1. Introduction
1.1. COVID-19-Related Viruses
SARS-CoV-2 was first observed among patients in November 2019 in Wuhan, China [
1,
2,
3,
4]. Its sequence was quickly determined, and SARS-CoV-2 was found to have approximately 80% genetic similarity with the 2003 SARS-CoV (SARS-CoV-1). A search for closely related CoVs pointed to a 2013 sample (bat-RaTG13) obtained in a cave in Yunnan that had a 96.4% sequence identity with the SARS-CoV-2 [
5,
6]. Interestingly, two sets of pangolin-CoV samples obtained were found to have approximately 90% similarities to SARS-CoV-2 [
7,
8,
9]. One set was obtained from smuggled pangolins confiscated in Guangdong (GD) province in 2019. The other samples were also from smuggled pangolins but from Guangxi (GX) province (2017-18) [
7,
8,
9,
10]. Later, similar data were found in bat and pangolin samples from Laos and Vietnam, respectively [
11,
12]. The series of CoVs found among bats in Laos were labelled BANAL, with one of the samples (BANAL-52) possessing a 96.8% genetic proximity to SARS-CoV-2 that surpasses RaTG13 [
3,
11].
The computational models used in this paper, Shell Disorder Models (SDMs), were built before the COVID-19 emergence. SDMs used AI (Neural Network) [
13,
14,
15,
16] that measures levels of disordered residues in two CoV shell proteins, membrane (M) and nucleocapsid (N) [
17]. A specific characteristics of the COVID-19-related viruses is that they all have abnormally hard outer shells (i.e., are characterized by low PID (Percentage of Intrinsic Disorder) values of their M proteins, PID
M), which might be associated with CoVs of burrowing animals, such as pangolins and rabbits [
18,
19,
20]. Viruses with hard outer shells, such as dengue and rabies viruses, are often associated with saliva that has powerful antimicrobial enzymes that could damage the virion [
21,
22,
23,
24]. It is believed that the reason for the high contagiousness of SARS-CoV-2 has to do with the ability of the virus to resist the onslaught of salivary antimicrobial enzymes as the result of its hard outer shell, which allows large amount of virus shedding by patients [
25]. This paradigm provides an important evolutionary connection between SARS-CoV-2 and pangolin, which is a burrowing animal. Viral transmission of burrowing animals is likely to involve feces that are buried for a long time and therefore necessitate the transmitted virus to persist in the ground, for which a hard outer shell id needed [
18,
19,
20].
The N protein, on the other hand, plays important roles in the replication process and thus greater N disorder provides the means necessary for more rapid and efficient replication [
17]. It is for this reason that greater disorder at the inner shell has been found to correlate with greater virulence in many viruses, such as DENV, Ebola virus (EBOV), SARS-CoV-1/2, and Nipah virus (NiV) [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. This prediction represents a part of the Shell Disorder Model (SDM). Upon an inspection of the PID
N of pangolin-CoVs, a larger range of the PID
N values is observed. Most interestingly, according to SDM, the pangolin-CoV 2017 [
18] is predicted to be attenuated, especially when compared to the Wuhan SARS-CoV-2 strain (Wuhan-Hu-1). Some of the co-authors of this paper published this observation in 2020 [
18]. Later, the other co-authors published papers, independently of the aforementioned 2020 computational study, which experimentally showed that Pang2017 is attenuated [
28,
29]. Similar characteristics were later found in the case of SARS-CoV-2 Omicron [
25,
26,
30,
31,
32,
33,
34]. Intriguingly, the GD pangolin-CoV from 2019 (Pang2019) did not show any such attenuation experimentally [
35]. Similar results involving greater aggressiveness can be found in some of the bat-CoV found in Laos, particularly BANAL-236 [
11]. Even as puzzling as these results are to some researchers, all these results did not come as a surprise to many of the co-authors as the SDMs had consistently predicted all these peculiarities.
1.2. Peculiar Relationships between SARS-CoV-2, Omicron, and Pangolin-CoVs
Since the first discovery of SARS-CoV-2 in 2019 (Wuhan-Hu-1), SARS-CoV-2 has undergone extensive mutagenesis. The variants are identified by mutations found at the S protein and given labels such as Alpha and Beta [
36,
37]. In November 2021, the Omicron variant was first detected in South Africa. Omicron is enigmatic by itself as the mutations found in the virus are unlike any other known variants [
38]. These observations raised important questions, such as where was the direct Omicron ancestor hiding all along? Why was it previously never noticed in the human population? Another characteristic of Omicron has to do with its attenuated nature. This observation was made very early in the spread in South Africa, when physicians observed relatively mild symptoms among patients infected by Omicron, especially when compared to the patients infected by the previous SARS-CoV-2 variants [
31,
32,
33,
34]. Upon inspection of the shell disorder, we were able to reproduce the mentioned observation of attenuation by detecting that the Omicron PID
N was similar to that of Pang2017. A group of us published the corresponding results in 2022 [
25,
26,
30]. The present study examines the computational and experimental data that are pertained to the peculiar relationships among pangolin-CoV, non-Omicron SARS-CoV-2, and Omicron.
2. Materials and Methods
2.1. Computational Biology: SDMs and Protein Intrinsic Disorder
The set of the Shell Disorder Models (SDMs) consist of three closely related models that measure the intrinsic disorder levels in the shell proteins of various viruses in order to predict their properties, such as spread and virulence. SDMs use PONDR®-VLXT (
www.pondr.com) [
15] algorithm to determine the levels of predicted order in proteins. Protein intrinsic disorder is defined as an entire protein or a portion of a protein that has no unique 3D structure. Disorder allows for many important protein roles, such as more efficient molecular recognition in protein-protein/RNA/glycoprotein/DNA/lipid binding [
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55]. PONDR®-VLXT is a neural network that is trained using known sequences of proteins that are ordered and disordered [
13,
14,
15]. It has been successfully used to study a large number of viruses, including Dengue virus (DENV), Ebola virus (EBOV), Nipah virus (NiV), HIV, influenza virulence, MERS-CoV, SARS-CoV-1, and SARS-CoV-2 [
56,
57,
58,
59,
60,
61]. PONDR®-VLXT predicts if each residue is ordered or disordered given the sequence of a protein. An important ratio that will be used extensively to determine the level of disorder of a protein is the percentage of intrinsic disorder (PID), which is defined as the number of disordered residues divided by the total number of residues multiplied by 100. Protein sequences for these analyses were taken from NCBI and UniProt [
62].
The database containing information on disorder and sequences of viral proteins was stored in MYSQL [
63] using JAVA [
64] and Python [
65]. The phylogenetic trees were constructed using CLUSTAL Omega, which is available at the EMBL-EBI website [
66]. The PONDR®-VLXT plots were generated using OpenOffice Spreadsheet [
67] and adapted for publication using GIMP [
68]. Statistical analyses were performed using R package [
69]. NCBI BLASTP (
https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins) [
70] was used to determine genetic similarities of proteins. The corresponding schematic diagrams were drawn using both OpenOffice [
67] and GIMP [
68].
2.2. Experimental Biology: Cells and Viruses
SARS-CoV-2 XBB.1.16 isolate/2023 was passaged three times in Vero cells, and Pang2017 (previously named GX_P2V) infectivity titer was determined by plaque assay. The Pangolin coronavirus was derived from a plaque clone of the early culture of Pang2017. This clone has a truncated 3´-UTR mutation and exhibits cross-neutralization epitopes with SARS-CoV-2 [
29,
71]. Vero cells were purchased from ATCC and cultured in Minimum Essential Medium (MEM, Gibco) supplemented with 10% vol/vol FBS (PAN) and 1% vol/vol Anti-Anti (Gibco). Viral infection was conducted by first removing the culture medium, then adding the virus stock at a multiplicity of infection (MOI) of 0.01, and rocking at room temperature for 2 hours. After two washes with phosphate-buffered saline (PBS, Hyclone), the cells were further incubated in MEM supplemented with 2% vol/vol FBS (PAN) and 1% vol/vol Anti-Anti (Gibco) at 37°C in a 5% CO
2 incubator. After 48 hours, the culture supernatant was harvested, aliquoted, and stored at -80°C.
