Submitted:
15 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
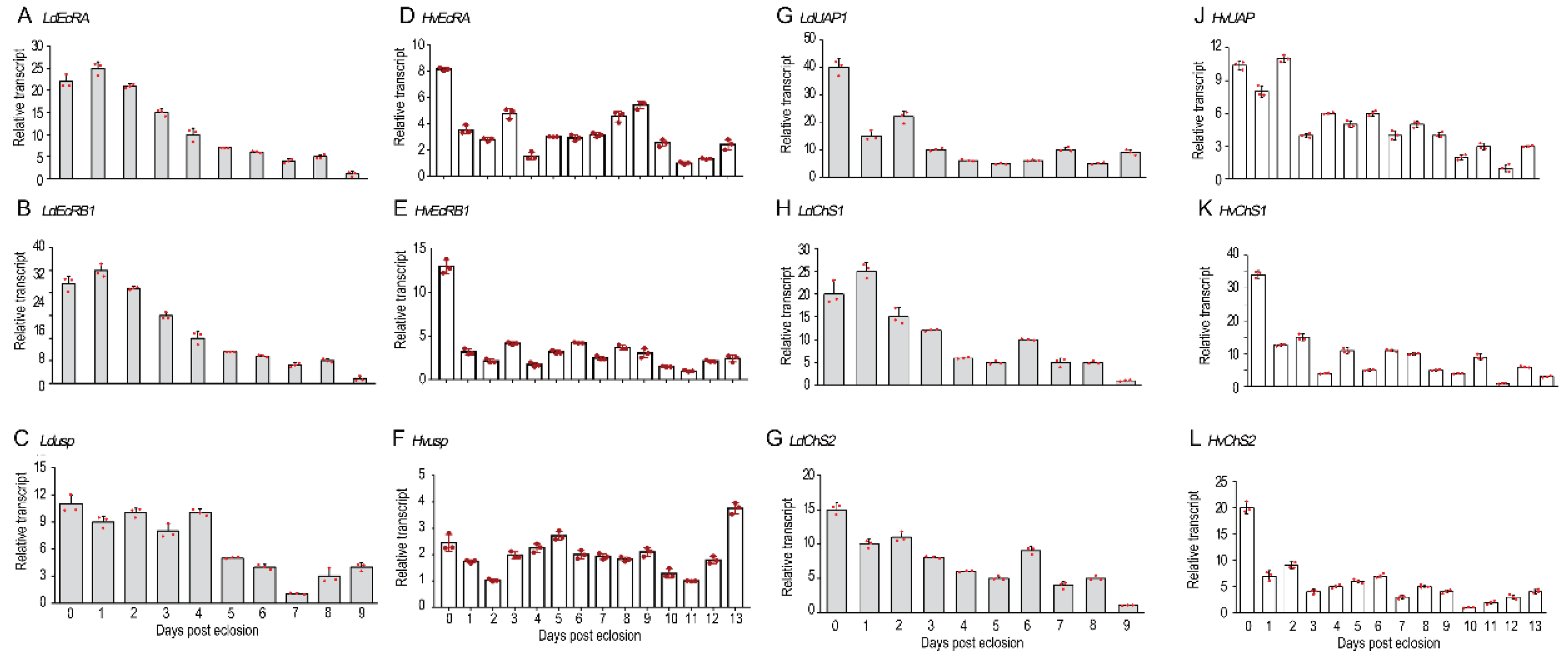
2.1. The Temporal Expression of Related Genes
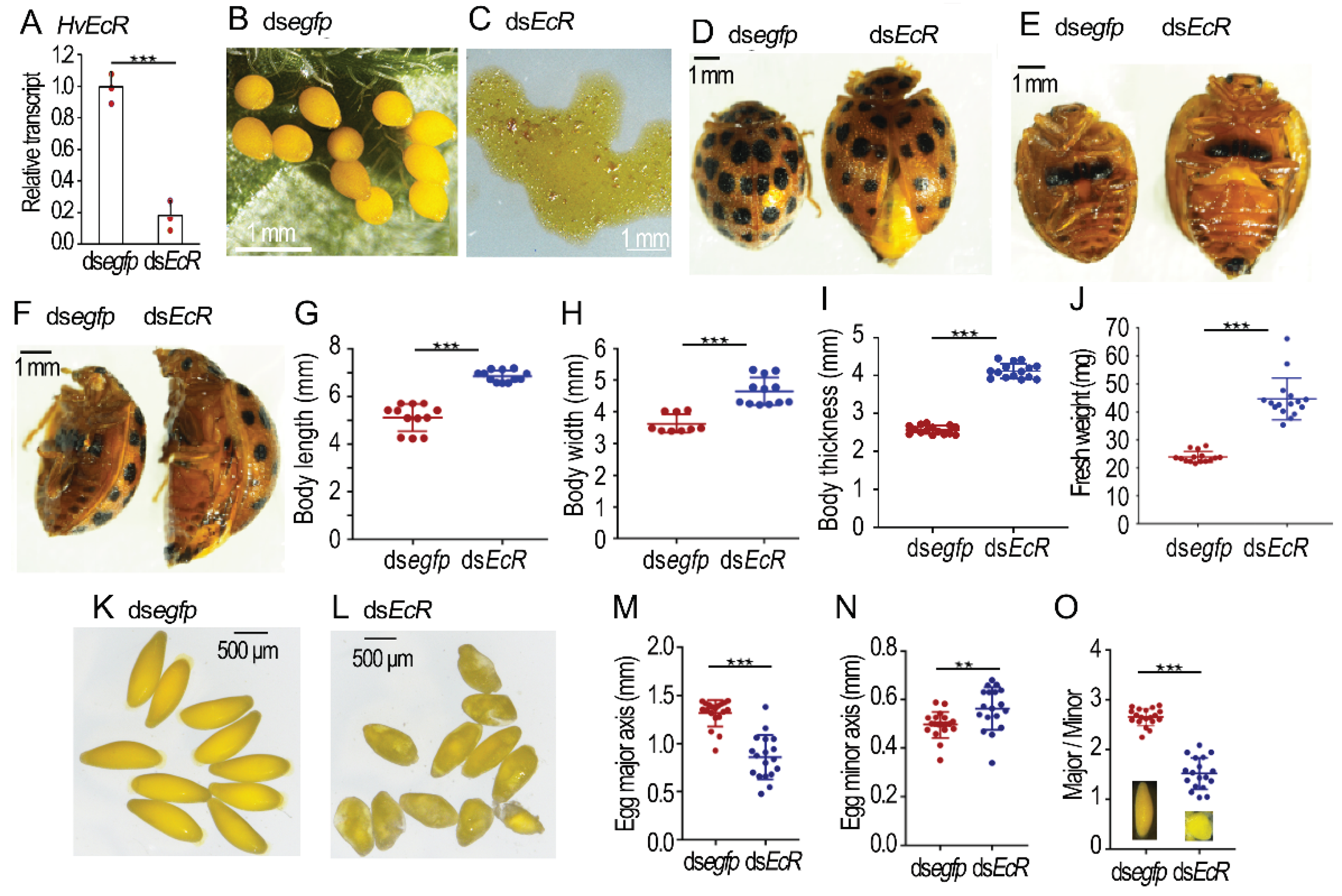
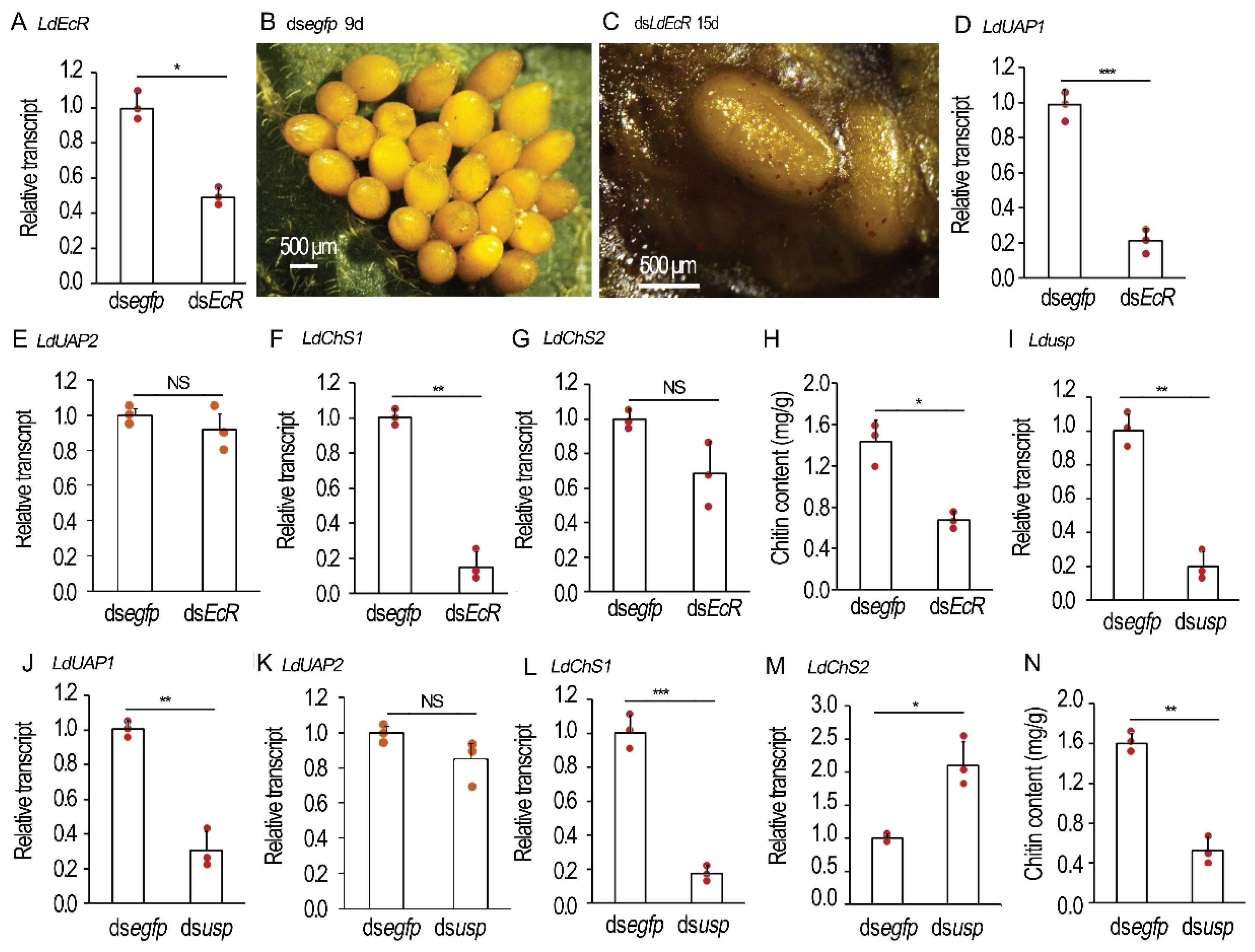
2.2. Knockdown of HvEcR Prevents Oviposition in H. vigintioctopunctata
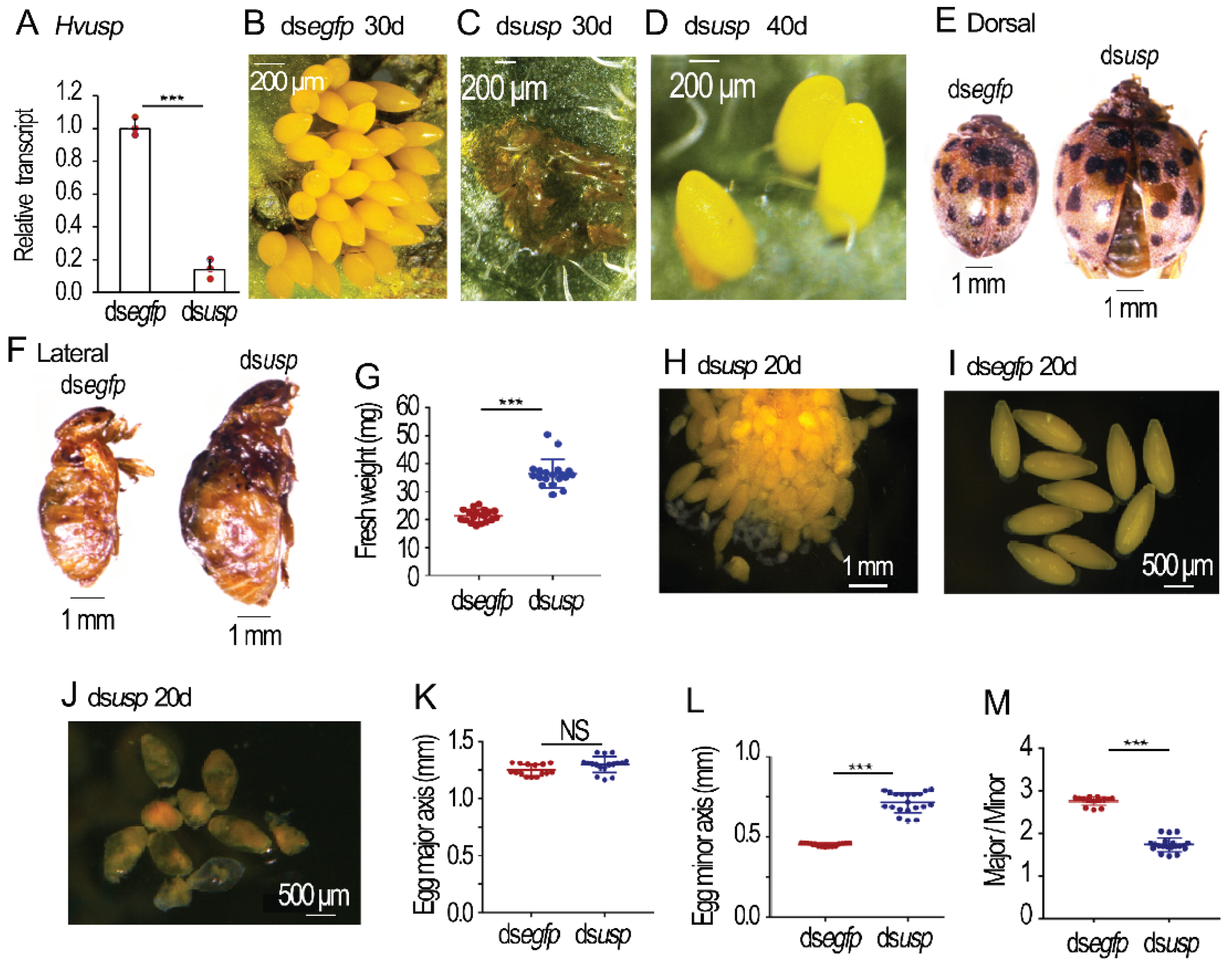
2.3. Depletion of Hvusp Affects Oogenesis in H. vigintioctopunctata
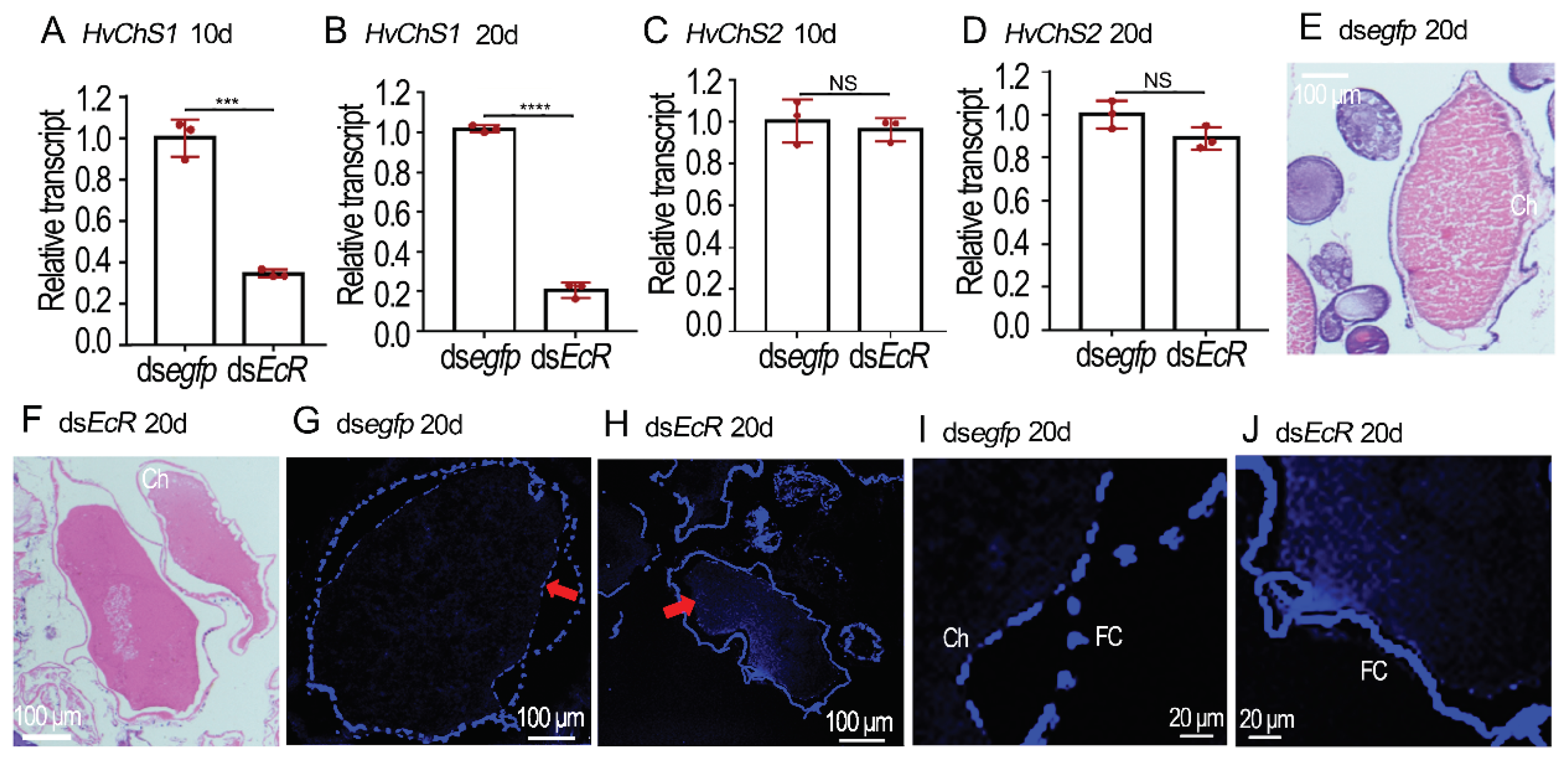
2.4. RNAi of HvEcR Affected CLS Deposition on H. vigintioctopunctata Oocytes
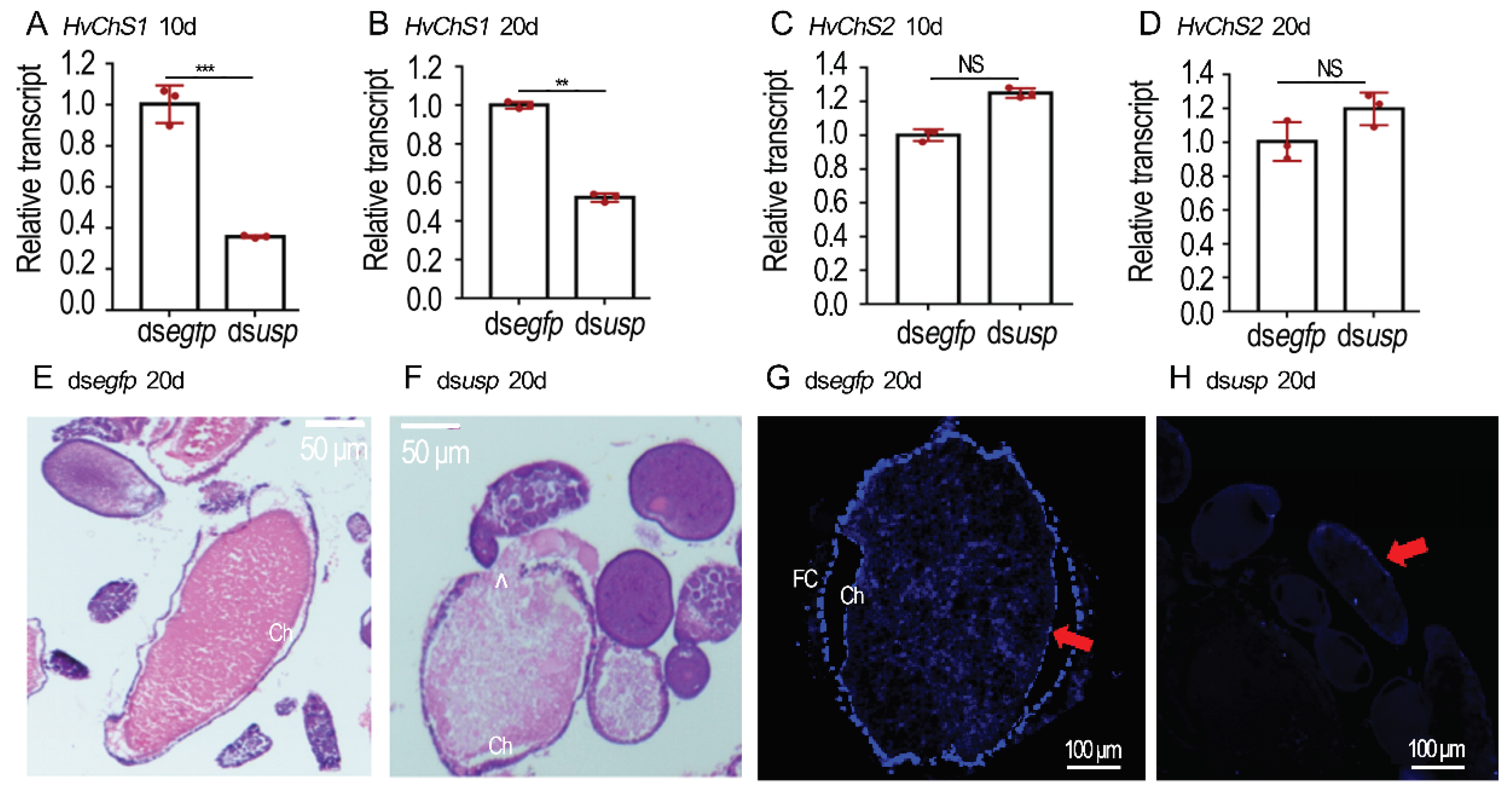
2.5. Silence of Hvusp Blocked CLS Deposition on H. vigintioctopunctata Oocytes
2.6. Shortage of 20E Signal Prevents CLS Contents in L. decemlineata Ovaries
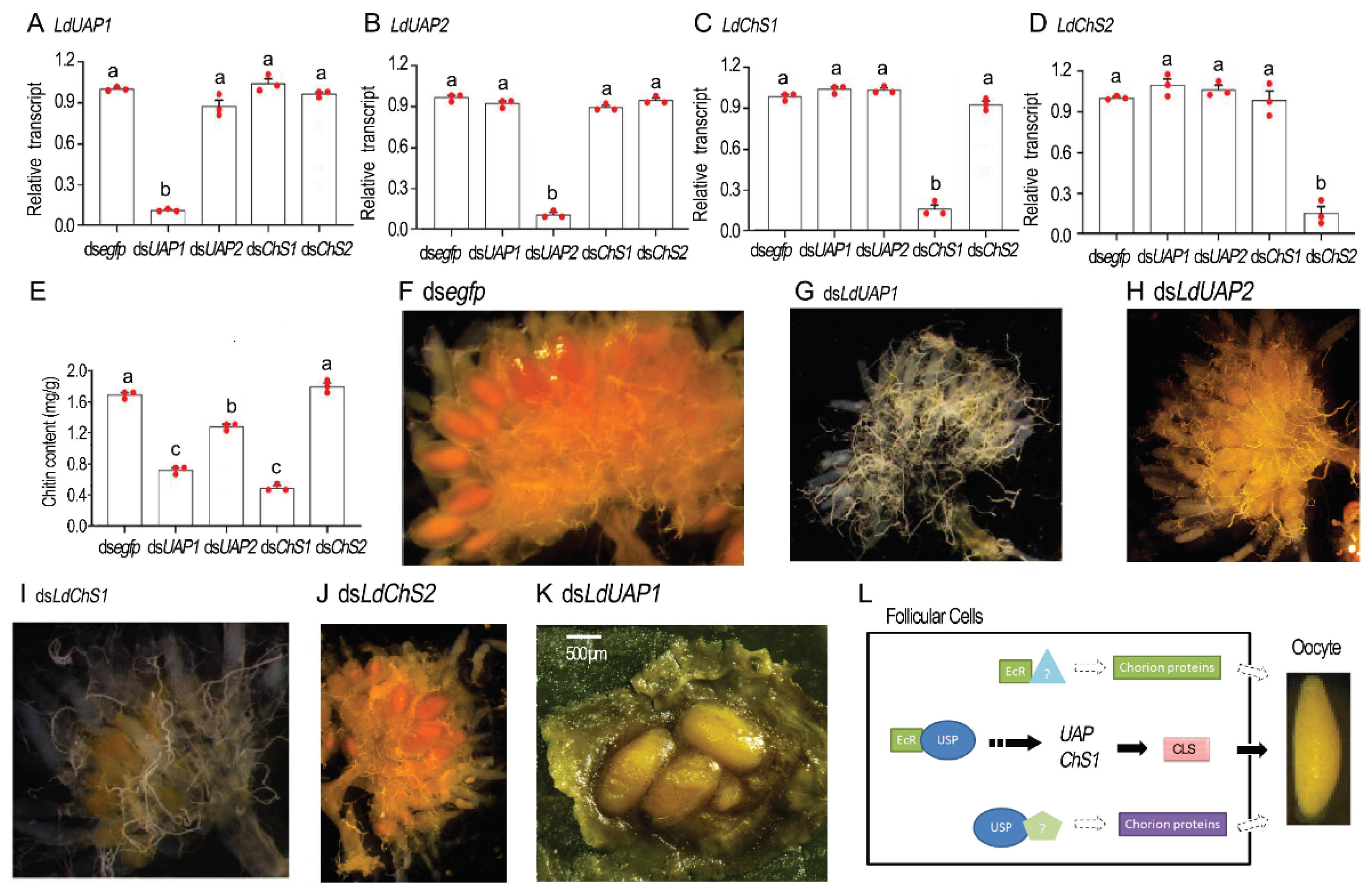
2.7. Chitin Defeciency Causes Similar Defects on L. decemlineata Oocytes
3. Discussion
3.1. 20 E Signal Triggers Choriogenesis
3.2. 20E Signal Triggers the Production of a CLS
3.3. Does 20E Signal Tune the Production of Chorion Proteins?
4. Materials and Methods
4.1. Insect Rearing
4.2. Synthesis of dsRNAs
4.3. Injection of dsRNAs
4.4. Quantitative Real-Time PCR
4.5. Hematoxylin-Eosin (HE) Staining
4.6. Chitin Staining with CALCOFLUOR
4.7. Chitin Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papantonis, A.; Swevers, L.; Iatrou, K. Chorion genes: A landscape of their evolution, structure, and regulation. Annu Rev Entomol 2015, 60, 177–194. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front Cell Dev Biol 2021, 8, 593613. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of endocrine system in the regulation of female insect reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sun, Z.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 2010, 40, 405–414. [Google Scholar] [CrossRef]
