, S.; Butt, A. , Effects of SARS-CoV-2 vaccination on blood donation and blood banks in India. Ann Med Surg (Lond) 2022, 78, 103772. [Google Scholar] [CrossRef]
- Roubinian, N. H.; Greene, J.; Liu, V. X.; Lee, C.; Mark, D. G.; Vinson, D. R.; Spencer, B. R.; Bruhn, R.; Bravo, M.; Stone, M.; Custer, B.; Kleinman, S.; Busch, M. P.; Norris, P. J. , Clinical outcomes in hospitalized plasma and platelet transfusion recipients prior to and following widespread blood donor SARS-CoV-2 infection and vaccination. Transfusion 2023, 64, 53–67. [Google Scholar] [CrossRef]
- Fertig, T. E.; Chitoiu, L.; Marta, D. S.; Ionescu, V.-S.; Cismasiu, V. B.; Radu, E.; Angheluta, G.; Dobre, M.; Serbanescu, A.; Hinescu, M. E.; Gherghiceanu, M. , Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Mörz, M. , A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kase, M.; Sano, H.; Kamijima, R.; Sano, S. , Persistent varicella zoster virus infection following mRNA COVID-19 vaccination was associated with the presence of encoded spike protein in the lesion. Journal of Cutaneous Immunology and Allergy 2022, 6, 18–23. [Google Scholar] [CrossRef]
- Castruita, J. A. S.; Schneider, U. V.; Mollerup, S.; Leineweber, T. D.; Weis, N.; Bukh, J.; Pedersen, M. S.; Westh, H. , SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS 2023, 131, 128–32. [Google Scholar] [CrossRef]
- Krauson, A. J.; Casimero, F. V. C.; Siddiquee, Z.; Stone, J. R. , Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines 2023, 8, 141. [Google Scholar] [CrossRef]
- Sano, S.; Yamamoto, M.; Kamijima, R.; Sano, H. , SARS-CoV-2 spike protein found in the acrosyringium and eccrine gland of repetitive miliaria-like lesions in a woman following mRNA vaccination. J Dermatol 2024. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. , mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Bitounis, D.; Jacquinet, E.; Rogers, M. A.; Amiji, M. M. , Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov 2024. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. , Adverse effects of COVID-19 vaccines and measures to prevent them. Virology Journal 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Y.; Rojas, M.; Beltran, S.; Polo, F.; Camacho-Dominguez, L.; Morales, S. D.; Gershwin, M. E.; Anaya, J. M. , Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J Autoimmun 2022, 132, 102898. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Galbusera, M.; Pezzotta, A.; Gastoldi, S.; Imberti, B.; Perna, A.; Ruggenenti, P.; Donadelli, R.; Benigni, A.; Remuzzi, G. , SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front Immunol 2022, 13, 827146. [Google Scholar] [CrossRef] [PubMed]
- Idrees, D.; Kumar, V. , SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochemical and Biophysical Research Communications 2021, 554, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Charnley, M.; Islam, S.; Bindra, G. K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M. D.; Han, K.; Berryman, J. T.; Reynolds, N. P. , Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nature Communications 2022, 13. [Google Scholar] [CrossRef]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G. J.; Kell, D. B.; Pretorius, E. , Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovascular Diabetology 2022, 21. [Google Scholar] [CrossRef] [PubMed]
- Nyström, S.; Hammarström, P. , Amyloidogenesis of SARS-CoV-2 Spike Protein. Journal of the American Chemical Society 2022, 144, 8945–50. [Google Scholar] [CrossRef]
- Chesney, A. D.; Maiti, B.; Hansmann, U. H. E. , SARS-COV-2 spike protein fragment eases amyloidogenesis of alpha-synuclein. J Chem Phys 2023, 159. [Google Scholar]
- Olajide, O. A.; Iwuanyanwu, V. U.; Adegbola, O. D.; Al-Hindawi, A. A. , SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Molecular Neurobiology 2021, 59, 445–58. [Google Scholar] [CrossRef]
- Oh, J.; Cho, W.-H.; Barcelon, E.; Kim, K. H.; Hong, J.; Lee, S. J. , SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Scientific Reports 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, B. C. V.; Weber, L.; Hueffer, K.; Weltzin, M. M. , SARS-CoV-2 spike ectodomain targets alpha7 nicotinic acetylcholine receptors. J Biol Chem 2023, 299, 104707. [Google Scholar]
- Buzhdygan, T. P.; DeOre, B. J.; Baldwin-Leclair, A.; Bullock, T. A.; McGary, H. M.; Khan, J. A.; Razmpour, R.; Hale, J. F.; Galie, P. A.; Potula, R.; Andrews, A. M.; Ramirez, S. H. , The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis 2020, 146, 105131. [Google Scholar] [CrossRef]
- Rhea, E. M.; Logsdon, A. F.; Hansen, K. M.; Williams, L. M.; Reed, M. J.; Baumann, K. K.; Holden, S. J.; Raber, J.; Banks, W. A.; Erickson, M. A. , The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nature Neuroscience 2020, 24, 368–78. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; Xu, Y.; Deng, W.; Gao, H.; Xue, J.; Song, Z.; Yu, P.; Han, Y.; Zhang, Y.; Sun, X.; Yu, X.; Qin, C. , SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduction and Targeted Therapy 2021, 6. [Google Scholar] [CrossRef]
- Trougakos, I. P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M. A. , Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends in Molecular Medicine 2022, 28, 542–54. [Google Scholar] [CrossRef] [PubMed]
- Halma, M. T. J.; Plothe, C.; Marik, P.; Lawrie, T. A. , Strategies for the Management of Spike Protein-Related Pathology. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Pastor-Barriuso, R.; Hernán, M. A. , The imprinting effect of covid-19 vaccines: an expected selection bias in observational studies. Bmj 2023. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Tam, A. R.; Valdez, R.; Gordon, A.; Liu, L.; Ho, D. D. , Deep immunological imprinting due to the ancestral spike in the current bivalent COVID-19 vaccine. Cell Rep Med 2023, 4, 101258. [Google Scholar] [CrossRef]
- Tortorici, M. A.; Addetia, A.; Seo, A. J.; Brown, J.; Sprouse, K.; Logue, J.; Clark, E.; Franko, N.; Chu, H.; Veesler, D. , Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans. Immunity 2024, 57, (4), 904-11 e4.