2.3. Experimental Biology: Viral One-Step Growth Curve
A day prior to infection, Vero cells were diluted in MEM complete medium at a density of 3.3×105 Vero/mL and seeded at 3 mL/well in six-well plates. Cells in the six-well plates were infected with virus stocks at MOI 0.1 and incubated at room temperature, with even distribution ensured by rocking. After thorough washing with PBS, cells were maintained in MEM supplemented with 2% vol/vol FBS and 1% vol/vol Anti-Anti and incubated at 37°C in a 5% CO2 cell incubator (PHCbi). At indicated time points (2, 4, 6, 8, 10, 12, 24, 36, 48, 72, 120 hours post-infection), 200 μL of the culture supernatant was collected and stored at -80°C. The lost volume was replenished with fresh pre-warmed medium with a consistent serum concentration of 2% FBS. This experiment was conducted in triplicate, and viral RNA in the supernatant was quantified by the following RT-qPCR.
Specifically, viral RNA was extracted using the cell/tissue total RNA extraction kit (Nobelab, China) as per the manufacturer's instructions and reverse transcribed using the HiScript III RT SuperMix for qPCR (+gDNA wiper) kit (Vazyme, China). Quantitative PCR targeting the viral N gene was performed using the QuantiNova Probe PCR Kit (QIAGEN, Germany). The primer and probe sequences for Pang2017 detection were: forward primer: TCTTCCTGCTGCAGATTTGGAT, reverse primer: TTACACATTAGGGCTCTTCCATATAGG, and probe: FAM-TGCAGACCACACAAGGCAGATGGGC-MGB. The primer and probe sequences for XBB.1.16 detection were: forward primer: TCTTCCTGCTGCAGATTTGGAT, reverse primer: ATTCTGCACAAGAGTAGACTATATATCGT, and probe: FAM-TGCAGACCACACAAGGCAGATGGGC-MGB. Following the manufacturer's instructions, primers and probes were used at final concentrations of 200 nM and 100 nM respectively. Plasmids containing amplification fragments of Pang2017 and XBB.1.16 were used as standards for quantifying the N gene of the two viruses.
2.4. Experimental Biology: Cytopathic Effect Analysis (CPE) and Plaque Assay
For CPE analysis, the virus infection procedure was same as described above. At designated time points, cell images were captured under an optical microscope (Olympus, Japan) using FCSnap software at a fixed magnification (100×). Plaque assay was conducted using methylcellulose (a semi-solid, low viscosity overlay) to determine plaque size and plaque-forming unit titers, as previously described [
29]. Briefly, Vero cells were seeded in six-well plates, and upon reaching full confluence, they were infected with serial dilutions of virus stock in virus culture medium with 2% FBS. Each well received 500 μL of the diluted virus, which was rocked at room temperature for 2 hours. Subsequently, the infection medium was discarded, cells were washed with PBS, and 3 mL of 1% wt/vol methylcellulose (prepared by mixing 2 × MEM with 2% methylcellulose at a 1:1 ratio, with FBS and Anti-Anti adjusted to 2% and 1% vol/vol respectively) was added. After incubating at 37°C in a 5% CO
2 incubator for 72 hours, the cells were fixed and stained for the plaque analyses.
3. Results
3.1. The Shell Disorder Models (SDMs)
The SDMs are a series of closely related models that measure the disorder propensity of the viral shell proteins in order to analyze and understand the behaviors of the viruses as seen in
Table 1. The parent model is the “Shapeshifter” model that was published in 2008, when it was the first to report the presence of an abnormally disordered outer shells (matrix) among a large number of HIV-1 variants [
16,
21]. We attributed the unsuccessful search for a HIV vaccine to this unique property, seldom seen among viruses. Another model was published in 2015 [
17,
24]. This model showed that there are strong correlations between disorder at the inner shell and the virulence of many viruses, including DENV, EBOV, and NiV [
17,
22,
24,
27,
72]. We shall see that it applies also to COVID-19-related viruses. The reason for the correlation between greater inner shell disorder (PID
N, percentage of intrinsically disordered residues in the N protein) and virulence has to do with the important roles that the inner shell proteins often play in the replication of the viruses [
73]. The replication processes require binding of the inner shell proteins to RNA/DNA and other viral and host proteins. The greater disorder provides means for more efficient binding and therefore faster replications. Many viruses take advantage of their ability to replicate more rapidly as a form of immune evasion. This “Trojan-horse” immune evasion involves the rapid replication of the virus via shell disorder even before the host immune system recognizes its presence. This strategy often, however, backfires on the virus by overwhelming the host body with abundant copies of infectious particles, which kill the host [
17,
60,
61].
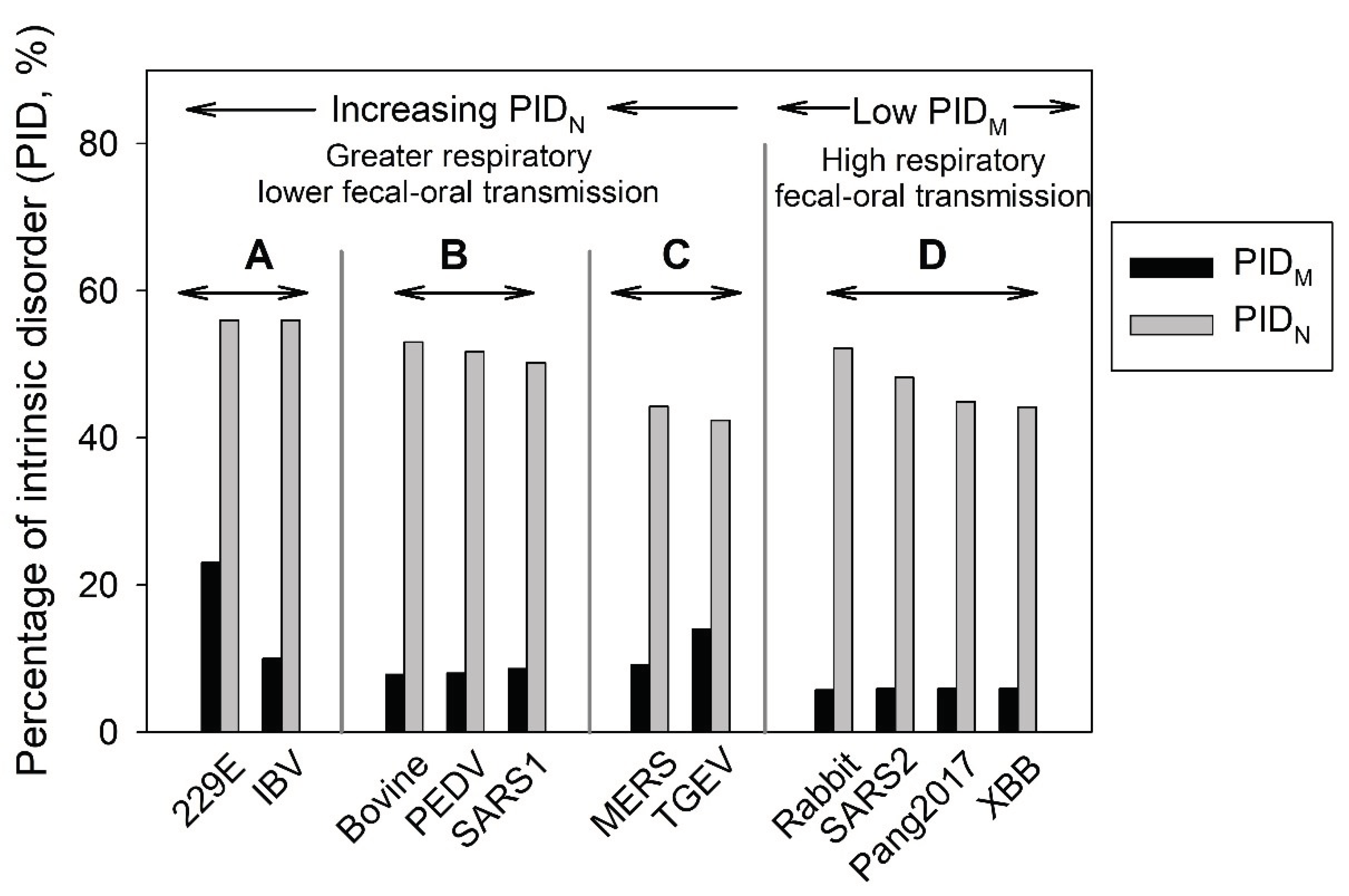
In 2012, the third SDM was published [
58]. This is the CoV Transmission Model, in which levels of fecal-oral and respiratory transmissions were correlated to the levels of disorder of the shell proteins. Initially, three groups of CoVs were categorized. With the arrival of SARS-CoV-2, a fourth group that is associated with burrowing animals was detected and added.