- Eid, D.M.; Chereddy, S.C.R.R.; Palli, S.R. The effect of E93 knockdown on female reproduction in the red flour beetle, Tribolium castaneum. Arch Insect Biochem Physiol 2020, 104, e21688. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, A.; Palli, S.R. The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum. J Insect Physiol 2010, 56, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Z.; Bi, J.; Feng, Q.; Beerntsen, B.T.; Song, Q. Neuropeptide bursicon influences reproductive physiology in Tribolium Castaneum. Front Physiol 2021, 12, 717437. [Google Scholar] [CrossRef] [PubMed]
- Swevers, L.; Iatrou, K. Early establishment and autonomous implementation of a developmental program controlling silk moth chorion gene expression. Dev Biol 1992, 150, 12–22. [Google Scholar] [CrossRef]
- Petri, W.H.; Wyman, A.R.; Kafatos, F.C. Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol 1976, 49, 185–199. [Google Scholar] [CrossRef]
- Tootle, T.L.; Williams, D.; Hubb, A.; Frederick, R.; Spradling, A.C. Drosophila eggshell production: Identification of new genes and coordination by Pxt. PLoS ONE 2011, 6, e19943. [Google Scholar] [CrossRef]
- Kafatos, F.C.; Spoerel, N.; Mitsialis, S.A.; Nguyen, H.T.; Romano, C.; Lingappa, J.R.; Mariani, B.D.; Rodakis, G.C.; Lecanidou, R.; Tsitilou, S.G. Developmental control and evolution in the chorion gene families of insects. Adv Genet 1987, 24, 223–242. [Google Scholar]
