Submitted:
14 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
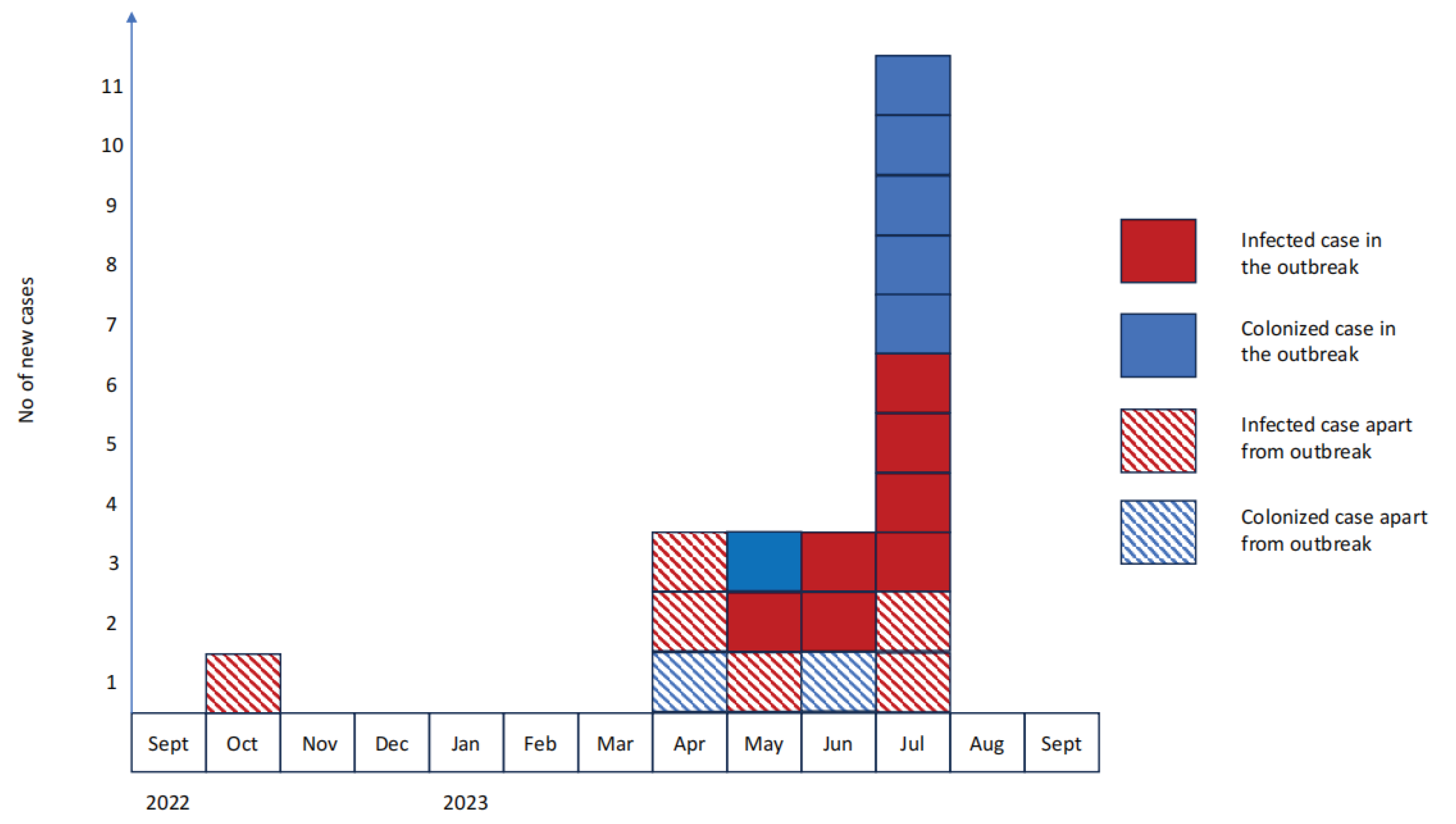
3.1. Case Series Description
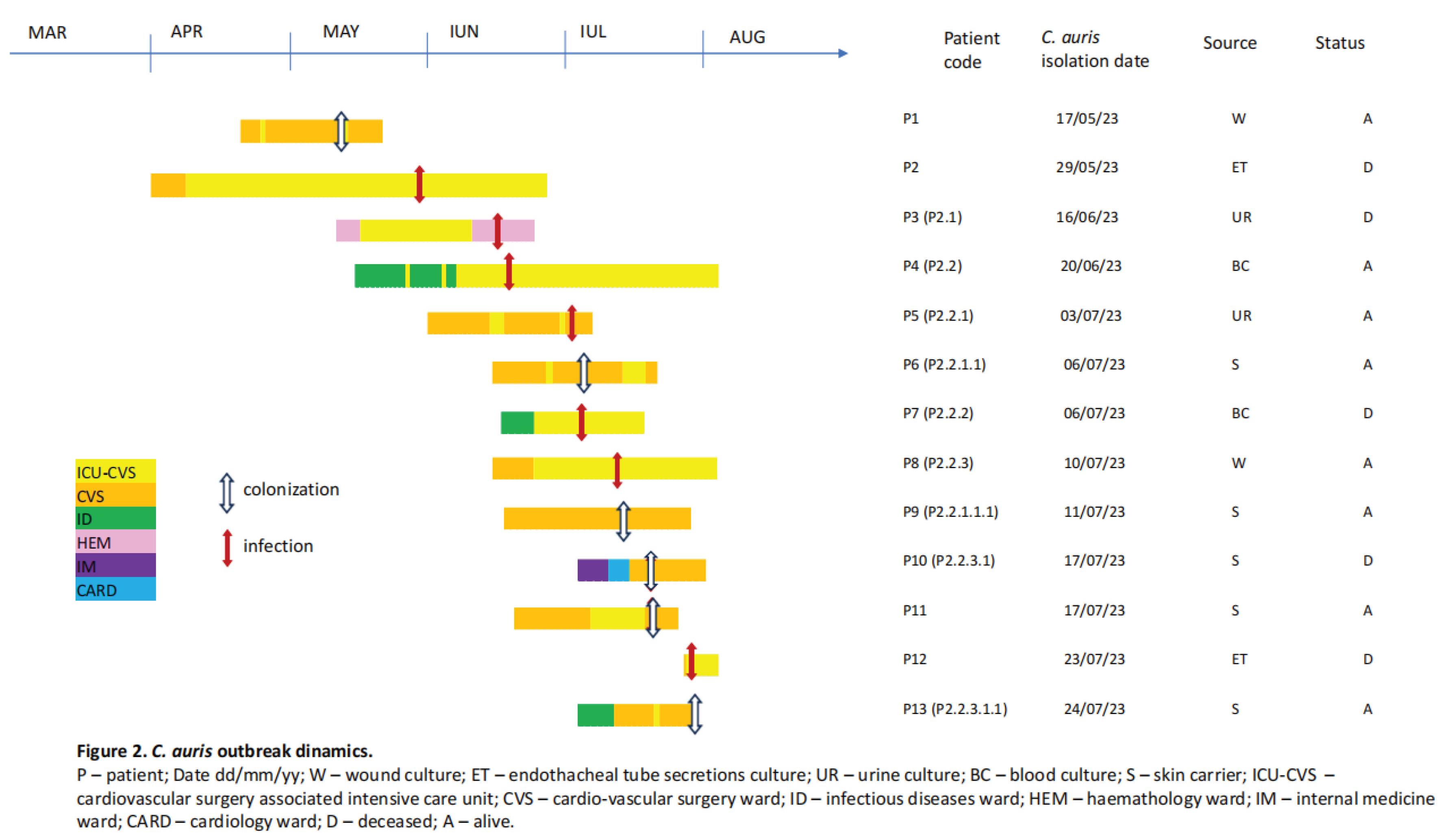
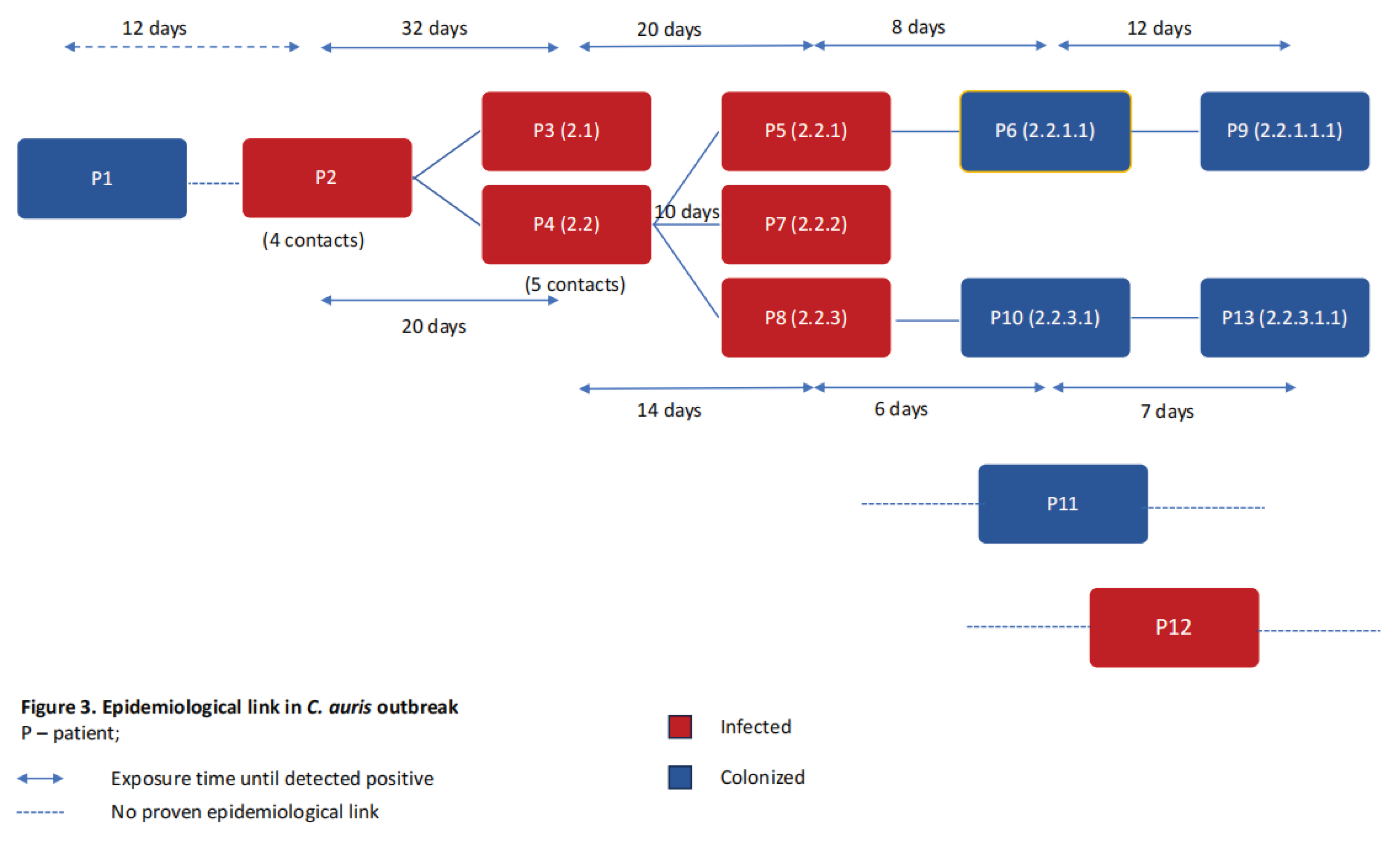
3.2. Outbreak Description
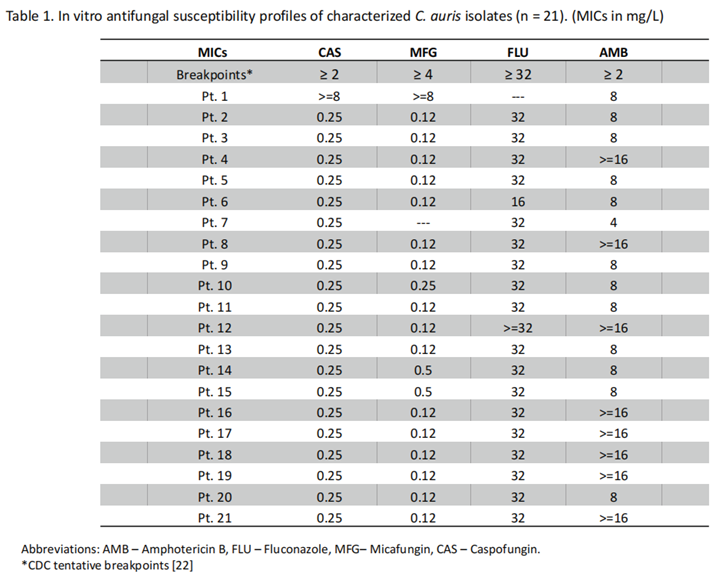
3.3. Antifungal Susceptibility Profile
3.4. Epidemiological Measures for Outbreak Control and Limitation
4. Discussions
4.1. C. auris Epidemiological Features
4.2. Present Therapies and New Directions
4.3. Unanswered Questions
5. Conclusions
References
- Arastehfar, A.; Lass-Flörl, C.; Garcia-Rubio, R.; Daneshnia, F.F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. J. Fungi 2020, 6, 138. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.C.; Fernández-Pereira, J.; Valentin, E.; Tormo-Mas, M.A.; Eraso, E.; Pemán, J.; de Groot, P.W. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. Int. J. Med Microbiol. 2018, 308, 812–818. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. Candida auris—the growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Chybowska, A.D.; Childers, D.S.; Farrer, R.A. Nine Things Genomics Can Tell Us About Candida auris. Front. Genet. 2020, 11, 351. [Google Scholar] [CrossRef]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira. A.; Antunes, F. Candida Auris, An Agent of Hospital-Associated Outbreaks: Which Challenging Issues Do We Need to Have in Mind?. Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef] [PubMed]
- Sekyere, J.O. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2019, 8, e00901. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida aurissp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D. ; Candida auris survey collaborative group Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. The Candida auris Alert: Facts and Perspectives. J. Infect. Dis. 2017, 217, 516–520. [Google Scholar] [CrossRef] [PubMed]
- A Pfaller, M.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Candida auris: a review of recent literature. GOV.UK. Available online: https://www.gov.uk/government/consultations/candida-auris-update-to-management-guidance/candida-auris-a-review-of-recent-literature. (accessed on 10 September 2023).
- Hardeep, K.; Khushbu, W.; Kusum, J.; Anamika, Y. Multidrug-resistant Candida auris: A global challenge. J. Appl. Biol. Biotechnol. 2021. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. (2022) Rapid risk assessment: Candida auris outbreak in healthcare facilities in northern Italy, 2019-2021; Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-candida-auris-outbreak-healthcare-facilities-northern-italy ; accessed on 10 September 2023.
- Borman, A.M.; Johnson, E.M. Candida auris in the UK: Introduction, dissemination, and control. PLOS Pathog. 2020, 16, e1008563. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO | Pan American Health Organization.; Epidemiological Alert: Candida auris outbreaks in health care services in the context of the COVID-19 pandemic; 2021. Available online: https://www.paho.org/en/documents/epidemiological-alert-candida-auris-outbreaks-health-care-services-context-covid-19 (accessed on 10 September 2023).
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit — Florida, July–August 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- CDC; Fungal Diseases and COVID-19; Available online: https://www.cdc.gov/fungal/covid-fungal.html accessed on 10 September 2023.