Figure 1 describes the 4 groups. CoVs in group A have higher PID
N values, which allow for higher respiratory transmission potentials. As aforementioned, the inner shell protein, N, is intimately involved in the replication process, and a higher disorder of this protein (greater PID
N) allows for more efficient interactions between N and the RNA and other proteins [
19,
59]. This greater efficiency leads to higher shedding of the virus that is necessary for respiratory transmission. The three groups, A-C, were categorized according to this principle, as seen in
Figure 1 [
17,
59]. Viruses in group A have the highest PID
N and have the higher respiratory transmission potentials but lower fecal-oral potentials. Conversely, the CoVs in group C have higher fecal-oral but lower respiratory transmissibility potentials. CoVs in group B, on the other hand, have intermediate levels of both fecal-oral and respiratory transmissibility potentials.
A fourth category, group D, was discovered when data pertaining to COVID-19 poured in [
19,
20,
26,
30]. This group consists of CoVs that have abnormally hard outer shells (i.e., their M proteins are characterized by low disorder content, low PID
M) that are usually associated with a burrowing animal host, such as rabbits and pangolin. The association between burrowing animals and hard outer shell of the virus has to do with the necessity of the virus to persist in feces that could be buried for a long time until encountered by another host [
18,
25,
26,
30]. It is believed that CoVs in this category has both high fecal-oral and respiratory transmission potentials. Because of their hard outer shells, the viruses are able to resist the onslaught of antimicrobial enzymes found in the saliva and mucus [
74,
75]. As in the case of COVID-19, the infected host body typically sheds large amounts of infectious particles. Since unlike SARS-CoV-2 very few CoVs have this feature [
76], COVID-19-related viruses are considered somewhat unique. We believe that this feature, being associated with the peculiarities of the physiological properties of the mammalian respiratory system (Mucociliary Clearance System) [
77,
78,
79], is responsible for the high infectivity of the virus [
25,
26,
30].
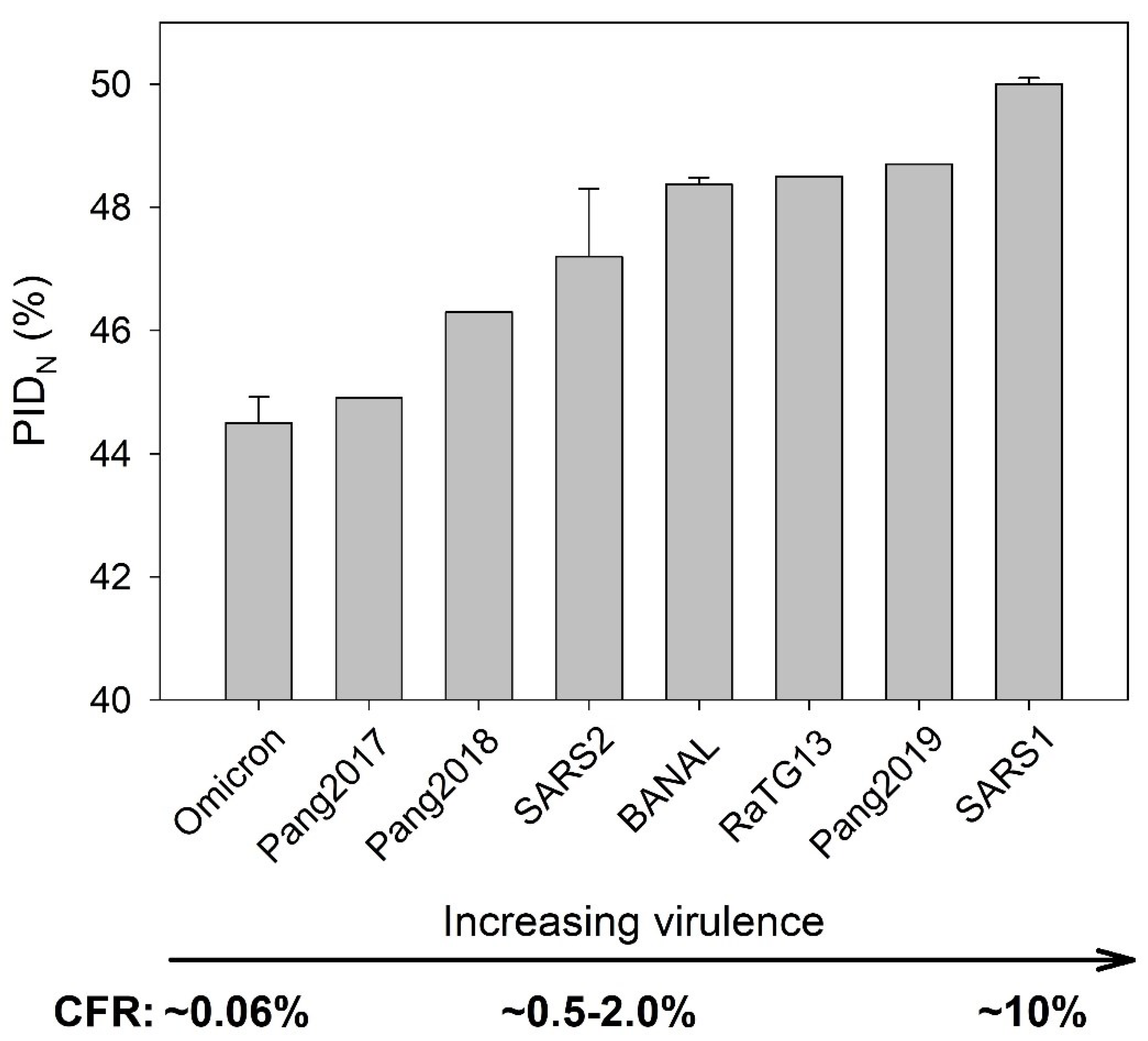
The three SDMs provide specific predictions, which are highly reproducible thus far. For instance, the SARS-CoV-1 is predicted to be more virulent but less transmissible than SARS-CoV-2. SARS-CoV-1 has a PID
N of 50 % and PID
M of 9%, whereas SARS-CoV-2 (Wuhan-Hu-1) has 48.2 % PID
N and 5.9% PID
M. The Virulence-SDM predicts that SARS-CoV-1 will be more pathogenic based on its higher PID
N, and this has been supported by the fact that the Case Fatality Rate (CFR) of SARS-CoV-1 is about 10%, while the Wuhan-Hu-1 was estimated to have the CFR of around 2%. The greater N disorder allows for more efficient replication of SARS-CoV-1 especially in vital organs, whereas the harder outer shell (lower PID
M) of SARS-CoV-2 causes greater shedding by the patients. The much higher level of shedding by COVID-19 patients has also been clinically observed [
76].