- Pusnik, J.; Zorn, J.; Monzon-Posadas, W. O.; Peters, K.; Osypchuk, E.; Blaschke, S.; Streeck, H. , Vaccination impairs de novo immune response to omicron breakthrough infection, a precondition for the original antigenic sin. Nat Commun 2024, 15, 3102. [Google Scholar] [CrossRef]
- Shrestha, N. K.; Burke, P. C.; Nowacki, A. S.; Simon, J. F.; Hagen, A.; Gordon, S. M. , Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine. Open Forum Infectious Diseases 2023, 10. [Google Scholar] [CrossRef]
- Arvin, A. M.; Fink, K.; Schmid, M. A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H. W. , A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–63. [Google Scholar] [CrossRef]
- Lee, W. S.; Wheatley, A. K.; Kent, S. J.; DeKosky, B. J. , Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 2020, 5, 1185–91. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; Ssebyatika, G.; Krey, T.; Falcone, V.; Schülein, C.; Peter, A. S.; Nganou-Makamdop, K.; Hengel, H.; Held, J.; Bogdan, C.; Überla, K.; Schober, K.; Winkler, T. H.; Tenbusch, M. , Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Science Immunology 2023, 8. [Google Scholar] [CrossRef]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. , Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Scientific Reports 2023, 13. [Google Scholar] [CrossRef]
- Uversky, V.; Redwan, E.; Makis, W.; Rubio-Casillas, A. , IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sakamoto, A.; Ozuru, R.; Kurihara, Y.; Itoh, R.; Ishii, K.; Shimizu, A.; Chou, B.; Nabeshima, S.; Hiromatsu, K. , The appearance of anti-spike receptor binding domain immunoglobulin G4 responses after repetitive immunization with messenger RNA-based COVID-19 vaccines. Int J Infect Dis 2024, 139, 1–5. [Google Scholar] [CrossRef]
- Espino, A. M.; Armina-Rodriguez, A.; Alvarez, L.; Ocasio-Malave, C.; Ramos-Nieves, R.; Rodriguez Martino, E. I.; Lopez-Marte, P.; Torres, E. A.; Sariol, C. A. , The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer-BioNTech Vaccine. Viruses 2024, 16. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Cowley, D.; Raszek, M.; Uversky, V. N.; Redwan, E. M. , Review: N1-methyl-pseudouridine (m1Psi): Friend or foe of cancer? Int J Biol Macromol 2024, (Pt 1) Pt 1, 131427. [Google Scholar] [CrossRef]
- Murata, K.; Nakao, N.; Ishiuchi, N.; Fukui, T.; Katsuya, N.; Fukumoto, W.; Oka, H.; Yoshikawa, N.; Nagao, T.; Namera, A.; Kakimoto, N.; Oue, N.; Awai, K.; Yoshimoto, K.; Nagao, M. , Four cases of cytokine storm after COVID-19 vaccination: Case report. Front Immunol 2022, 13, 967226. [Google Scholar] [CrossRef]
- Masset, C.; Kervella, D.; Kandel-Aznar, C.; Fantou, A.; Blancho, G.; Hamidou, M. , Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney International 2021, 100, 465–66. [Google Scholar] [CrossRef]
- Patel, A. H. , Acute Liver Injury and IgG4-related Autoimmune Pancreatitis following mRNA based COVID-19 vaccination. Hepatology Forum 2022. [Google Scholar] [CrossRef]
- Aochi, S.; Uehara, M.; Yamamoto, M. , IgG4-related Disease Emerging after COVID-19 mRNA Vaccination. Internal Medicine 2023, 62, 1547–51. [Google Scholar] [CrossRef]
- Katsikas Triantafyllidis, K.; Giannos, P.; Mian, I. T.; Kyrtsonis, G.; Kechagias, K. S. , Varicella Zoster Virus Reactivation Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2021, 9. [Google Scholar] [CrossRef]
- Lensen, R.; Netea, M. G.; Rosendaal, F. R. , Hepatitis C Virus Reactivation Following COVID-19 Vaccination - A Case Report. Int Med Case Rep J 2021, 14, 573–76. [Google Scholar]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. , Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9. [Google Scholar] [CrossRef]
- Fathy, R. A.; McMahon, D. E.; Lee, C.; Chamberlin, G. C.; Rosenbach, M.; Lipoff, J. B.; Tyagi, A.; Desai, S. R.; French, L. E.; Lim, H. W.; Thiers, B. H.; Hruza, G. J.; Fassett, M.; Fox, L. P.; Greenberg, H. L.; Blumenthal, K.; Freeman, E. E. , Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venereol 2022, 36, e6–e9. [Google Scholar] [CrossRef]
- Gringeri, M.; Battini, V.; Cammarata, G.; Mosini, G.; Guarnieri, G.; Leoni, C.; Pozzi, M.; Radice, S.; Clementi, E.; Carnovale, C. , Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines 2022, 21, 675–84. [Google Scholar] [CrossRef]
- Hertel, M.; Heiland, M.; Nahles, S.; von Laffert, M.; Mura, C.; Bourne, P. E.; Preissner, R.; Preissner, S. , Real-world evidence from over one million COVID-19 vaccinations is consistent with reactivation of the varicella-zoster virus. Journal of the European Academy of Dermatology and Venereology 2022, 36, 1342–48. [Google Scholar] [CrossRef]
- Shafiee, A.; Amini, M. J.; Arabzadeh Bahri, R.; Jafarabady, K.; Salehi, S. A.; Hajishah, H.; Mozhgani, S.-H. , Herpesviruses reactivation following COVID-19 vaccination: a systematic review and meta-analysis. European Journal of Medical Research 2023, 28. [Google Scholar] [CrossRef]
- Culver, J. , Preventing transmission of blood-borne pathogens: a compelling argument for effective device-selection strategies. Am J Infect Control 1997, 25, 430–3. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, E. M.; Williams, I. T.; Shapiro, C. N.; Chamberland, M. E. , Risk and Management of Blood-Borne Infections in Health Care Workers. Clinical Microbiology Reviews 2000, 13, 385–407. [Google Scholar] [CrossRef]
- Ison, M. G.; Grossi, P.; Practice, A. S. T. I. D. C. o. , Donor-derived infections in solid organ transplantation. Am J Transplant 2013, 13 Suppl 4, 22–30. [Google Scholar] [CrossRef]
- Fishman, J. A.; Grossi, P. A. , Donor-derived infection--the challenge for transplant safety. Nat Rev Nephrol 2014, 10, 663–72. [Google Scholar] [CrossRef]