- Moreira, M.F.; Dos Santos, A.S.; Marotta, H.R.; Mansur, J.F.; Ramos, I.B.; Machado, E.A.; Souza, G.H.M.F.; Eberlin, M.N.; Kaiser, C.R.; Kramer, K.J.; et al. A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem Mol Biol 2007, 37, 1249–1261. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Mu, L.-L.; Jin, L.; Anjum, A.A.; Li, G.-Q. Silencing uridine diphosphate N-acetylglucosamine pyrophosphorylase gene impairs larval development in Henosepilachna vigintioctopunctata. Pest Manag Sci 2022, 78, 3894–3902. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Mu, L.-L.; Jin, L.; Anjum, A.A.; Li, G.-Q. RNAi for chitin synthase 1 rather than 2 causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Pestic Biochem Physiol 2021, 178, 104934. [Google Scholar] [CrossRef]
- Shi, J.-F.; Mu, L.-L.; Chen, X.; Guo, W.-C.; Li, G.-Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). International Journal of Biological Sciences 2016, 12, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-F.; Fu, J.; Mu, L.-L.; Guo, W.-C.; Li, G.-Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylase genes LdUAP1 and LdUAP2 are specialized for synthesis of chitin in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochemistry and Molecular Biology 2016, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-F.; Mu, L.-L.; Guo, W.-C.; Li, G.-Q. Identification and hormone induction of putative chitin synthase genes and splice variants in Leptinotarsa decemlineata (Say). Archives of Insect Biochemistry and Physiology 2016, 92, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 2008, 38, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.G.; Rezende, G.L.; Lamers, G.E.; van der Zee, M. The extraembryonic serosa protects the insect egg against desiccation. P Roy Soc B Biol Sci 2013, 280, 20131082. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Jin, L.; Li, G.-Q. The timings of vitellogenesis and choriogenesis in the Henosepilachna vigintioctopunctata oocytes. Journal of Asia-Pacific Entomology 2024, 27, 102183. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Jin, L.; Li, G.-Q. RNAi-mediated functional analysis reveals the regulation of oocyte vitellogenesis by ecdysone signaling in two Coleoptera species. Biology 2023, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.R.; Beeman, R.W. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix, respectively. Insect Mol Biol 2005, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Benrabaa, S.; Orchard, I.; Lange, A.B. A critical role for ecdysone response genes in regulating egg production in adult female Rhodnius prolixus. PLoS ONE 2023, 18, e0283286. [Google Scholar] [CrossRef] [PubMed]
- Benrabaa, S.A.M.; Orchard, I.; Lange, A.B. The role of ecdysteroid in the regulation of ovarian growth and oocyte maturation in Rhodnius prolixus, a vector of Chagas disease. J Exp Biol 2022, 225, jeb244830. [Google Scholar] [CrossRef]
- Knapp, E.M.; Li, W.; Singh, V.; Sun, J. Nuclear receptor Ftz-f1 promotes follicle maturation and ovulation partly via bHLH/PAS transcription factor Sim. Elife 2020, 9, e54568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, Q.; Xu, C.; Wang, R.; Wang, H.; Song, Y.; Zeng, R. Regulation of NlE74A on vitellogenin may be mediated by angiotensin converting enzyme through a fecundity-related SNP in the brown planthopper, Nilaparvata lugens. Comp Biochem Physiol 2018, 225, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, Y.; Gao, H.; Lin, X. The direct interaction between E93 and Kr-h1 mediated their antagonistic effect on ovary development of the brown planthopper. Int J Mol Sci 2019, 20, 2431. [Google Scholar] [CrossRef] [PubMed]