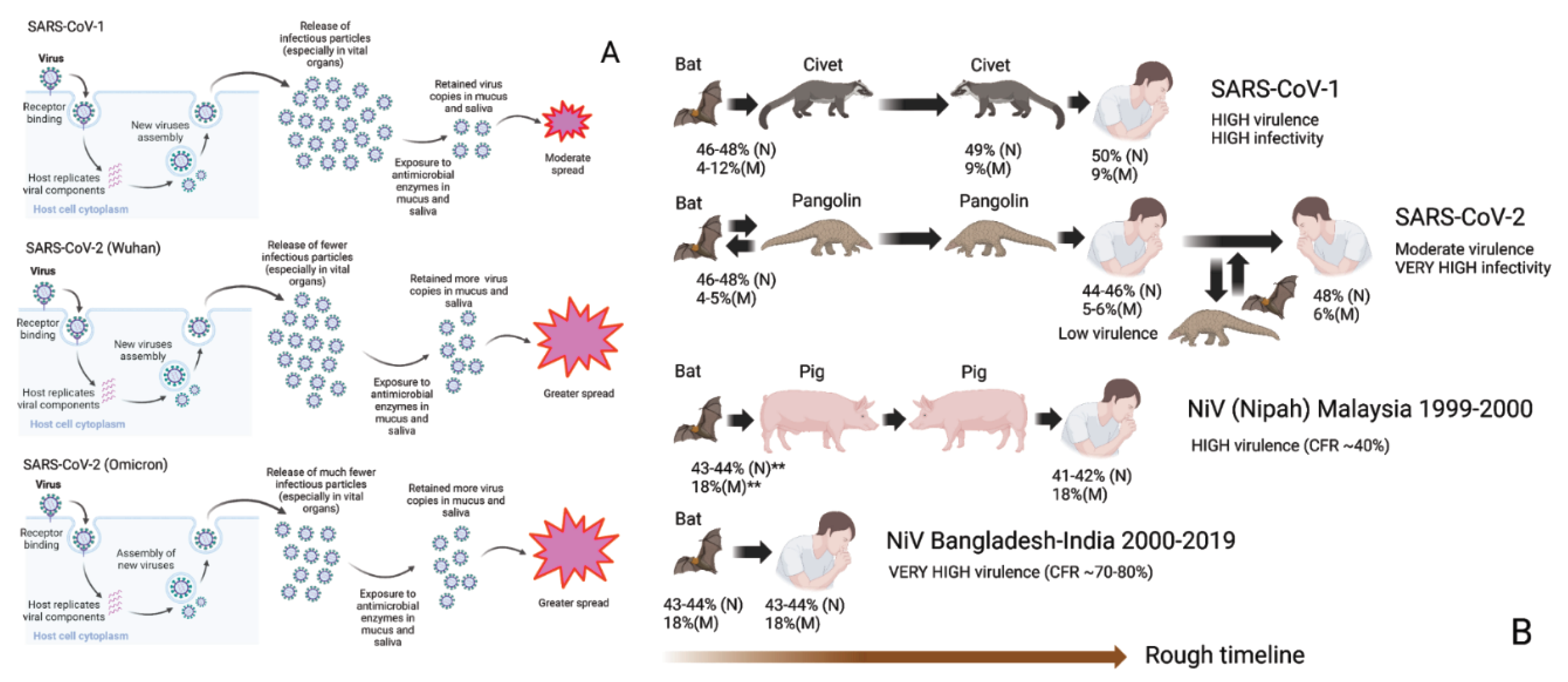
Figure 2A summarizes how SARS-CoV-2 is able to shed more infectious particles yet remain relatively less virulent than SARS-CoV-1. It produces less copies in vital organs but because of its hard outer shell, the virus is able to resist the anti-microbial enzymes and thus sheds more particles, unlike SARS-CoV-1.
Figure 2B illustrates the strong relationship between COVID-19 and pangolin. Intermediary animal hosts often provide a venue for attenuation, as in the case of pigs in the Malaysian Nipah outbreak in 1999-2000 because of the behavior of the animals [
72,
80]. Likewise, SARS-CoV-2 was more efficiently attenuated because it is likely to have been passed between humans and pangolins for at least several years before the appearance of Wuhan-Hu-1 [
18]. This is likely also the case in Omicron [
25,
26,
30]. Further evidence of interspecies transmissions can be found by the facts that SARS-CoV-2 is capable of infecting a large number of mammalian species [
81,
82], and that bats often dwell alongside pangolins in caves and underground burrows [
83].
3.2. Phylogenetic Study Using M Reveals Intimate Relationship Between Pangolin-CoV and SARS-CoV-2/Omicron
We have seen that a common characteristic of all thus far known COVID-19-related viruses is the abnormally hard outer shell (low PID
M), with the high rigidity of M being tied to the high COVID-19 infectivity and the evolutionary close relationship between SARS-CoV-2 and a burrowing animal, pangolin [
25,
26,
30]. We have also seen that Omicron presented the enigma of being different from all previous SARS-CoV-2 variants, which may suggest that Omicron had been hiding in an animal host. The phylogenetic tree using M shown in
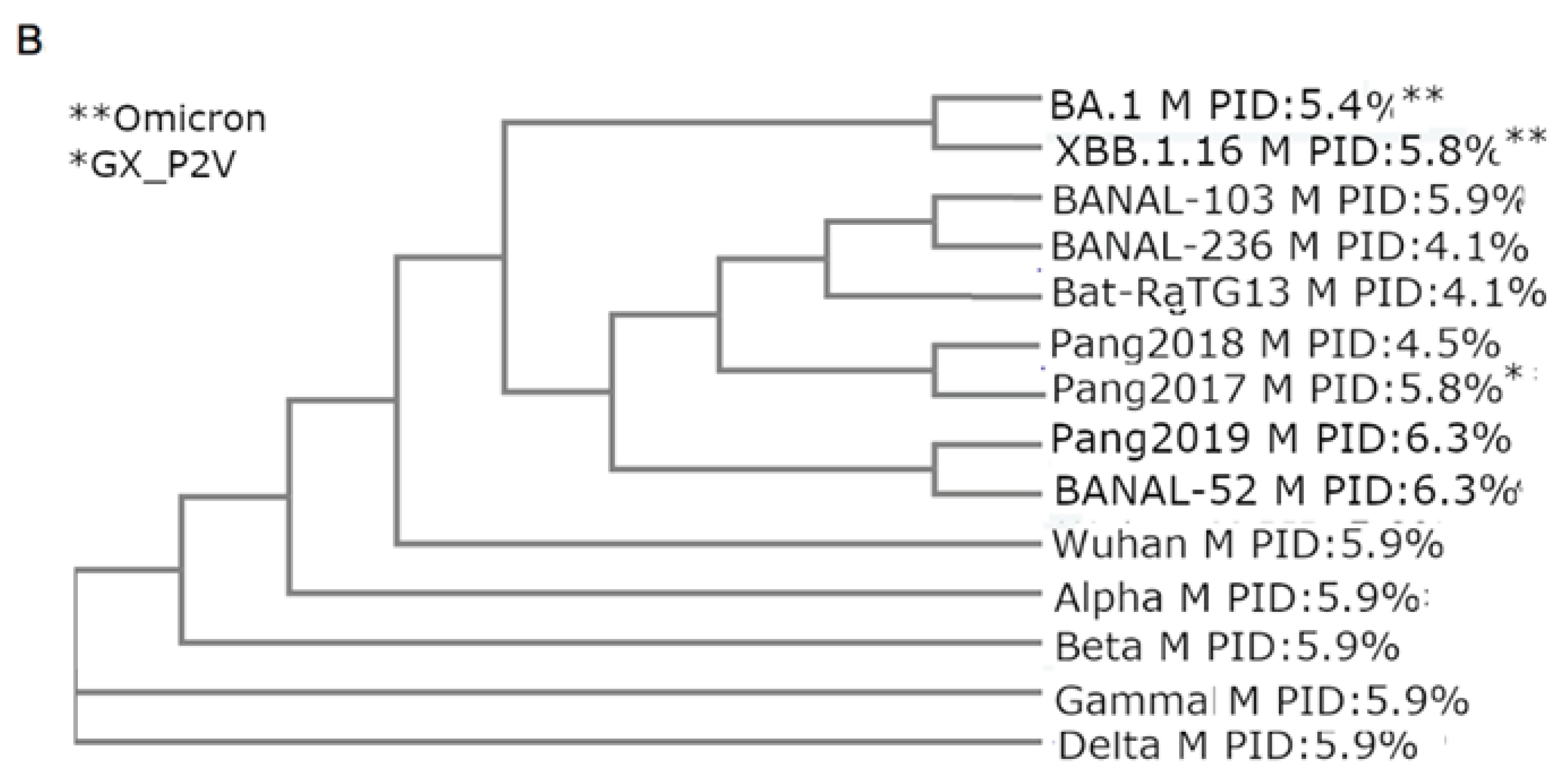
Figure 3 provides evidence to support this scenario by grouping both Omicron XBB and BA2 closer to pangolin-CoVs and bat-CoVs, rather than the other SARS-CoV-2 variants [
25,
26,
30]. A previous study using M and different software and algorithms involving distance optimization resulted in an even closer relationship between Omicron and pangolins. The phylogenetic studies using M are the likeliest to present the most accurate trees, because M needs to be highly conserved given its rigidity. A highly conserved protein provides greater accuracy in phylogenetic studies since studies have shown that current phylogenetic algorithms often handle recombination poorly [
84]. In fact, previous phylogenetic studies using M were shown to offer a unique perspective on the evolution of SARS-CoV-2 as related to pangolin-CoVs in comparison to other studies.
3.3. Omicron and Pang2017: Low PIDN and Attenuation
Evidence of the SARS-CoV-2 attenuation by its association with pangolins can be found in
Table 2. Most of the bat-CoVs have higher PID
N values (48.5%) than Wuhan-Hu-1 (48.2%). The pangolin-CoVs have a much wider range of PID
N values, which suggest that pangolins may provide a suitable environment for the attenuation of the virus, since a harder inner shell does also provide some further protection to the virion [
22,
23,
24]. We see also in
Table 2, the range of PID
N values of SARS-CoV-2 including Omicron resemble more closely those of pangolin-CoVs, rather than those of bat-CoVs even if the latter is genetically closer.
3.4. Omicron Has a Lower PIDN Similar to Pango2017 but has a lower PIDM: Attenuation and Faster Spread
Further evidence that levels of N disorder have a strong correlation (r=0.9 P < 0.05) with virulence can be found in
Figure 4, where a clear Omicron attenuation is seen. The underlying theoretical link is found in the Virulence-Inner SDM, in which the greater disorder at the inner shell protein allows faster replication of the virus that often leads to higher virulence. Because CFRs of SARS-CoV-1 and SARS-CoV-2 variants are available, the correlation between the PID
N values and virulence can be calculated. More data became available when CFR (<0.2%) of patients, especially non-vaccinated ones, infected by Omicrons became available [
33,
37]. This can be compared to the other earlier SARS-CoV-2 variants (2-0.5%) [
61]. Even though
