1. Introduction
Benign prostatic hyperplasia (BPH) is caused by the presence of disproportionate cellularity in both the stromal and epithelial components. Several theories suggest multiple components that causes progression to BPH including, hormonal pathophysiology, which is considered as the most appropriate reason, stem cell replication, anti-apoptotic activity, embryonic stromal awakening. The enzyme 5-α-Reductase, located mostly in the prostate tissue, converts Testosterone into dihydrotestosterone (DHT). Androgen binds to the androgen receptor. The prostate tissue contains one of the androgen receptors, isozyme-2 while isozyme-1 is primarily found in the skin and liver. [
1] After DHT binds to the receptor, a protein complex is formed that phosphorylates and modifies translation. Stem cells work in need of the hour and maintain prostatic cellular integrity by causing hyperplasia. [
2] If this proliferation persists, the stromal to epithelial ratio is influenced. In BPH, it results in increasing the stromal to epithelial ratio from 2:1 to 5:1. BPH is caused by the hyperproliferation of stromal to epithelial components in prostate tissue. [
3] Defect in cellular apoptotic activity when joined with hyper-proliferation of prostate tissue leads to increase in hyperplasia. [
4] Prostatic stroma reawakens selectively in the transition-peri-urethral zone (TPZ) because failed distal ducts literally require renovation, which mesenchymal stem cells perform in response to deterioration. This process in TPZ reawakens inductive stroma, causing an increase in the number of prostatic cells, which leads to BPH. [
5] According to literature, first-degree relatives of patients who have undergone BPH surgery are at a significantly increased risk of having BPH. Previous researches has shown that patients with no documented cases of BPH in 1st degree relatives have a 17% chance of developing BPH during their lifetime, compared to a 66% lifetime risk in patients with this condition reported in 1st degree relatives. [
6] Progressive BPH puts pressure on the urethra and lead to the narrowing of its lumen which eventually cause Lower urinary tract symptoms (LUTS). Approximately 50% male above the age of 60 and 80% male above the age of 90 suffer from certain degree of LUTS; which significantly affects their quality of life (QOL). [
7] The quantification of LUTS is accomplished with the use of the International Prostate Symptom Score (IPSS). IPSS consists of a total number of eight questions, with seven questions dedicated to evaluating LUTS and one question specifically designed to assess the individual's QOL.
Patients with LUTS are initially prescribed with medicinal therapy in the first stage of treatment. The prostate gland is made up of smooth muscle and α-1-receptors, found in the prostatic urethra, prostatic capsule, bladder trigone, and prostate gland itself. Tamsulosin, Doxazosin, Terazosin, or Alfuzosin are examples of alpha blockers that are frequently used as a first line treatment for LUTS. They greatly diminish voiding LUTS and enhance urine flow rate. [
8,
9] DHT is the biologically active form of Testosterone and it is strongly associated with the proliferation of stromal and epithelial components, which in the end results in BPH. The class of 5-α-reductase inhibitors consists of Finasteride and Dutasteride. Their function is to inhibit the conversion of Testosterone to DHT, resulting in a reduction in prostate weight. Plasma half-life of finasteride is 6-8 hours and tissue binding is 4-5 days. The primary isozyme of 5-α-reductase is isozyme-2. It is present in the prostate epithelium. Finasteride selectively targets isozyme-2. Finasteride has been observed to reduce the expression of mean vascular density (MVD) and vascular endothelial growth factor (VEGF), thereby exerting an inhibitory effect on angiogenesis in prostatic tissue. It diminishes the vascularity of the prostate and minimizes the occurrence of hematuria. [
10,
11] Patients who do not respond well to medicinal treatment are referred to surgical interventions. Surgical management of BPH in divided into minimally invasive and invasive. Temperature and power are delivered at different strength which produces variable grades of coagulative necrosis in minimally invasive techniques. Vaporization of prostatic tissue is achieved in invasive techniques. Invasive techniques are TURP, transurethral vaporization of the prostate (TUVP), potassium-titanyl-phosphate photo selective vaporization of the prostate (KTP-PVP) while minimal invasive techniques includes transurethral needle ablation (TUNA), transurethral microwave thermotherapy (TUMT) and high-intensity focused ultrasound (HiFU). TURP is considered the gold standard surgical technique. It delivers beneficial outcomes. It is cost-effective, easily accessible, and has a short learning curve. TURP enhances urine flow rate (Qmax) and substantially reduces LUTS. [