- Gujar, H.; Palli, S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci Rep 2016, 6, 35546. [Google Scholar] [CrossRef]
- Mansur, J.F.; Alvarenga, E.S.; Figueira-Mansur, J.; Franco, T.A.; Ramos, I.B.; Masuda, H.; Melo, A.C.; Moreira, M.F. Effects of chitin synthase double-stranded RNA on molting and oogenesis in the Chagas disease vector Rhodnius prolixus. Insect Biochem Mol Biol 2014, 51, 110–121. [Google Scholar] [CrossRef]
- Lou, Y.H.; Pan, P.L.; Ye, Y.X.; Cheng, C.; Xu, H.J.; Zhang, C.X. Identification and functional analysis of a novel chorion protein essential for egg maturation in the brown planthopper. Insect Mol Biol 2018, 27, 393–403. [Google Scholar] [CrossRef]
- Lou, Y.H.; Shen, Y.; Li, D.T.; Huang, H.J.; Lu, J.B.; Zhang, C.X. A mucin-like protein is essential for oviposition in Nilaparvata lugens. Front Physiol 2019, 10, 551. [Google Scholar] [CrossRef]
- Ahmed, S.; Seo, K.; Kim, Y. An ovary-specific mucin is associated with choriogenesis mediated by prostaglandin signaling in Spodoptera exigua. Arch Insect Biochem Physiol 2021, 106, e21748. [Google Scholar] [CrossRef]
- Margaritis, L.H. The egg-shell of Drosophila melanogaster III. Covalent crosslinking of the chorion proteins involves endogenous hydrogen peroxide. Tissue Cell 1985, 17, 553–559. [Google Scholar] [CrossRef]
- Mindrinos, M.N.; Petri, W.H.; Galanopoulos, V.K.; Lombard, M.F.; Margaritis, L.H. Crosslinking of the Drosophila chorion involves a peroxidase. Wilehm Roux Arch Dev Biol 1980, 189, 187–196. [Google Scholar] [CrossRef]