Figure 4 includes pangolin-CoV and bat-CoV, we do not really know the CFR of these viruses. However,
Figure 4 makes prediction on the likely CFR of such viruses if they infect human population, and, indeed, as we will see later, the PID
N is found to correlate with aggressiveness of the virus under experimental conditions.
3.5. The Role of N in CoV-Transmission SDM and Virulence-Inner Shell Disorder Model
Based on the available data for SARS-CoV-1 and SARS-CoV-2 variants, a correlation between PID
N values and virulence can be calculated. This analysis revealed a strong correlation (r=0.8). This is in sharp contrast to
Figure 1, where PID
N has strong correlation to the mode of transmission especially in groups A-C. The two correlations are based on the same theoretical principle. The Virulence-Inner SDM as seen
Figure 4 entails higher PID
N for greater pathogenesis of the virus because higher disorder levels in N facilitates faster replication of the virus through greater protein-protein/RNA/Lipid binding efficiency [
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55] and large amounts of a virus in vital organs often kill the host [
17,
60,
61].
3.6. All Known COVID-19 Viruses have Hard Outer Shell: Evolutionary Association with Pangolins
While it is easy to assume that bat-CoVs, such as RaTG13 and BANAL-52, are most closely related to SARS-CoV-2, such an assumption could be misplaced as many other considerations, such as recombination, are involved. There are also evolutionary patterns that we need to be concerned about. Several papers have already noted that parts of the S protein of pangolin-CoVs most closely resemble those of SAR-CoV-2 [
85]. We have also seen that the range of PID
N values of pangolin-CoVs resembles that of SARS-CoV-2, especially when Omicron is included. There is yet another mystery that involves the origin of Omicron. When Omicron first emerged, it was already noted that it did not resemble any other previous variant, and the types of mutations found in it have previously never been seen. If so, where was Omicron hiding? The phylogenetic tree using M in
Figure 4 offers some clues by showing the surprisingly close relationship between pangolin-CoVs and Omicron. M is likelier to offer greater reliability in phylogenetic studies of COVID-19-related viruses. In fact, as we have seen in
Figure 1 and
Table 2, all COVID-19 CoVs have abnormally hard M, which implies that M is likelier to be the most of highly conserved proteins which makes it the best choice in such studies.
Figure 3 shows that Omicron (BA.1.44 and XBB.1.16) are clustered together.
3.7. Correlation Between Viral Growth and N Disorder of COVID-19-Related Viruses
We noticed that, in
Figure 4, the CFRs for pangolin-CoVs and bat-CoVs are not available, as human infections have not been observed for those viruses even though laboratory studies have shown that they are capable of infecting a wide range of animals including humans [
81]. Given the strong correlation between CFRs and virulence, the SDMs predict that aggressiveness of the various COVID-19-related viruses will correlate with the PID
N values. This is because the SDMs involve the paradigm that greater N disorder allows for more rapid replication of virus copies, which leads to greater virulence. An examination of experiments by other laboratories and our associated laboratory provides evidence of a correlation (r=8) between aggressiveness of the virus and PID
N levels as seen in
Table 3.
Hou et al. [
35] showed that under certain circumstances, Pang2019 replicates more efficiently in respiratory cells than SARS-CoV-2. Temman et al. [
11] showed that BANAL-236 has about the same replication rates as SARS-CoV-2, when Vero-E6 or Caco-3 cells are used. Ogando et al. [
86] demonstrated that SARS-CoV-1 replicates fifty times faster than SARS-CoV-2 in Vero-E6 cells. Guo et al. [
28] and Lu et al. [
29] have found that Pang2017 is attenuated especially when compared to non-Omicron SARS-CoV-2 variants. As for Omicron, numerous clinical and experimental studies [
31,
32,
33,
34] have shown it to be milder than other variants. The viral growths of XBB.1.16 and Pang2017 are addressed later in this paper. All these data are used to construct
Table 3.