12] Hemorrhage is considered to be the most common TURP consequence, resulting from the trauma associated with the surgical procedure. Blood transfusions may be necessary for patients afterward. Previously in TURP patients, blood loss was estimated by weak evidences such as color of irrigation fluid, degree of hematuria duration of catheterization and requirement of blood transfusion. A novel technique to determine the amount of blood loss is by using hemoglobin in irrigation fluid, volume of irrigation fluid and pre-operative hemoglobin value; and then placing them in an equation to get results. It will provide the amount of blood loss in milliliters (ml). [
13]
The fluid for irrigation and bladder wash is collected. At the end of surgery small amount of irrigation fluid is collected and hemoglobin in irrigation fluid is determined by hemoglobin analyzer. Pre-operative assessment includes the complete blood count (CBC) from which the value of pre-operative hemoglobin is obtained. Resected prostate tissue is collected in a container and weight is measured in grams (g) on a digital weighing machine. [
14] The administration of Finasteride prior to surgery for 2 weeks may provide benefits by reducing the amount of blood loss that occurs during TURP. Multiple studies have indicated that the combination of finasteride and TURP is highly advantageous in reducing the blood loss associated with TURP procedures. [
15] A study conducted in Shiraz University of Medical Sciences in Iran shows preoperative treatment with finasteride for two weeks before TURP reduces the blood loss after TURP. [
16] While a study from India conducted in 2021 also documents that preoperative use of Finasteride significantly reduces perioperative and postoperative TURP bleeding. [
17] Interestingly almost all of the earlier researches on the use of various medications, such as finasteride, dutasteride, or tranexamic acid, to reduce blood loss in TURP patients employed haemoglobin and hematocrit differences between pre-op and post-op measurements to determine blood loss. Since fluid resuscitation alters plasma concentration and hemodilution, haemoglobin concentration and hematocrit percentage efficiency are never thought to be trustworthy. Subsequently, other researchers measured the intraoperative blood loss using the urine-strip approach; the results showed that there was some correlation between the urine-strip and spectrophotometer and the earlier techniques. Based on the haemoglobin in blood, the haemoglobin in irrigation fluid, and the volume of irrigation fluid used, this study's unique method was implemented. Blood loss is calculated using a formulation, and while this process is very precise, it still has to be refined through additional study and testing.
Over one-third of individuals with BPH require TURP. The physiological reserves are lower as a result of ageing and decreased levels of physical exercise. This is among the causes for which certain individuals need blood transfusions after surgery. We require methods to lower down peri-operative blood loss. The purpose of our study was to look into the possible effect of finasteride on TURP blood loss decrease during intraoperative procedures.
2. Materials and Methods
This prospective randomized control trial was conducted in the Department of Urology and Kidney Transplantation at the Faisalabad Medical University Allied Hospital from September 2022 to February 2023. After ethical approval was taken, 120 patients were enrolled on the basis of inclusion criteria that consists of i) Age 40-80 years. ii) Prostate weight 40-80g. iii) Severe LUTS. iv) Refractory urinary retention. While the Exclusion criteria consists i) Patients who previously used 5-α-reductase inhibitors before. ii) Patient with >III ASA. iii) Neurogenic Bladder. iv) Untreated UTI.
Patients were assessed in Urology OPD and those who fall under the treatment line of TURP were prescribed with Finasteride for 2 weeks and got admitted in Urology ward at 13th day of medicine. Written informed consents were taken and surgical pre-requisites were completed. Each subject was evaluated by demographic data, general history, detailed urological history, physical examination and investigations. Urological history includes LUTS, hematuria, history of catheterization, urinary tract infection and any prostatic or urethral surgery. Physical examination included general examination, examination of the renal region, external genitalia and digital rectal examination while investigations consist of complete blood count, renal function tests, serum PSA. Ultrasound of KUB with post void residual volume was done to evaluate the size, volume and texture of prostate was done. Patient division was done into 2 groups; Group-A and Group-B. Group-A consists of patients who underwent TURP with pre-operative 2 weeks use of Finasteride while Group-B patients underwent TURP without it. Categorization was done using even and odd serial numbers. Even serial numbers were given to the patients in invention group i.e. Group-A & odd serial numbers were given to the patients in control group. On the designated date surgeries were performed. TURP was done with standard protocols, under spinal anesthesia and in lithotomy position. After urethra-cystoscopy, findings were noted, later on with monopolar diathermy TURP was performed with a resectoscope and 5% dextrose irrigation fluid. After completing the resection, hemostasis was secured. A 3-way Foley catheterization was done, irrigation started and resection time was noted in minutes (min).