- Lu, J.B.; Zhang, M.Q.; Li, L.C.; Zhang, C.X. DDC plays vital roles in the wing spot formation, egg production, and chorion tanning in the brown planthopper. Arch Insect Biochem Physiol 2019, 101, e21552. [Google Scholar] [CrossRef]
- Wang, P.; Granados, R.R. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc Natl Acad Sci U S A 1997, 94, 6977–6982. [Google Scholar] [CrossRef] [PubMed]
- Ze, L.-J.; Wang, P.; Peng, Y.-C.; Jin, L.; Li, G.-Q. Silencing tyrosine hydroxylase or dopadecarboxylase gene disrupts cuticle tanning during larva-pupa-adult transformation in Henosepilachna vigintioctopunctata. Pest Manag Sci 2022, 78, 3880–3893. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Wang, X.; Ding, Q.; Said, F.; Gao, X.; Desneux, N.; Song, D. RNAi-mediated knockdown of chitin synthase 1 (CHS1) gene causes mortality and decreased longevity and fecundity in Aphis gossypii. Insects 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Harethardottir, H.M.; Male, R.; Nilsen, F.; Dalvin, S. Chitin synthases are critical for reproduction, molting, and digestion in the salmon louse (Lepeophtheirus salmonis). Life 2021, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Rios, T.; Bomfim, L.; Ramos, I. The transition from vitellogenesis to choriogenesis triggers the downregulation of the UPR sensors IRE1 and PERK and alterations in the ER architecture in the follicle cells of the vector Rhodnius prolixus. Cell Tissue Res 2022, 387, 63–74. [Google Scholar] [CrossRef]
- Bernardi, F.; Romani, P.; Tzertzinis, G.; Gargiulo, G.; Cavaliere, V. EcR-B1 and Usp nuclear hormone receptors regulate expression of the VM32E eggshell gene during Drosophila oogenesis. Dev Biol 2009, 328, 541–551. [Google Scholar] [CrossRef]