The repetitive interspecies transmission may provide insight into the mechanisms by which SARS-CoV-2 acquires its unusual multi-species adaptation [
81]. It also reiterates the suggestion made in our previous publications that an attenuated COVID-19-related virus may have entered the human population in 2017 or earlier. An entry of an attenuated virus could easily have been mistaken for a mild cold by the medical community especially if the spread is slow.
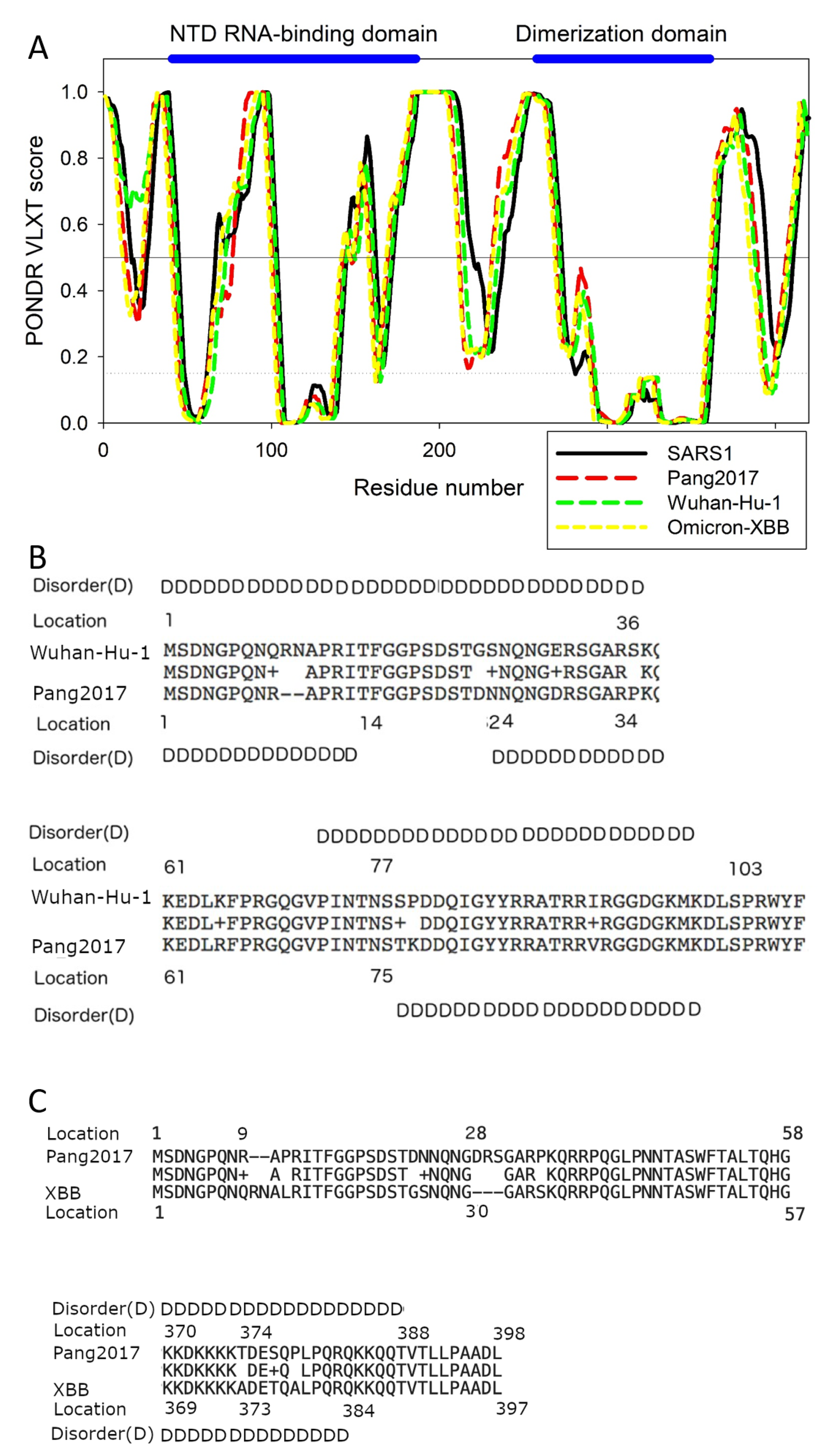
3.8. Molecular Analysis SARS-CoV-2's Evolution within Animals Affects its Virulence and Human Spread
Results of more detailed computational analysis of N proteins from SARS-CoV-1, Wuhan-Hu-1, Omicron XBB.1.16, and pangolin-CoV-2017 can be found in
Figure 5.
Figure 5A represents the PONDR
® VLXT-generated intrinsic disorder profiles of these proteins, where the X-axis represents the locations of the residues and the Y-axis shows the corresponding PONDR
® VLXT scores. Regions with the scores of 0.5 and above are considered as predicted intrinsically disordered regions.
While
Figure 4 and
Table 2 tell us that the virulence of SARS-CoV-1, Wuhan-Hu-1, Omicron and Pang2017 is related to the levels of their respective PID
N values,
Figure 5 points out the exact locations of the differences in the N disorder. As seen in
Figure 5A,B, the NTD (N-terminal domain) responsible for the RNA binding by N protein is located within the locations 44-186 region, dimerization domain occupies 258-361 region, whereas the NLS (Nuclear Localization Signal) motif lies in the 366-381 region [
26,
60,
87]. While the viral RNA binds to other regions of N protein, the NTD RNA-binding domain is arguably the main RNA-binding region of this protein. We know from
Figure 4 that SARS-CoV-1 is more virulent than Wuhan-Hu-1 as the former is associated with higher PID
N.
Figure 5B,C shows that the higher levels of the intrinsic disorder can be found both inside and outside the NTD RNA-binding region of the Wuhan-Hu-1 N protein. The N protein is found both within the cytoplasm and nucleus, where it could manipulate the cell cycle to the virus's advantage, and the NLS site provides means for this protein to enter the nucleus. Importantly, the region encompassing this motif is predicted to be highly disordered in all four proteins analyzed in this study.
A comparison of disorder profiles in
Figure 5A suggests that while the disorder differences between SARS-CoV-1 and Wuhan-Hu-1 can be found in regions at or near the NLS, NTD RNA-binding domain and dimerization domain, the differences between Wuhan-Hu-1 and Pang2017 lie mainly in and around their NTD RNA-binding domain but not in other regions. This implies that the attenuation of Pang2017 is likely to arise from the modulation of the protein-RNA binding, not protein-protein or protein-membrane interactions, which provide us with the ideas on ways to further attenuate Pang2017.
Figure 5B allows tracing the source of the discrepancies in disorder by sequence comparison. There is a large ordered region in the Pang2017 NTD (residues 14-23), not seen in Wuhan-Hu-1 (
Figure 5A). It should be noted that even though this ordered region is not inside the NTD RNA-binding domain (i.e. residues 44-187), it is adjacent to it and is therefore likely to affect the binding potential.
The residues responsible for this disorder discrepancy can be seen in
Figure 5B. At position 9, a mutation took place involving replacement of an R (Arginine) by a Q (Glutamine) in the case of SARS-CoV-2. This point mutation is followed by two insertions of R (Arginine) and N (Asparagine). Therefore, instead of one disorder-promoting residue seeing in Pang2017, this region of SARS-CoV-2 N protein has three disorder-promoting residues.