Irrigation fluid was gathered for quantification and 10ml of it was taken in 10ml hydremic syringe. Hemoglobin (Hb) in irrigation fluid was determined and blood loss was calculated with following;
Calculated blood loss (mL) = Hb in irrigation fluid (g/dL) x Irrigation fluid volume (mL)
Resected tissue weight was measured on weighing machine in grams (g) and blood loss per gram of prostate tissue was calculated with following;
All the data was noted on the designed Performa, processed and analyzed with SPSS 25 and Microsoft Excel worksheet.
3. Results
Our study revealed statistically significant reduction in blood loss in patients with pre-operative 2 weeks use of Finasteride later undergone TURP.
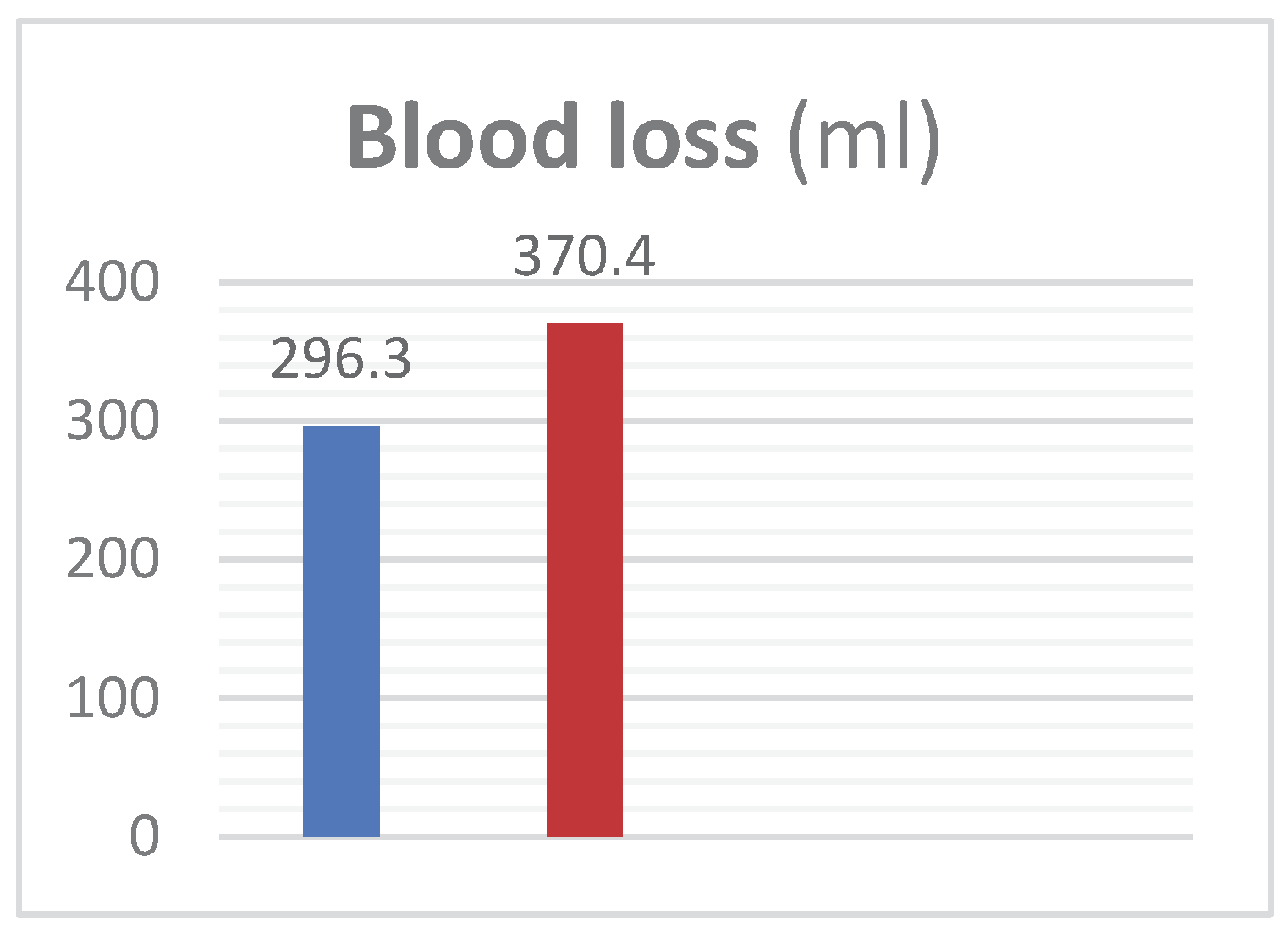
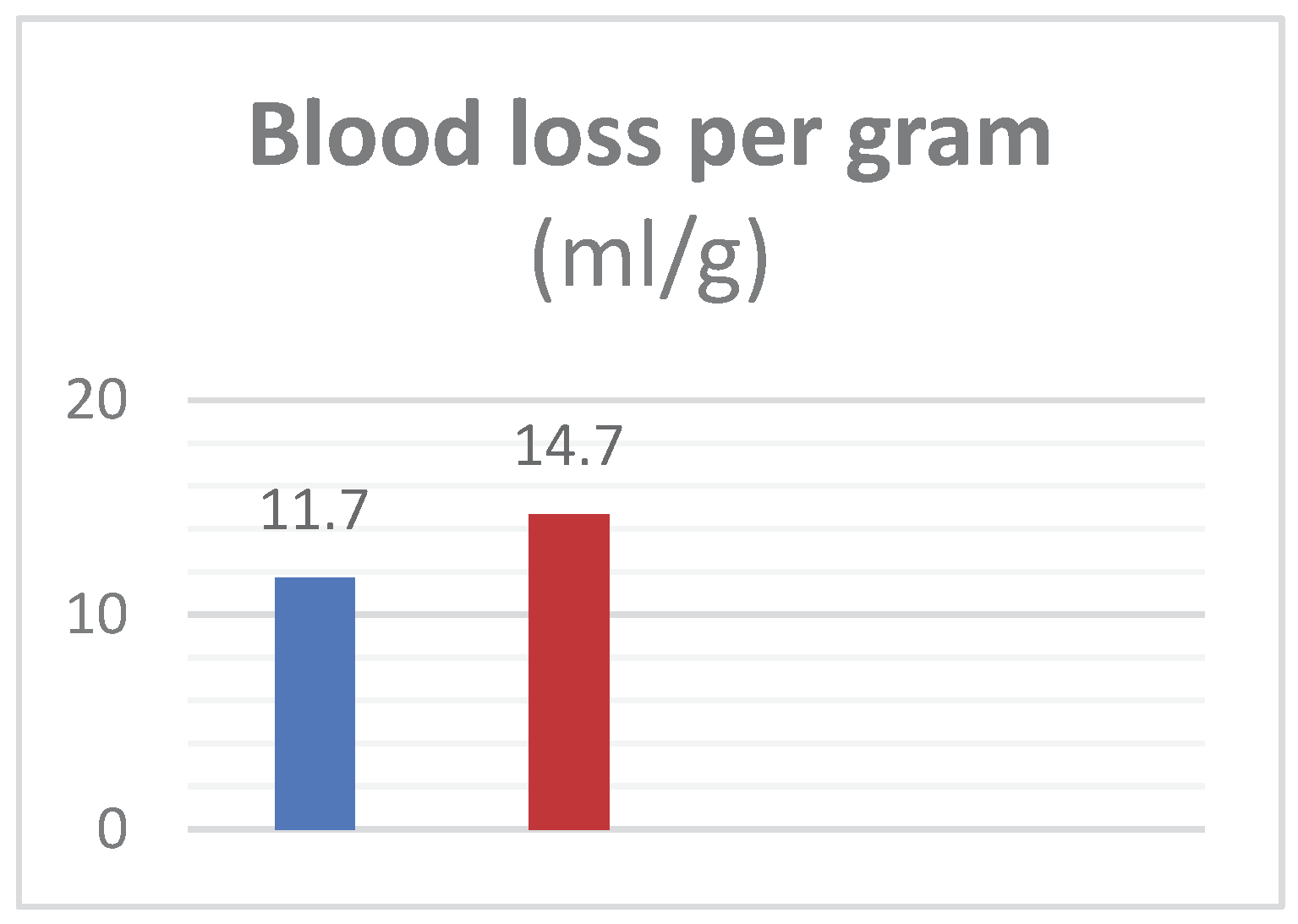
Group-A mean age was 63.6±13.66 years and in Group B was 63.3±13.2 years with mean prostate weight of 61.38±9.6g in Group-A and 61.8±10.4g in Group-B. Mean pre-operative Hb was 12.4±1.38g/dL in Group-A while 12.36±1.59g/dL in Group-B. Irrigation fluid was 13508±976ml in Group-A versus 14633±1258 in Group-B. Hb in irrigation fluid, mean value in Group-A was 0.26±0.047g/dL and it was 0.30±0.042g/dL in Group-B. Blood loss in Group-A patients was seen 296±75.2ml while 370.4±64.2ml