- Dutko-Gwóźdź, J.; Gwóźdź, T.; Orłowski, M.; Greb-Markiewicz, B.; Duś, D.; Dobrucki, J.; Ozyhar, A. The variety of complexes formed by EcR and Usp nuclear receptors in the nuclei of living cells. Mol Cell Endocrinol 2008, 294, 45–51. [Google Scholar] [CrossRef]
- Costantino, B.F.; Bricker, D.K.; Alexandre, K.; Shen, K.; Merriam, J.R.; Antoniewski, C.; Callender, J.L.; Henrich, V.C.; Presente, A.; Andres, A.J. A novel ecdysone receptor mediates steroid-regulated developmental events during the mid-third instar of Drosophila. PLoS Genet 2008, 4, e1000102. [Google Scholar] [CrossRef]
- Sharma, V.; Pandey, A.K.; Kumar, A.; Misra, S.; Gupta, H.P.K.; Gupta, S.; Singh, A.; Buehner, N.A.; Ravi Ram, K. Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor. PLoS Genet 2017, 3, e1006788. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sakai, A.; Magata, F.; Ogura, T.; Miyashita, M.; Miyagawa, H. Molecular cloning of the ecdysone receptor and the retinoid X receptor from the scorpion, Liocheles australasiae. FEBS J 2007, 274, 6191–6203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Zhang, Z.L.; Robinson, G.E.; Palli, S.R. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem 2007, 282, 37605–37617. [Google Scholar] [CrossRef] [PubMed]
- Bodofsky, S.; Koitz, F.; Wightman, B. Conserved and exapted functions of nuclear receptors in animal development. Nucl Receptor Res 2017, 4, 101305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Miura, K.; Chen, L.; Raikhel, A.S. Cyclicity of mosquito vitellogenic ecdysteroid-mediated signaling is modulated by alternative dimerization of the RXR homologue Ultraspiracle. Proc Natl Acad Sci U S A 2003, 100, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Y.; Deng, P.; Zhang, Q.; Li, A.