- Bahakel, H. K.; Pellet Madan, R.; Danziger-Isakov, L. , Approach to suspected donor-derived infections. Front Pediatr 2023, 11, 1265023. [Google Scholar] [CrossRef]
- Tobin, G. J.; Trujillo, J. D.; Bushnell, R. V.; Lin, G.; Chaudhuri, A. R.; Long, J.; Barrera, J.; Pena, L.; Grubman, M. J.; Nara, P. L. , Deceptive imprinting and immune refocusing in vaccine design. Vaccine 2008, 26, 6189–99. [Google Scholar] [CrossRef]
- Gatto, D.; Brink, R. , The germinal center reaction. J Allergy Clin Immunol 2010, 126, (5), 898-907; quiz 08-9.
- Seneff, S.; Nigh, G. , Worse Than the Disease? Reviewing Some Possible Unintended Consequences of the mRNA Vaccines Against COVID-19. International Journal of Vaccine Theory, Practice, and Research 2021, 2, (1), 38-79.
- Bernardini, A.; Gigli, G. L.; Janes, F.; Pellitteri, G.; Ciardi, C.; Fabris, M.; Valente, M. , Creutzfeldt-Jakob disease after COVID-19: infection-induced prion protein misfolding? A case report. Prion 2022, 16, 78–83. [Google Scholar] [CrossRef]
- Lukiw, W. J.; Jaber, V. R.; Pogue, A. I.; Zhao, Y. , SARS-CoV-2 Invasion and Pathological Links to Prion Disease. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. , Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Makhoul, K.; Beeber, T.; Cordero, R.; Khan, A.; Saliaj, M. , Prion Disease After COVID-19: A Case Report. Am J Case Rep 2023, 24, e940564. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.-C.; Moret-Chalmin, C.; Montagnier, L. , Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection. International Journal of Vaccine Theory, Practice, and Research 2023, 3, (1), 727-70.
- Seneff, S.; Kyriakopoulos, A. M.; Nigh, G.; McCullough, P. A. , A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review. Cureus 2023. [Google Scholar] [CrossRef] [PubMed]
- Perez, J. C.; Lounnas, V.; Montagnier, M. , The Omicron Variant Breaks the Evolutionary Lineage of Sars-Cov2 Variants. International Journal of Research -GRANTHAALAYAH 2021, 9, (12), 108-32.
- Chapman, C. W., S. , Project risk management: Process, techniques and insight. Wiley: London, UK, 2003.
- Aven, T. , Risk assessment and risk management: Review of recent advances on their foundation. European Journal of Operational Research 2016, 253, 1–13. [Google Scholar] [CrossRef]
- Watson, N.; Brandel, J.-P.; Green, A.; Hermann, P.; Ladogana, A.; Lindsay, T.; Mackenzie, J.; Pocchiari, M.; Smith, C.; Zerr, I.; Pal, S. , The importance of ongoing international surveillance for Creutzfeldt–Jakob disease. Nature Reviews Neurology 2021, 17, 362–79. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S. M.; Simberg, D. , Pro-inflammatory concerns with lipid nanoparticles. Molecular Therapy 2022, 30, 2109–10. [Google Scholar] [CrossRef] [PubMed]
- Tahtinen, S.; Tong, A.-J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M. J.; Freund, E. C.; Amir, Z. A.; de la Cruz, C. C.; Haley, B.; Blanchette, C.; Schartner, J. M.; Ye, W.; Yadav, M.; Sahin, U.; Delamarre, L.; Mellman, I. , IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nature Immunology 2022, 23, 532–42. [Google Scholar] [CrossRef] [PubMed]
- Halma, M. T. J.; Rose, J.; Lawrie, T. , The Novelty of mRNA Viral Vaccines and Potential Harms: A Scoping Review. J 2023, 6, 220–35. [Google Scholar] [CrossRef]
- Bakos, T.; Meszaros, T.; Kozma, G. T.; Berenyi, P.; Facsko, R.; Farkas, H.; Dezsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; Glatter, K. A.; Radovits, T.; Szenasi, G.; Szebeni, J. , mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S. M. , Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Molecular Therapy 2021, 29, 898–900. [Google Scholar] [CrossRef]
- Faizullin, D.; Valiullina, Y.; Salnikov, V.; Zuev, Y. , Direct interaction of fibrinogen with lipid microparticles modulates clotting kinetics and clot structure. Nanomedicine 2020, 23, 102098. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. , Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 2021, 18, 666–82. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H. C.; Pavli, A.; Tsakris, A. , Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T. C. , Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Molecular Neurobiology 2022, 59, 1850–61. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O'Keeffe, E.; Zaporojan, L.; O'Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; Martin-Loeches, I.; Long, A.; Cheallaigh, C. N.; Conlon, N.; Doherty, C. P.; Campbell, M. , Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat Neurosci 2024. [Google Scholar]
- Houston, F.; Foster, J. D.; Chong, A.; Hunter, N.; Bostock, C. J. , Transmission of BSE by blood transfusion in sheep. Lancet 2000, 356, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Hunter, N.; Foster, J.; Chong, A.; McCutcheon, S.; Parnham, D.; Eaton, S.; MacKenzie, C.; Houston, F. , Transmission of prion diseases by blood transfusion. J Gen Virol 2002, (Pt 11) Pt 11, 2897–905. [Google Scholar] [CrossRef]
- Seki, Y.; Yamazaki, Y.; Inoue, Y.; Wakabayashi, C.; Seto, S. , How HIV infected haemophiliacs in Japan were informed of their HIV-positive status. AIDS Care 2002, 14, 651–64. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, C. A.; Hewitt, P. E.; Knight, R. S.; Amar, K.; Cousens, S.; Mackenzie, J.; Will, R. G. , Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 2004, 363, 417–21. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, J. , Tainted Blood and Vengeful Spirits: The Legacy of Japan’s Yakugai Eizu (AIDS) Trial. Culture, Medicine and Psychiatry 2005, 29, (1), 5-31.