in Group-B. Operative time also reduced; in Group-A TURP took 42.9±7.9min while in Group B it took 49.2±6.6min. Blood loss per gram of resected tissue was 11.7±1.3ml/g in Group-A and 14.7±2.69ml/g in Group-B. Results showed that Finasteride reduced intra-operative blood loss and blood loss per gram in TURP.
3.1. Figures, Tables and Scheme
Table 1.
- Continues variables of this study consists of blood loss (ml) and blood loss per gram (ml/g).
Table 1.
- Continues variables of this study consists of blood loss (ml) and blood loss per gram (ml/g).
| Quantitative Variables (Discrete): |
|---|
| Variables Group-A (Mean ± SD) Group-B (Mean ± SD) P-Value |
|---|
Blood loss (ml) 296.3±75.2 370.4±64.2 <0.001
Blood loss (ml/g) 11.7±1.3 14.7±2.69 <0.001 |
Table 2.
- Discrete variables of this study consists of age, prostate weight, operative time, pre-op Hb, volume of irrigation fluid, Hb in irrigation fluid and resected tissue weight.
Table 2.
- Discrete variables of this study consists of age, prostate weight, operative time, pre-op Hb, volume of irrigation fluid, Hb in irrigation fluid and resected tissue weight.
| Quantitative Variables (Continues): |
|---|
| Variables Group-A (Mean ± SD) Group-B (Mean ± SD) P-Value |
|---|
Age (years) 63.6±13.66 63.3±13.2 <0.001