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Ecdysone receptor isoforms play distinct roles in larval-pupal-adult transition in Leptinotarsa decemlineata. Insect Sci 2020, 27, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Y.; Deng, P.; Li, A.; Zhang, Q.; Mu, L.-L.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Functional characterization of ultraspiracle in Leptinotarsa decemlineata using RNA interference assay. Insect Mol Biol 2019, 28, 676–688. [Google Scholar] [CrossRef]
- Xu, P.; Ze, L.-J.; Kang, W.-N.; Wu, J.-J.; Jin, L.; Ali, A.A.; Li, G.-Q. Functional divergence of white genes in Henosepilachna vigintioctopunctata revealed by RNA interference. Insect Molecular Biology 2020, 29, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Ze, L.-J.; Xu, P.; Kang, W.-N.; Wu, J.-J.; Jin, L.; Anjum, A.A.; Li, G.-Q. Disruption of ommochrome biosynthesis affects eye coloration, phototaxis and climbing in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Q.; Guo, W.-C.; Wan, P.-J.; Zhou, L.-T.; Ren, X.-L.; Tursun, A.; Fu, K.-Y.; Li, G.-Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Research Notes 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, S.; Guo, M.; Ye, C.; Qiu, B.; Wu, J.; Yang, C.; Pan, H. Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front Physiol 2018, 9, 1614. [Google Scholar] [CrossRef]
- Reissig, J.L.; Strominger, J.L.; Leloir, L.F. A modified colorimetric method for the estimation of N-acetylamino sugars. Journal of Biological Chemistry 1955, 217, 959–966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