- Hewitt, P. E.; Llewelyn, C. A.; Mackenzie, J.; Will, R. G. , Creutzfeldt–Jakob disease and blood transfusion: results of the UK Transfusion Medicine Epidemiological Review study. Vox Sanguinis 2006, 91, 221–30. [Google Scholar] [CrossRef]
- McLeod, N. P.; Nugent, P.; Dixon, D.; Dennis, M.; Cornwall, M.; Mallinson, G.; Watkins, N.; Thomas, S.; Sutton, J. M. , Evaluation of efficacy of prion reduction filters using blood from an endogenously infected 263K scrapie hamster model. Transfusion 2015, 55, 2390–7. [Google Scholar] [CrossRef]
- Seed, C. R.; Hewitt, P. E.; Dodd, R. Y.; Houston, F.; Cervenakova, L. , Creutzfeldt-Jakob disease and blood transfusion safety. Vox Sanguinis 2018, 113, 220–31. [Google Scholar] [CrossRef] [PubMed]
- Tighe, P. J.; Ryder, R. R.; Todd, I.; Fairclough, L. C. , ELISA in the multiplex era: Potentials and pitfalls. PROTEOMICS – Clinical Applications 2015, 9, (3-4), 406-22.
- Macklin, A.; Khan, S.; Kislinger, T. , Recent advances in mass spectrometry based clinical proteomics: applications to cancer research. Clinical Proteomics 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baudys, J.; Bundy, J. L.; Solano, M.; Keppel, T.; Barr, J. R. , Comprehensive Analysis of the Glycan Complement of SARS-CoV-2 Spike Proteins Using Signature Ions-Triggered Electron-Transfer/Higher-Energy Collisional Dissociation (EThcD) Mass Spectrometry. Analytical Chemistry 2020, 92, 14730–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. , Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduction and Targeted Therapy 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Mustafa Hellou, M.; Górska, A.; Mazzaferri, F.; Cremonini, E.; Gentilotti, E.; De Nardo, P.; Poran, I.; Leeflang, M. M.; Tacconelli, E.; Paul, M. , Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: a systematic review and meta-analysis. Clinical Microbiology and Infection 2021, 27, 341–51. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.-S. , Proteomics technologies for cancer liquid biopsies. Molecular Cancer 2022, 21. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.; Liu, S.; Ren, X.; Shi, D.; Ba, Y.; Huo, Y.; Zhang, W.; Ma, L.; Liu, Y.; Yang, Y.; Cheng, N. , The screening value of RT-LAMP and RT-PCR in the diagnosis of COVID-19: systematic review and meta-analysis. J Virol Methods 2022, 300, 114392. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Gadhave, K.; Kapuganti, S. K.; Kumar, P.; Brotzakis, Z. F.; Saumya, K. U.; Nayak, N.; Kumar, A.; Joshi, R.; Mukherjee, B.; Bhardwaj, A.; Thakur, K. G.; Garg, N.; Vendruscolo, M.; Giri, R. , Amyloidogenic proteins in the SARS-CoV and SARS-CoV-2 proteomes. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Pan, K. M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R. J.; Cohen, F. E. , Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proceedings of the National Academy of Sciences 1993, 90, 10962–66. [Google Scholar] [CrossRef]
- 133. Langeveld, Jan P. M.; Wang, J. J.; Van de Wiel, Dick F. M.; Shih, Giles C.; Garssen, G. J.; Bossers, A.; Shih, Jason C. H., Enzymatic Degradation of Prion Protein in Brain Stem from Infected Cattle and Sheep. The Journal of Infectious Diseases 2003, 188, (11), 1782-89.