Prostate weight (grams) 61.38±9.6 61.8±10.4 <0.001
Operative time (min) 42.9±7.9 49.2±6.6 <0.001
Pre-op Hb (g/dL) 12.4±1.38 12.36±1.59 <0.001
Vol. of irrigation fluid (ml) 13508±976 14633±1258 <0.001
Hb in irrigation fluid (g/dL) 0.26±0.047 0.30±0.042 <0.001
Resected tissue weight (g) 25.11±5.28 26.6±4.78 <0.001 |
Figure 1.
– Exhibits the mean peri-operative blood loss (ml) in patients of Group A and B.
Figure 1.
– Exhibits the mean peri-operative blood loss (ml) in patients of Group A and B.
Table 3.
– Tabulated form of mean peri-operative blood loss (ml) with standard deviation in patients of Group A and B.
Table 3.
– Tabulated form of mean peri-operative blood loss (ml) with standard deviation in patients of Group A and B.
| Mean blood loss in (ml) |
|---|
| Group Mean ± SD Group Mean ± SD P-value |
|---|
| A 296.3±75.2 B 370.4±64.2 <0.001 |
Figure 2.
- Exhibits the mean peri-operative blood loss per gram (ml/g) of prostate tissue in patients of Group A and B.
Figure 2.
- Exhibits the mean peri-operative blood loss per gram (ml/g) of prostate tissue in patients of Group A and B.
Table 4.
– Tabulated form of mean peri-operative blood loss per gram (ml/g) of prostate tissue with standard deviation in patients of Group A and B.
Table 4.