- Stanciu, A.M.; Florea, D.; Surleac, M.; Paraschiv, S.; Oțelea, D.; Tălăpan, D.; Popescu, G.A. First report of Candida auris in Romania: clinical and molecular aspects. Antimicrob. Resist. Infect. Control. 2023, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- CDC; Antifungal Susceptibility Testing and Interpretation. | Candida auris | Fungal Diseases |2022; Available online: https:// www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html ; (accessed on 1 September 2023).
- Troeman, D. P. R., Hazard, D., Timbermont, L., Malhotra-Kumar, S., van Werkhoven, C. H., Wolkewitz, M., Ruzin, A., Goossens, H., Bonten, M. J. M., Harbarth, S., Sifakis, F., Kluytmans, J. A. J. W., ASPIRE-SSI Study Team, Vlaeminck, J., Vilken, T., Xavier, B. B., Lammens, C., van Esschoten, M., Paling, F. P., Recanatini, C., … Van den Abeele, A. M. (2023). Postoperative Staphylococcus aureus Infections in Patients With and Without Preoperative Colonization. JAMA network open, 6(10), e2339793. https://doi.org/10.1001/jamanetworkopen.2023.39793 Erratum in: JAMA Netw Open. 2024 Mar 4;7(3):e244564. PMID: 37906196; PMCID: PMC10618839.
- ORDIN 1738 29/06/2022 - Portal Legislativ. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/257428 (accessed on 7 March 2024).
- National Institute of Public Health; Information and recommendation regarding the risk induced by colonization/ infection with Candida auris and measures to be applied in medical facilities; 2022. Available online: https://insp.gov.ro/download/informare-unitati-sanitare-privind-candida-auris/ ; (accessed on 29 February 2024).
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLOS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Piatti, G.; Sartini, M.; Cusato, C.; Schito, A.M. Colonization by Candida auris in critically ill patients: role of cutaneous and rectal localization during an outbreak. J. Hosp. Infect. 2021, 120, 85–89. [Google Scholar] [CrossRef]
- Schwartz, I.; Smith, S.; Dingle, T. Something wicked this way comes: What health care providers need to know about Candida auris. Can. Commun. Dis. Rep. 2018, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Rossow, J.; Ostrowsky, B.; Adams, E.; Greenko, J.; McDonald, R.; Vallabhaneni, S.; Forsberg, K.; Perez, S.; Lucas, T.; A Alroy, K.; et al. Factors Associated With Candida auris Colonization and Transmission in Skilled Nursing Facilities With Ventilator Units, New York, 2016–2018. Clin. Infect. Dis. 2020, 72, e753–e760. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New Clonal Strain ofCandida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2017, 31, e00029-17. [Google Scholar] [CrossRef] [PubMed]
- Al Maani, A.; Paul, H.; Al-Rashdi, A.; Wahaibi, A.A.; Al-Jardani, A.; Al Abri, A.M.A.; AlBalushi, M.A.H.; Al-Abri, S.; Al Reesi, M.; Al Maqbali, A.; et al. Ongoing challenges with healthcare-associated Candida auris outbreaks in Oman. J. Fungi 2019, 5, 101. [Google Scholar] [CrossRef]
- Araúz, A.B.; Caceres, D.H.; Santiago, E.; Armstrong, P.; Arosemena, S.; Ramos, C.; Espinosa-Bode, A.; Borace, J.; Hayer, L.; Cedeño, I.; et al. Isolation of Candida auris from 9 patients in Central America: Importance of accurate diagnosis and susceptibility testing. Mycoses 2017, 61, 44–47. [Google Scholar] [CrossRef]
- Forgács, L.; Borman, A.M.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]
- Tian, S.; Bing, J.; Chu, Y.; Chen, J.; Cheng, S.; Wang, Q.; Zhang, J.; Ma, X.; Zhou, B.; Liu, L.; et al. Genomic epidemiology of Candida auris in a general hospital in Shenyang, China: a three-year surveillance study. Emerg. Microbes Infect. 2021, 10, 1088–1096. [Google Scholar] [CrossRef]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef]