- Prusiner, S. B.; Groth, D. F.; McKinley, M. P.; Cochran, S. P.; Bowman, K. A.; Kasper, K. C. , Thiocyanate and hydroxyl ions inactivate the scrapie agent. Proceedings of the National Academy of Sciences 1981, 78, 4606–10. [Google Scholar] [CrossRef]
- Race, R. E.; Raymond, G. J. , Inactivation of Transmissible Spongiform Encephalopathy (Prion) Agents by Environ LpH. Journal of Virology 2004, 78, 2164–65. [Google Scholar] [CrossRef] [PubMed]
- Peretz, D.; Supattapone, S.; Giles, K.; Vergara, J.; Freyman, Y.; Lessard, P.; Safar, J. G.; Glidden, D. V.; McCulloch, C.; Nguyen, H.-O. B.; Scott, M.; DeArmond, S. J.; Prusiner, S. B. , Inactivation of Prions by Acidic Sodium Dodecyl Sulfate. Journal of Virology 2006, 80, 322–31. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Kiba, Y.; Yu, J.; Hsu, K.; Chen, S.; Ishii, A.; Yokogawa, T.; Suzuki, R.; Inoue, Y.; Kitamura, M. , Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Tai, M. W.; Sweet, B. V. , Nattokinase for prevention of thrombosis. Am J Health Syst Pharm 2006, 63, 1121–3. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Yao, J.; Sparks, S.; Wang, K. Y. , Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; McGowan, E. M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. , Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, A.; Risch, L.; Weber, M.; Thiel, S.; Jungert, K.; Pichler, M.; Wohlwend, N.; Lung, T.; Ritzler, M.; Hillmann, D.; Copeland, S.; Renz, H.; Paprotny, M.; Risch, M. , Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study. Clin Chem Lab Med 2020, 59, e49–e51. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Yorsaeng, R.; Posuwan, N.; Puenpa, J.; Wanlapakorn, N.; Sudhinaraset, N.; Sripramote, M.; Chalongviriyalert, P.; Jirajariyavej, S.; Kiatpanabhikul, P.; Saiyarin, J.; Soudon, C.; Thienfaidee, O.; Palakawong Na Ayuthaya, T.; Brukesawan, C.; Chirathaworn, C.; Intharasongkroh, D.; Chaiwanichsiri, D.; Issarasongkhram, M.; Kitphati, R.; Mungaomklang, A.; Nagavajara, P.; Poovorawan, Y. , Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci Rep 2021, 11, 23216. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Vande Weygaerde, Y.; Andre, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. , Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol 2021, 136, 104765. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J. C.; Macartney, K.; Naus, M.; Grange, Z.; Escolano, S.; Sepulveda, G.; Shetty, A.; Pillsbury, A.; Sullivan, C.; Naveed, Z.; Janjua, N. Z.; Giglio, N.; Perala, J.; Nasreen, S.; Gidding, H.; Hovi, P.; Vo, T.; Cui, F.; Deng, L.; Cullen, L.; Artama, M.; Weintraub, E.; Lu, H.; Clothier, H. J.; Batty, K.; Paynter, J.; Petousis-Harris, H.; Buttery, J.; Black, S.; Hviid, A. , COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024. [Google Scholar] [CrossRef]
- Stroup, D. F.; Berlin, J. A.; Morton, S. C.; Olkin, I.; Williamson, G. D.; Rennie, D.; Moher, D.; Becker, B. J.; Sipe, T. A.; Thacker, S. B. , Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, (15), 2008-12.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. G.; Group, P. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010, 8, 336–41. [Google Scholar] [CrossRef]
- Murad, M. H.; Montori, V. M.; Ioannidis, J. P.; Jaeschke, R.; Devereaux, P. J.; Prasad, K.; Neumann, I.; Carrasco-Labra, A.; Agoritsas, T.; Hatala, R.; Meade, M. O.; Wyer, P.; Cook, D. J.; Guyatt, G. , How to read a systematic review and meta-analysis and apply the results to patient care: users' guides to the medical literature. JAMA 2014, 312, 171–9. [Google Scholar] [CrossRef]
- Gould, C. V.; Free, R. J.; Bhatnagar, J.; Soto, R. A.; Royer, T. L.; Maley, W. R.; Moss, S.; Berk, M. A.; Craig-Shapiro, R.; Kodiyanplakkal, R. P. L.; Westblade, L. F.; Muthukumar, T.; Puius, Y. A.; Raina, A.; Hadi, A.; Gyure, K. A.; Trief, D.; Pereira, M.; Kuehnert, M. J.; Ballen, V.; Kessler, D. A.; Dailey, K.; Omura, C.; Doan, T.; Miller, S.; Wilson, M. R.; Lehman, J. A.; Ritter, J. M.; Lee, E.; Silva-Flannery, L.; Reagan-Steiner, S.; Velez, J. O.; Laven, J. J.; Fitzpatrick, K. A.; Panella, A.; Davis, E. H.; Hughes, H. R.; Brault, A. C.; St George, K.; Dean, A. B.; Ackelsberg, J.; Basavaraju, S. V.; Chiu, C. Y.; Staples, J. E.; Yellow Fever Vaccine Virus, T.; Transfusion Investigation, T. , Transmission of yellow fever vaccine virus through blood transfusion and organ transplantation in the USA in 2021: report of an investigation. Lancet Microbe 2023, 4, e711–e21. [Google Scholar] [CrossRef]
- Yaqoob, I.; Salah, K.; Jayaraman, R.; Al-Hammadi, Y. , Blockchain for healthcare data management: opportunities, challenges, and future recommendations. Neural Computing and Applications 2021, 34, 11475–90. [Google Scholar] [CrossRef]
- Musamih, A.; Salah, K.; Jayaraman, R.; Arshad, J.; Debe, M.; Al-Hammadi, Y.; Ellahham, S. , A Blockchain-Based Approach for Drug Traceability in Healthcare Supply Chain. IEEE Access 2021, 9, 9728–43. [Google Scholar] [CrossRef]
- WHO, International Health Regulations (2005). 2nd edn. In World Health Organization: Geneva, 2008.
- Bakanidze, L.; Imnadze, P.; Perkins, D. , Biosafety and biosecurity as essential pillars of international health security and cross-cutting elements of biological nonproliferation. BMC Public Health 2010, 10, (Suppl 1).
- WHO, Global Covid-19 Vaccination Strategy in a Changing World July 2022 update. In World Health Organization: Geneva, 2022.