– Tabulated form of mean peri-operative blood loss per gram (ml/g) of prostate tissue with standard deviation in patients of Group A and B.
| Mean blood loss in (ml/g) |
|---|
| Group Mean ± SD Group Mean ± SD P-value |
|---|
| A 11.7±1.3 B 14.7±2.69 <0.001 |
3.3. Formatting of Mathematical Components
4. Discussion
BPH is one of the most common urological disorders in males beyond 5th decade of life. Patients who fail to exhibit a positive response to therapy are eventually offered with the option of undergoing surgical procedures. Accurately 15 studies comprising 6659 patients were included in Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) to compare and demonstrate the efficacy of various surgical treatments for BPH. The literature states that laparoscopic prostatectomy (LSP) and open simple prostatectomy (OSP) are inferior to robot assisted simple prostatectomy (RASP). Indeed, RASP offers a minimal error or failure rate, it is not widely available and comes at significant expenses to both the payer and the patient. While LSP needs strong laparoscopic skills and has a lengthy learning curve, OSP presents with considerable post-op problems. On the other hand, electrosurgery is widely available and reasonably priced. TURP is still the most commonly used surgical option for the treatment of BPH. A Worthington report from 2017 states that TURP has been the most common operation for BPH for 40 years, with over 25,000 procedures carried out each year. It is generally a successful operation, but has well-documented risks for the patient. During this surgical treatment, the most frequent complication seen is intraoperative bleeding. Finasteride belongs to a class of drugs that permanently blocks the 5-α-reductase and in turn causes a massive reduction in DHT and leads to reduction of VEGF and MVD. Putcher and mithel revealed that 5-α-reductase inhibitor stop the conversion of testosterone to DHT which in turn leads to decrease in VEGF and MVD.
In our trial we granted finasteride to the candidates of TURP 2 weeks before surgery, believing that this duration would be sufficient to reduce MVD and angiogenesis in prostatic tissue. Later, this hypothesis got supported by our study conclusion. Smith et al stated that finasteride has proven potent in reducing BPH associated hematuria and even in the preparation of TURP. Abdelbaki et al reveal a statistically significant reduction in operative time as compared to control group in patients undergoing TURP with pre-operative use of finasteride. Our trial state is opposite conclusion from Lund et al, according to whom pre-operative finasteride use does not effect on intra-operative blood loss.
According to the results of our trial, administering Finasteride two weeks before TURP reduces peri-operative blood loss and blood loss per gram.5. Conclusions
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Author Contributions
Dr. Aurangzeb being the chief investigator; conceived the idea and discussed with Dr. Moin Anwar who polished the plan and made a ground map. Dr. Aurangzeb planned and carried out the experiments. Dr. Kamran Liaqat, Dr. Rehan Idrees and Dr. Mubashar Ibrar carried out the simulations under the supervision of chief investigator. Dr. Ahmed Junaid and Dr. Murtaza Ikhlaq contributed to sample preparation. Dr. Fatima Ashraf contributed to the interpretation of the results. She analyzed and finalized the results using IBM SPSS statistics (version 25) and Microsoft Excel sheet from Microsoft Corporation. Dr. Zia Mahmood took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding
All the medications and investigations, surgical interventions and peri-operative equipment are available for admitted patients in Allied Hospital Faisalabad. As the entity is Government funded so no burden was put on the patients. The chief investigators Dr. Aurangzeb and his team were self-funded and completed this study on their own.
Institutional Review Board Statement
The study was approved by the Institutional Review Board (or Ethics Committee) of FAISALABAD MEDICAL UNIVERSITY, ALLIED HOSPITAL FAISALABAD.
Informed Consent Statement
Prior to enrolling participants in the study, informed consent was obtained from each patient. They were provided with comprehensive information about the study's purpose and their right to withdraw at any time without repercussions.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
All authors declare that they have no conflict of interest to disclose.
References
- Reynard J (2004). Does anticholinergic medication have a role for men with lower urinary tract symptoms/benign prostatic hyperplasia either alone or in combination with other agents? Curr Opin Urol 14:13–16.
- Nicholson, T., Ricke WA. 2011. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 82(4-5): 184–199. [CrossRef]
- Prajapati A, Gupta S, Mistry B, Gupta S. Prostate stem cells in the development of benign prostate hyperplasia and prostate cancer: emerging role and concepts. Biomed Res Int. 2013;2013:107954.