- Ruiz, G.B.; Lorenz, A. What do we know about the biology of the emerging fungal pathogen of humans Candida auris? Microbiol. Res. 2021, 242, 126621. [Google Scholar] [CrossRef]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzón, A.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; Brugnaro, P.; Solinas, M.; Scarparo, C.; Panese, S. Candida auris as an Emergent Public Health Problem: A Current Update on European Outbreaks and Cases. Healthcare 2023, 11, 425. [Google Scholar] [CrossRef]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Xin, H.; Mohiuddin, F.; Tran, J.; Adams, A.; Eberle, K. Experimental Mouse Models of Disseminated Candida auris Infection. mSphere 2019, 4, e00339–19. [Google Scholar] [CrossRef] [PubMed]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Alfouzan, W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms 2021, 9, 807. [Google Scholar] [CrossRef]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘New kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; McKloud, E.; Townsend, E.M.; Sherry, L.; Delaney, C.; Jones, B.L.; Williams, C.; Ramage, G. The comparative efficacy of antiseptics against Candida auris biofilms. Int. J. Antimicrob. Agents 2018, 52, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Sansom, S.; Gussin, G.M.; Singh, R.D.; Bell, P.B.; Jinal, E.B.; Makhija, J.; Froilan, R.; Saavedra, R.; Pedroza, R.; Thotapalli, C.; et al. Multicenter evaluation of contamination of the healthcare environment near patients with Candida auris skin colonization. Antimicrob. Steward. Heal. Epidemiology 2022, 2, s78–s79. [Google Scholar] [CrossRef]
- de Almeida, J.N.; Brandão, I.B.; Francisco, E.C.; de Almeida, S.L.R.; Dias, P.d.O.; Pereira, F.M.; Ferreira, F.S.; de Andrade, T.S.; Costa, M.M.d.M.; Jordão, R.T.d.S.; et al. Axillary Digital Thermometers uplifted a multidrug-susceptible Candidaauris outbreak among COVID-19 patients in Brazil. Mycoses 2021, 64, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.K.; Clancy, C.J. Lanyards as Source of a Candida auris Outbreak: As You Investigate the Environment, Do Not Overlook Hand Hygiene*. Crit. Care Med. 2021, 49, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.A.M.; Wyncoll, D.F.; Patel, A.B.; Ceesay, Y.M.; Newsholme, W.F.; Chand, M.F.; Mitchell, H.; Tan, M.B.; Edgeworth, J.D. Cloth Lanyards as a Source of Intermittent Transmission of Candida auris on an ICU*. Crit. Care Med. 2021, 49, 697–701. [Google Scholar] [CrossRef]
- Ong, C.W.; Chen, S.C.; Clark, J.E.; Halliday, C.L.; Kidd, S.E.; Marriott, D.J.; Marshall, C.L.; Morris, A.J.; Morrissey, C.O.; Roy, R.; et al. Diagnosis, management and prevention of Candida auris in hospitals: position statement of the Australasian Society for Infectious Diseases. Intern. Med. J. 2019, 49, 1229–1243. [Google Scholar] [CrossRef]
- Ilie, M.I. CANDIDA AURIS: THE UNWELCOME SUPERFUNGUS. FARMACIA 2023, 71, 225–235. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kumar, V.A.; Sharma, C.; Prakash, A.; Agarwal, K.; Babu, R.; Dinesh, K.R.; Karim, S.; Singh, S.K.; Hagen, F.; et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 33, 919–926. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First Three Reported Cases of Nosocomial Fungemia Caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Mizusawa, M.; Miller, H.; Green, R.; Lee, R.; Durante, M.; Perkins, R.; Hewitt, C.; Simner, P.J.; Carroll, K.C.; Hayden, R.T.; et al. Can Multidrug-Resistant Candida auris Be Reliably Identified in Clinical Microbiology Laboratories? J. Clin. Microbiol. 2017, 55, 638–640. [Google Scholar] [CrossRef]
- Odds, F.C.; Bernaerts, R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J. Clin. Microbiol. 1994, 32, 1923–1929. [Google Scholar] [CrossRef]
- de Jong, A.W.; Dieleman, C.; Carbia, M.; Tap, R.M.; Hagen, F. Performance of Two Novel Chromogenic Media for the Identification of Multidrug-Resistant Candida auris Compared with Other Commercially Available Formulations. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Shin, J.H.; Byun, S.A.; Choi, M.J.; Won, E.J.; Lee, D.; Lee, S.Y.; Chun, S.; Lee, J.H.; Choi, H.J.; et al. Candida auris Clinical Isolates from South Korea: Identification, Antifungal Susceptibility, and Genotyping. J. Clin. Microbiol. 2019, 57, 01624–18. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; Berkow, E.L.; Lockhart, S.R.; Andes, D.R. Pharmacodynamic Optimization for Treatment of Invasive Candida auris Infection. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Treatment and Management of C. auris Infections and Colonization | Candida auris | Fungal Diseases | CDC. (2022) Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html (accessed on 3 February 2024.