- Wu, Y. C.; Chen, C. S.; Chan, Y. J. , The outbreak of COVID-19: An overview. J Chin Med Assoc 2020, 83, 217–20. [Google Scholar] [CrossRef]
- Beeckman, D. S. A.; Rudelsheim, P. , Biosafety and Biosecurity in Containment: A Regulatory Overview. Front Bioeng Biotechnol 2020, 8, 650. [Google Scholar] [CrossRef]
- Berg, P.; Baltimore, D.; Brenner, S.; Roblin, R. O.; Singer, M. F. , Summary statement of the Asilomar conference on recombinant DNA molecules. Proc Natl Acad Sci U S A 1975, 72, 1981–4. [Google Scholar] [CrossRef]
- Barinaga, M. , Asilomar revisited: lessons for today? Science 2000, 287, 1584–5. [Google Scholar] [CrossRef] [PubMed]
- Krimsky, S. , From Asilomar to industrial biotechnology: risks, reductionism and regulation. Sci Cult (Lond) 2005, 14, 309–23. [Google Scholar] [CrossRef] [PubMed]
- Gregorowius, D.; Biller-Andorno, N.; Deplazes-Zemp, A. , The role of scientific self-regulation for the control of genome editing in the human germline: The lessons from the Asilomar and the Napa meetings show how self-regulation and public deliberation can lead to regulation of new biotechnologies. EMBO Rep 2017, 18, 355–58. [Google Scholar] [CrossRef]
- Taylor, D. M. , Inactivation of TSE agents: safety of blood and blood-derived products. Transfus Clin Biol 2003, 10, 23–5. [Google Scholar] [CrossRef]
- Klein, M. A.; Frigg, R.; Flechsig, E.; Raeber, A. J.; Kalinke, U.; Bluethmann, H.; Bootz, F.; Suter, M.; Zinkernagel, R. M.; Aguzzi, A. , A crucial role for B cells in neuroinvasive scrapie. Nature 1997, 390, 687–90. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A. , Leukocyte depletion for safe blood transfusion. Biotechnol J 2009, 4, 1140–51. [Google Scholar] [CrossRef]
- Schmidt, A.; Refaai, M.; Kirkley, S.; Blumberg, N. , Proven and potential clinical benefits of washing red blood cells before transfusion: current perspectives. International Journal of Clinical Transfusion Medicine 2016, Volume 4, 79–88. [Google Scholar] [CrossRef]
- Cardigan, R.; New, H. V.; Tinegate, H.; Thomas, S. , Washed red cells: theory and practice. Vox Sanguinis 2020, 115, 606–16. [Google Scholar] [CrossRef]
- Palmqvist, M.; Von Schreeb, J.; Älgå, A. , Autotransfusion in low-resource settings: a scoping review. BMJ Open 2022, 12. [Google Scholar] [CrossRef]
- Mulroney, T. E.; Pöyry, T.; Yam-Puc, J. C.; Rust, M.; Harvey, R. F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A. P.; Stoneley, M.; Sawarkar, R.; Mentzer, A. J.; Lilley, K. S.; Smales, C. M.; von der Haar, T.; Turtle, L.; Dunachie, S.; Klenerman, P.; Thaventhiran, J. E. D.; Willis, A. E. , N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2023. [Google Scholar] [CrossRef]
- Alameh, M. G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J. R.; Gaudette, B. T.; Soliman, O. Y.; Pine, M.; Hicks, P.; Manzoni, T. B.; Knox, J. J.; Johnson, J. L.; Laczko, D.; Muramatsu, H.; Davis, B.; Meng, W.; Rosenfeld, A. M.; Strohmeier, S.; Lin, P. J. C.; Mui, B. L.; Tam, Y. K.; Kariko, K.; Jacquet, A.; Krammer, F.; Bates, P.; Cancro, M. P.; Weissman, D.; Luning Prak, E. T.; Allman, D.; Locci, M.; Pardi, N. , Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, (12), 2877-92 e7.
- Guimaraes, L. E.; Baker, B.; Perricone, C.; Shoenfeld, Y. , Vaccines, adjuvants and autoimmunity. Pharmacol Res 2015, 100, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Jara, L. J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. , Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clinical Rheumatology 2022, 41, 1603–09. [Google Scholar] [CrossRef] [PubMed]
- Kaulen, L. D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H. M.; Nagel, S.; Wildemann, B.; Bendszus, M.; Wick, W.; Schönenberger, S. , Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. European Journal of Neurology 2021, 29, 555–63. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; Dias, C.; Sousa, M. J.; Radice, A.; Eriksson, C.; Hultgren, O.; Viander, M.; Khamashta, M.; Regenass, S.; Andrade, L. E. C.; Wiik, A.; Tincani, A.; Rönnelid, J.; Bloch, D. B.; Fritzler, M. J.; Chan, E. K. L.; Garcia-De La Torre, I.; Konstantinov, K. N.; Lahita, R.; Wilson, M.; Vainio, O.; Fabien, N.; Sinico, R. A.; Meroni, P.; Shoenfeld, Y. , International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Annals of the Rheumatic Diseases 2014, 73, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z. X.; Miller, J. S.; Zheng, S. G. , An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev 2021, 20, 102743. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J. L.; Fung, A. W. S.; Mattman, A.; Quach, T. T. T.; Gauiran, D. T. V.; Carruthers, M. N.; Chen, L. Y. C. , Clinical utility of serum IgG4 measurement. Clin Chim Acta 2020, 506, 228–35. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.; Stone, J. H. , Clinical Perspectives on IgG4-Related Disease and Its Classification. Annu Rev Med 2022, 73, 545–62. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.; Royse, C. F.; Terkawi, A. S. , Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth 2017, (Suppl 1) (Suppl 1), S80–S89. [Google Scholar] [CrossRef]