- Torrealba N, Rodríguez-Berriguete G, Vera R, Fraile B, Olmedilla G, Martínez-Onsurbe P, Sánchez-Chapado M, Paniagua R, Royuela M. 2020. Homeostasis: apoptosis and cell cycle in normal and pathological prostate. Aging Male. (5):335-345. [CrossRef]
- Brennen WN, Isaacs JT. 2018. Mesenchymal stem cells and the embryonic reawakening theory of BPH. Nat Rev Urol. 15(11):703-715. [CrossRef]
- Sanda MG, Beaty TH, Stutzman RE, Childs B, Walsh PC. 1994. Genetic susceptibility of benign prostatic hyperplasia. J Urol. 152(1):115-9. [CrossRef]
- Ng M, Leslie SW, Baradhi KM. Benign Prostatic Hyperplasia. 2024 Jan 11.
- Plochocki A, King B. 2022. Medical Treatment of Benign Prostatic Hyperplasia. Urol Clin North Am. 49(2):231-238. [CrossRef]
- Dunn CJ, Matheson A, Faulds DM. 2002. Tamsulosin: a review of its pharmacology and therapeutic efficacy in the management of lower urinary tract symptoms. Drugs Aging. 19(2):135-61.
- Upreti R, Naredo G, Faqehi AM, Hughes KA, Stewart LH, Walker BR, Homer NZ, Andrew R. 2015. Simultaneous pharmacokinetic and pharmacodynamic analysis of 5α-reductase inhibitors and androgens by liquid chromatography tandem mass spectrometry. Talanta. 131:728-35. [CrossRef]
- Steiner JF. Clinical pharmacokinetics and pharmacodynamics of finasteride. Clin Pharmacokinet. 1996 Jan;30(1):16-27. [CrossRef]
- Leslie SW, Chargui S, Stormont G. Transurethral Resection Of The Prostate. 2022 Nov 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;
- Tosukhowong., Dissayabutra., Ungjaroenwathana. & Bunyaratavej. 2007. Estimation of blood loss in transurethral resection of prostate (TUR-P) by urine-strip. J Med Assoc Thai, 90(11), 2409-2415.
- Hakenberg OW, Helke C, Manseck A, Wirth MP. 2001. Is there a relationship between the amount of tissue removed at transurethral resection of the prostate and clinical improvement in benign prostatic hyperplasia. Eur Urol. 39(4):412-7. [CrossRef]
- Yudhistira Pradnyan Kloping, Niwanda Yogiswara, Yusuf Azmi. The role of preoperative dutasteride in reducing bleeding during transurethral resection of the prostate: A systematic review and meta-analysis of randomized controlled trials. Asian Journal of Urology, Volume 9, Issue 1, 2022, Pages 18-26 . [CrossRef]
- Aminsharifi A, Salehi A, Noorafshan A, Aminsharifi A, Alnajar K. Effect of Preoperative Finasteride on the Volume or Length Density of Prostate Vessels, Intraoperative, Postoperative Blood Loss during and after Monopolar Transurethral Resection of Prostate: A Dose Escalation Randomized Clinical Trial Using Stereolog Methods. Urol J. 2016 Mar 5;13(1):2562-8. PMID: 26945662.
- Dutt UK, Kumar S, Dorairajan LN, Badhe BA, Manikandan R, Singh S. Effect of preoperative finasteride on perioperative blood loss during transurethral resection of the prostate and on microvessel density in patients with benign prostatic hyperplasia: An open label randomized controlled trial. Urol Ann. 2021 Jul-Sep;13(3):199-204. [CrossRef]
- Minutoli, L., Altavilla, D., Marini, H. et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci 21, 19 (2014). [CrossRef]
- Worthington, J., Taylor, H., Abrams, P. et al. A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral vaporesection of the prostate (ThuVARP) versus transurethral resection of the prostate (TURP) in the National Health Service (NHS) – the UNBLOCS trial: a study protocol for a randomised controlled trial. Trials 18, 179 (2017). [CrossRef]
- Crocerossa F, Cantiello F, Bagalá L, Sicoli F, Carbonara U, Manfredi C, Falagario U, Veccia A, Pandolfo SD, Napolitano L, Ferro M, Di Dio M, Mondaini N, Damiano R. Clinical Effects of Oral Supplementation of Gamma-Cyclodextrin Curcumin Complex in Male Patients with Moderate-To-Severe Benign Prostatic Hyperplasia-Related Lower Urinary Tract Symptoms. Urol Int. 2023;107(10-12):924-934. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).