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of High-Level Echinocandin Resistance in a Patient With Recurrent Candida auris Candidemia Secondary to Chronic Candiduria. Open Forum Infect. Dis. 2019, 6, ofz262. [Google Scholar] [CrossRef]
- O'Brien, B.; Liang, J.; Chaturvedi, S.; Jacobs, J.L.; Chaturvedi, V. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe 2020, 1, e193–e194. [Google Scholar] [CrossRef] [PubMed]
- Balla, N.; Kovács, F.; Balázs, B.; Borman, A.M.; Bozó, A.; Jakab. ; Tóth, Z.; Kobaissi, O.; Majoros, L.; Kovács, R. Synergistic Interaction of Caspofungin Combined with Posaconazole against FKS Wild-Type and Mutant Candida auris Planktonic Cells and Biofilms. Antibiotics 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- John, L.L.; Thomson, D.D.; Bicanic, T.; Hoenigl, M.; Brown, A.J.; Harrison, T.S.; Bignell, E.M. Heightened Efficacy of Anidulafungin When Used in Combination with Manogepix or 5-Flucytosine against Candida auris In Vitro. Antimicrob. Agents Chemother. 2023, 67, e0164522. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.; Forgács, L.; Locke, J.B.; Kardos, G.; Nagy, F.; Kovács, R.; Szekely, A.; Borman, A.M.; Majoros, L. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2019, 74, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.Y.; Lewis, J.S.; Thompson, G.R. Rezafungin: a novel antifungal for the treatment of invasive candidiasis. Futur. Microbiol. 2021, 16, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Huband, M.D.; Rhomberg, P.R.; Bien, P.A.; Castanheira, M. Activities of Manogepix and Comparators against 1,435 Recent Fungal Isolates Collected during an International Surveillance Program (2020). Antimicrob. Agents Chemother. 2022, 66, e0102822. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Pappas, P.G.; Boffard, K.; Paruk, F.; Bien, P.A.; Tawadrous, M.; Ople, E.; Wedel, P.; Oborska, I.; Hodges, M.R. Clinical Efficacy and Safety of a Novel Antifungal, Fosmanogepix, in Patients with Candidemia Caused by Candida auris : Results from a Phase 2 Trial. Antimicrob. Agents Chemother. 2023, 67, e0141922. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Jaggavarapu, S.; Burd, E.M.; Weiss, D.S. Micafungin and amphotericin B synergy against Candida auris. Lancet Microbe 2020, 1, e314–e315. [Google Scholar] [CrossRef]
- CDC; Infection Prevention and Control for Candida auris. (2021) Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html (accessed on 10 September 2023).
- European Centre for Disease Prevention and Control. Candida auris in healthcare settings – Europe; (2016). Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Candida-in-healthcare-settings_19-Dec-2016.pdf (accessed on 10 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