- Semmler, A.; Mundorf, A. K.; Kuechler, A. S.; Schulze-Bosse, K.; Heidecke, H.; Schulze-Forster, K.; Schott, M.; Uhrberg, M.; Weinhold, S.; Lackner, K. J.; Pawlitzki, M.; Meuth, S. G.; Boege, F.; Ruhrländer, J. , Chronic Fatigue and Dysautonomia following COVID-19 Vaccination Is Distinguished from Normal Vaccination Response by Altered Blood Markers. Vaccines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Bashir, M. S.; Joyce, K.; Rashid, H.; Laher, I.; Elshazly, S. , An Update on COVID-19 Vaccine Induced Thrombotic Thrombocytopenia Syndrome and Some Management Recommendations. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E. J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; Asleh, R.; Amir, O.; Meir, K.; Cohen, D.; Dichtiar, R.; Novick, D.; Hershkovitz, Y.; Dagan, R.; Leitersdorf, I.; Ben-Ami, R.; Miskin, I.; Saliba, W.; Muhsen, K.; Levi, Y.; Green, M. S.; Keinan-Boker, L.; Alroy-Preis, S. , Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. New England Journal of Medicine 2021, 385, 2140–49. [Google Scholar] [CrossRef]
- Nakahara, T.; Iwabuchi, Y.; Miyazawa, R.; Tonda, K.; Shiga, T.; Strauss, H. W.; Antoniades, C.; Narula, J.; Jinzaki, M. , Assessment of Myocardial (18)F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated Patients. Radiology 2023, 308, e230743. [Google Scholar] [CrossRef]
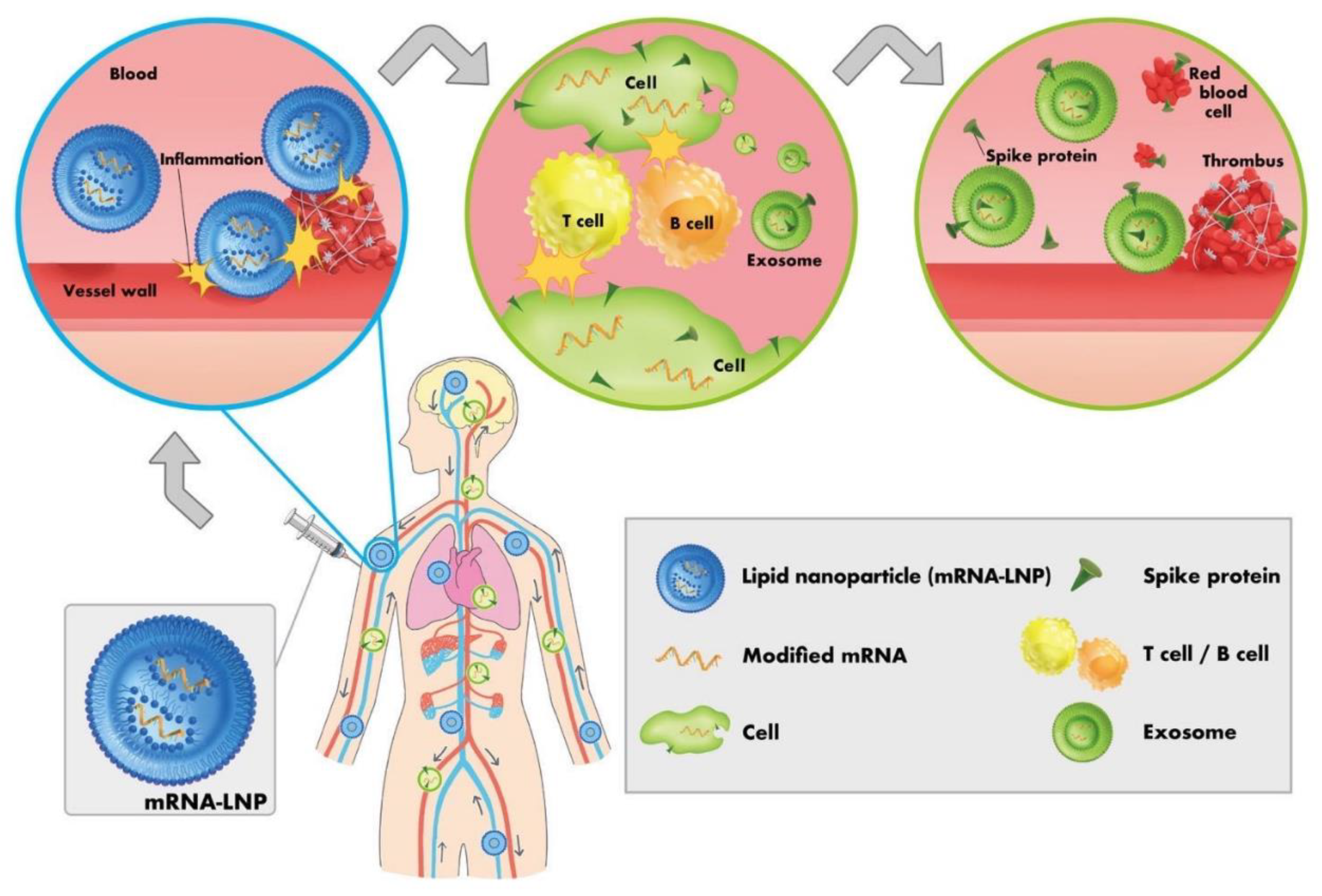
| Concerns | Description | References | |
|---|---|---|---|
| 1 | Spike protein contamination | The spike protein, which is the antigen of SARS-CoV-2 and genetic vaccines, has already been found to have various toxicities, including effects on red blood cells and platelet aggregation, amyloid formation, and neurotoxicity. It is essential to recognize that the spike protein itself is toxic to humans. It has also been reported that the spike protein can cross the blood–brain barrier. Therefore, it is essential to remove the genetic vaccine-derived spike protein itself from blood products. | [22,29,45,56,57,58,59,60,61] |
| 2 | Contamination with amyloid aggregates and microthrombi formed by spike proteins | Amyloid aggregation and development of microthrombi formed by the spike proteins into visible thrombi is yet unknown. However, once formed, amyloid aggregates may not be readily cleared and therefore need to be removed from blood products. These amyloid aggregates have been shown to be toxic. | [52,53,131] |
| 3 | Events attributable to decreased donor immune system and immune abnormalities due to immune imprinting or class switch to IgG4, etc., resulting from multiple doses of genetic vaccines | In cases where the immune function of a donor is impaired by vaccination with genetic vaccines, there is a risk that the donor might have an (subclinical) infectious disease or has developed viremia or other conditions after being infected with a pathogenic virus, even in the absence of subjective symptoms. Therefore, healthcare professionals who perform surgical procedures, including blood sampling and organ transplantation, as well as use blood products, should exercise caution while handling the blood of genetic vaccine recipients to prevent infections through blood. All healthcare professionals should be informed of these risks. | [64,65,66,67,68,71,72,73,74,75,76,81,82,83,84,85,87,88,89,90,91,92] |
| 4 | Presence of lipid nanoparticles (LNPs) and pseudouridinated mRNA (mRNA vaccines only) | If the blood donated by recipients of mRNA vaccines is collected without a sufficient deferral period after genetic vaccination, LNPs and pseudouridinated mRNA may remain in the blood. LNPs are highly inflammatory and have been found to be thrombogenic, posing a risk to transfusion recipients. Furthermore, LNPs have potent adjuvant activity and pose a risk of inducing Adjuvant-Induced Autoimmune Syndrome (ASIA syndrome). An additional risk is that if the pseudouridinated mRNA is incorporated into the recipient's blood while still packaged in LNPs, further spike protein may be produced in the recipient's body. Additionally, if modified mRNAs persist in the body for a prolonged period of time, they can cause a decrease in immune functions. | [23,40,44,76,106,107,108,109,110,111,167,169] |