An important point to be made in this paper is that SDMs have determined that Pang2017 and Omicron have similar levels of attenuation based on their PID
N values. Even though the viruses have similar levels of N disorder, slight differences can be detected depending on the specific Omicron subvariant used. Two Omicron subvariants, BA.1 and XBB.1.16 are used but XBB.1.16 is our point of reference both computationally and experimentally. As seen in
Table 2, BA.1.44 and XBB.1.16 have PID
N values of 44.7% and 44.2%, whereas Pang2017 PID
N is 44.8%. The small difference between the PID
N values of XBB.1.16 and Pang2017 could explain the subtle but perhaps important experimental discrepancy between their viral growth in Vero cell as we shall see later. A comparison of the PONDR
® VLXT plots in
Figure 5 reveals two regions responsible for much of the mentioned discrepancy. One region sits at the NTD RNA-binding domain, whereas the other involves the NLS domain. The site at the NLS domain responsible for much of the discrepancy lies near position 384, where Pang2017 has slightly greater disorder.
Figure 5C tells us that the mutations 374TA373, 377ST376, and 379PA378. The mutations generally involve replacement of a more polar residue for a less polar one in the case of Pang2017. Polar residues tend to cause greater disorder. The existence of higher local disorder, even if the difference is not very large, at or near the NLS is likely to indicate that Pang2017 has slightly greater ability to penetrate the nuclear of the host cell during replication as greater disorder provides means for better protein-protein interactions and, thus, allows some added advantage during replication.
A different story can be found at the NTD RNA-Binding domain. Despite the fact that pang2017 N being generally slightly more disordered, XBB.1.16 is seen as having greater disorder around location 70 (
Figure 5). This greater local disorder level may allow XBB.1.16 to have higher RNA-binding efficiency, which is likely to be reflected in our experimental part as we will see later. An examination of the sequences using
Figure 5C tells us that this greater disorder is likely arising from a sequence insertion and deletion at locations 28 and 9 respectively for Pang2017.
Introduction of SARS-CoV-1 into humans may be linked to an intermediary involving Civet cats. Because of the higher virulence of SARS-CoV-1, in comparison to SARS-CoV-2 and the high genetic similarity of SARS-CoV-1 and Civet-CoV, it can be suspected that SARS-CoV-1 entered civets for a short while before moving to humans. The lower virulence of SARS-CoV-2 leads us to believe that the virus may have been incubating in an intermediary involving a burrowing animal, such as pangolin for a relatively longer period of time. The case of NiV illustrates this point. The virus involved in the 1999-2000 Malaysian outbreak is less virulent than the viruses involved in outbreaks in Bangladesh-India, because the former involved an intermediary; i.e., farm pigs.
3.9. Comparison of Cytopathic Effects, One-Step Growth Curve, and Plaque Size of Pang2017 and SARS-CoV-2 XBB.1.16 in Vero Cells
Vero cells were infected with Pang2017 and XBB.1.16 at an MOI of 0.1. At 36 hours post-infection, no notable changes were observed in cells infected with Pang2017, while those infected with XBB.1.16 exhibited pronounced cytopathic effects. By 48 hours post-infection, cells infected by both viruses began to round up, shrink, and detach. Between 48-72 hours post-infection, a greater number of cytopathic cells were observed in the Pang2017 infected group, but the monolayer density was considerably higher than in cells infected with XBB.1.16.
Using qPCR to measure the viral RNA load in the culture supernatant, the one-step growth curves of the two viruses were determined. Overall, both viruses displayed similar growth curves, with their log phase spanning between 12-36 hours. Between 8 to 10 hours post-infection, the RNA copy number of Pang2017 rose by approximately one log, while there was no significant change in the XBB.1.16 RNA copy number. From 10 to 48 hours post-infection, the Pang2017 RNA load in the supernatant was consistently higher than that of XBB.1.16. By 48 hours post-infection, the RNA loads of the two viruses converged. Between 48 and 120 hours, Pang2017 did not proliferate noticeably, whereas XBB.1.16 increased by approximately 0.9 log.
In
Figure 6, the results from the plaque assay indicated that the size of plaques formed by Pang2017 was about 60% of those formed by XBB.1.16. This suggests that Pang2017's invasive ability in cells is slightly inferior to that of XBB.1.16. As seen in
Figure 6, Pang2017 and XBB.1.16 exhibit similar growth characteristics in Vero cells. However, in the later stages of infection (after 48 hours), Pang2017's cytotoxicity and proliferation ability are slightly lower than those of XBB.1.16. XBB.1.16 is a currently prevalent SARS-CoV-2 variant, and compared to the wild-type strain, its pathogenicity is considered reduced. On the other hand, Pang2017 is not a human pathogen and is highly attenuated in animals. The biological comparative analysis of the two viruses aligns with the known virulence of each virus.
4. Discussion
4.1. COVID-19 Special Relationship with Pangolin-CoVs: Can Be Found in the Abnormally Hard M: Burrowing Animal
The fact that pangolin-CoVs have generally an approximately 90% genetic similarity to SARS-CoV-2, as compared to RaTG13 and BANAL-236 of about 96% causes many scientists to jump into conclusion that pangolin-CoVs are not that closely related to SARS-CoV-2, not to mention being a recent ancestor. Such a conjecture, however, hides the intricacies of genetics and evolution especially with regard to the likelihood of the presence of recombination [
84], which inevitably leads to gross errors. Indeed, at least one laboratory has noticed that portions of the S proteins of pangolin-CoVs resemble most closely those of the SARS-CoV-2 S protein. In the present study, a complex computational and experimental approach to look at different viral proteins is used to study this enigma.
The computational side of our study that involves SDMs has uncovered a specific feature, seldom found in CoVs, with the exception of CoVs of burrowing animals, such as rabbit-CoV (
Figure 1). This tell-tale feature has to do with the fact that SDMs have uncovered that all thus far known COVID-19-related viruses have hard outer shell (low PID
M). The presence of this unique and peculiar property is corroborated independently by another laboratory that showed that SARS-CoV-2 is much more persistent in environments away from light than all tested CoVs [
88].
The presence of this hard outer shell presents a few intriguing questions like: Why is this even present? How does this affect the transmissibility of the virus? The hallmark hard M arises from SARS-CoV-2 close evolutionary relationship with a burrowing animal, pangolin. CoVs of burrowing animals need to rely on feces that have been buried for a long time to facilitate its transmission [
18,
25,
26,
30]. A hard outer shell, and sometimes even inner shell, is required for the virus to stay viably active in the feces for a long time as the outer and, to some extent, inner shells protect the virion from damage [
18,
19,
20,
74,
75]. It is therefore, not surprising that SARS-CoV-2 has been experimentally shown to be more resilient away from light than the available referenced CoVs used as controls [
88].
4.2. Evidence of an Even Closer Relationship Between Omicron and Pangolin-CoVs
The harder outer (lower PID
M) and inner (lower PID
N) shells result, however, in the different manifestation of the disease caused by the virus. A harder N (lower N disorder) causes greater attenuation of the virus, as N is intimately involved in the replication process, whereas a harder M provides greater resistance to the anti-microbial enzymes found in saliva and mucus. This is the reason why SARS-CoV-2 was highly infectious, unlike SARS-CoV-1. This SDM prediction has been reproduced by [
76], who clinically showed that COVID-19 patients shed much larger amounts of viral particles than 2003 SARS-CoV-1 patients. Furthermore, the shared rigidity of M among COVID-19 variants provides a unique opportunity for a glimpse into the evolution of SARS-CoV-2 via phylogenetic studies because phylogenetic studies are often affected by the potential errors in the presence of recombination, but M, on the other hand, may be able to alleviate this problem by the fact that it is likely to be very conserved given its rigidity. Indeed, the phylogenetic trees using M have been somewhat different from the trees created using other CoV proteins or entire genome. As seen in
Figure 3A, our phylogenetic tree places SARS-CoV-2 (SARS2) in the close proximity to Pang2019 and, thereby, suggesting a very close evolutionary relationship between pangolin-CoVs and SARS-CoV-2.