| 5 | Contamination with aggregated red blood cells or platelets | The spike protein causes red blood cells and platelets to aggregate; these aggregates will be carried into the recipient's blood unless they are physically removed from the blood product before transfusion. | [7,8,9,10,11,50] |
| 6 | Memory B cells producing IgG4 as well as IgG4 produced from them | Large amounts (serum concentration typically above 1.25–1.4 g/L) of non-inflammatory IgG4-positive plasma cells can cause chronic inflammation, such as fibroinflammatory disease. | [78,79,80,173,174] |
| Concerns | Description | References | |
|---|---|---|---|
| 1 | Spike protein content in blood | Immunochemical techniques include enzyme-linked immunosorbent assay, immunophenotyping, mass spectrometry, liquid biopsy, and a combination of liquid biopsy and proteomics. We propose initially conducting mass spectrometry because it can directly measure the protein itself. | [28,29,124,125,126,127,129] |
| 2 | Spike protein mRNA in blood | PCR and/or liquid biopsy are the options. If mRNA for the spike protein is detected, lipid nanoparticles (LNPs) may be present (mRNA vaccines only). | [127,128,130] |
| 3 | Spike protein DNA in blood | PCR and liquid biopsy are the options. This test is necessary because AstraZeneca's viral vector is a DNA vaccine. For mRNA vaccines, it is believed that pseudouridinated mRNA is not reverse transcribed, but this test is required if the spike protein remains in the body for a prolonged period. | [127,128] |
| 4 | Autoimmune disorders | Long-term persistence of the spike protein in the blood increases the risk of autoimmune disease. Therefore, it would be useful to assess for autoimmune disease using antinuclear antibodies as biomarkers in people who are positive for the spike protein, taking into account the results of interviews regarding the subjective symptoms. | [27,169,171,172] |
| 5 | Post-vaccination syndrome (PVS) | A history of vaccination with genetic vaccines and COVID-19, current and previous medical history, and presence of subjective symptoms (e.g., headache, chest pain, shortness of breath, malaise) should be obtained from blood donors and formally recorded. The type of questions included in the interview are critical to facilitate diagnosis and treatment of COVID-19 PVS, as more people are complaining of psychiatric and neurological symptoms after genetic vaccination. | [15,175,176] |
| 6 | Proteins resulting from frameshifting of pseudouridinated mRNA | Although it is not yet clear whether proteins other than the spike protein are translated from pseudouridinated mRNAs, mass spectrometry may be useful in confirming this. | [166] |
| 7 | Amyloid aggregates and thrombi | Common markers of thrombosis, such as D-dimer, should be first used. Once the major components of amyloid aggregates and thrombi have been identified, their use as biomarkers is proposed. Understanding the composition of amyloid aggregates will be important in the future, as amyloid aggregates have been reported to be toxic. Understanding the composition of amyloid aggregates may provide clues to how amyloid is broken down. | [52,53,131,177] |
| 8 | Origin of spike protein | This test will help determine whether the spike protein is from the genetic vaccine or from SARS-CoV-2. Potential candidates include nucleocapsid. | [4,5,41,128] |
| 9 | Immunosuppression | It may be necessary to analyze immunoglobulin subclasses (such as the amount of IgG4) if immunosuppression from multiple doses of the genetic vaccine is a concern. | [71,72,73,74] |
| 10 | Anti-nucleocapsid antibodies | The presence or absence and amount of anti-nucleocapsid antibodies as well as antibody isotypes may be an indicator(s) for distinguishing whether these are caused by genetic vaccines or long COVID. | [141,142,143] |
| 11 | Others | Occurrence of myocarditis and pericarditis after genetic vaccination has been reported in various countries. Therefore, those with subjective symptoms should also be assessed for myocarditis markers, such as cardiac troponin T. | [18,19,29,144,178,179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