An even more intriguing picture is seen in
Figure 3B showing that Omicron is more closely related to bat-CoVs and pangolin-CoVs than the other SARS-CoV-2 variants. How could this be so? A hint to the answer is likely to involve an already mentioned mystery pertaining to fact that Omicron has been found to be very genetically different to all previous variants [
38]. This lead to some scientists believing that Omicron had been hiding in an animal host, such as mice [
89]. According to SDMs, mice is theoretically a possibility but mice have dual evolutions depending on the species. Mice and rats in urban settings have evolved to live in homes of humans since the dawn of civilization, but those in rural environments live in burrows [
90]. We have therefore reasons to believe that mice and rats, unlike pangolins, may not provide the most ideal environments for the hardening of M and N even if the scenario is possible according to SDMs.
While the phylogenetic tree in
Figure 3A,B using Clustal Omega places SARS-CoV-2 and Omicron in very close proximity to pangolin-CoVs and pangolin-CoVs/bat-CoVs respectively, another M phylogenetic study using Clustalw with distance optimization has placed Omicron even closer to pangolin-CoVs. Phylogenetic studies using M are not the only evidence of a closer relationship between Omicron and pangolin-CoVs. For instance,
Table 2 shows that the original Omicron subvariant, BA.1, has an even harder M (5.4%) than later XBB.1.16 (5.8%) and that the former has a much lower SARS-CoV-2 genetic similarity (98.7%) than the other variants (99.1%), which brings it closer to that of pangolin-CoVs (98.2%). Curiously, the later Omicron subvariant, XBB.1.16 has a PID
M (5.9%) and M genetic similarity (99.1%) that resemble the other SARS-CoV-2 variants even though the phylogenetic study using M clusters XBB.1.16 and BA.1 together. Apparently, as time goes on, Omicron began to pick up bits of genetic materials from another variant, presumably, Delta. Evidence of the even closer relationship between Omicron and pangolin-CoVs could suggest a greater probability that Omicron may have been hiding among pangolins before it surfaced among humans in South Africa.
4.3. Range of SARS-CoV-2 N Disorder Matches that Pangolin-CoVs2017, Not Bat-CoVs
Yet another piece of evidence that depicts the close relationship between pangolin-CoVs and SARS-CoV-2/Omicron can be found when we inspect the peculiarities of disorder distribution in N. The pattern of N disorder of pangolin-CoVs closely matches that of SARS-CoV-2 in terms of range when you include Omicron. This is in sharp contrast to the COVID-19-related to bat-CoVs as seen in
Table 3. We are able to see that both Omicron and pangolin-CoVs are able to reach a lower threshold of 44.5-44.8, which is not seen in bat-CoVs. The reason for the larger range of PID
N values of pangolin-CoV can be traced to its fecal-oral transmission route. As mentioned, even though a hard outer shell is essential to protect the virus from a harsh environment, a harder inner shell (lower N disorder) often adds additional protection. This also has been seen in many other viruses including rabies and DENV [
21,
22,
24]. We see, however, the COVID-19-related bat-CoV PID
N remaining within the range seen in bat-CoVs in general as bats are flying animals that require higher N PIDs for the respiratory viral transmission. Apparently, SARS-CoV-2, especially Omicron, was able to acquire its greater range of PID
N likely from its closer relationship with pangolin-CoV.
When the PID
N values of pangolin-CoVs were first calculated upon then available data, it became clear that the PID
N of Pang2017 is as low as 44.8%. For this reason, the SDMs predicted the Pang2017 to be attenuated and provided a window for a silent spread as an attenuated virus can easily escape the attention of the medical community. Then came the Omicron outbreak that first started in South Africa. Unlike, previous variants, Omicron PID
N (BA1, 44.7%) was found to be quite similar to Pang2017 (44.8%) and, unsurprisingly, Omicron was clinically and experimentally found to be milder than previous variants. Similarly, at least two laboratories have experimentally shown that Pang2017 is attenuated. In agreement with SDMs, as seen in
Table 3, no such attenuation was seen in BANAL-236 (PID
N: 48.6%) and Pang2019 (PID
N: 48.6%), which were instead shown to be even more aggressive than Wuhan-Hu-1 in certain cells.
4.4. Differences in Pang2017 and XBB.1.16 N Disorder Patterns Can Explain Subtle Discrepancy in Experimental Results for the Two Viruses
While viral titrations have been done for Pang2017, Pang2019, SARS_CoV-1 and BANAL-236 have been performed in comparison to non-Omicron SARS-CoV-2, none has yet to be done for Pang2017 with Omicron until now with this paper. While
Figure 6 shows that XBB.1.16 and Pang2017 have similar cytopathic effects, viral growth and plaque formation even if there are subtle differences. SDMs attribute the similarities to the similarity in PID
N values (Pang2017 PID
N: 44.7%, XBB PID
N: 44.5%), a more careful study using disorder and molecular analysis is needed to understand the subtle differences. The PONDR
® VLXT plots of
Figure 5 tell us that even though Pang2017 has a slight advantage in N disorder (44.7%), much of the greater disorder lies in the NLS region, not within the NTD RNA-binding region. This basically means that Pang2017 N is more efficient in entering the nucleus of the host cell to change the cell-cycle to its advantage, whereas XBB.1.16 N has greater ability to bind to RNA. These properties can be seen in the viral titration curve. The higher RNA copies of Pang2017 seen at 9-48 hours after initial infection is likely the result of the greater ability of the Pang2019 N to enter the nucleus to delay the cell-cycle, while the higher disorder at NTD RNA-binding region of XBB.1.16 N allows for the slightly greater viral replication after 48 hours. We need to remember that all SARS-CoV-2 variants including XBB has a Furin cleavage site (FCS) that is missing in all pangolin-CoVs and COVID-19-related bat-CoVs. For this reason, the presence of FCS in XBB.1.16 but not in Pang2019 is likely to add to the slightly higher efficiency of the former. Even so, it is important to note that FCS is not likely to have given much advantage given the result we have. This note may be important given the debate on the importance of FCS in infectiousness. The disorder patterns detected using molecular analysis can be used for further attenuation, which will be useful for future vaccine development.
5. Summary and Conclusions
The evolutionary footprint of pangolin-CoVs can be found in all COVID-19-related viruses in the form of a hard outer shell (abnormally low PIDM values), which is necessity for viral transmission via buried feces of a burrowing animal, pangolin. The fact that all thus far known COVID-19-related SARS-CoV-2 variants have abnormally hard M, seldom seen in any CoV with the exception of CoVs related to a burrowing animal like rabbits, is itself evidence that not only do pangolin-CoVs play a major evolutionary role in all COVID-19-related viruses but also pangolins are likely to serve as a major reservoir for COVID-19-related viruses. Bat-CoVs, such as RaTG13 and BANAL, have the highest genetic similarities to SARS-CoV-2 at approximately 96% in contrast to the approximately 90% in Pangolin-CoVs. To jump to conclusion, however, that SARS-CoV-2 came directly from bats would be to ignore the probability that SARS-CoV-2 had been moving to and fro between pangolins and humans for a long time. We have further evidence of this. The seemingly greater genetic difference in the pangolin-CoVs thus found may be a reflection of the greater diversity of pangolin-CoVs. In fact, the diversity in N disorder of SARS-CoV-2 more closely resembles that of pangolin-CoVs, not bat-CoVs. The emergence of Omicron is highly enigmatic as it is genetically very different from the other SARS-CoV-2 variants. Phylogenetic studies using M under two different algorithms show that Omicron including XBB.1.16 is closer to pangolin-CoVs than the other variants. This provides further evidence that Omicron could have been hiding in pangolin all the while. Yet further suggestion that Omicron could have been hiding in a burrowing animal is seen by two factors. The first is the observation that came when it was noticed that the early Omicron subvariant BA.1 has an even harder outer shell than the other SARS-CoV-2 (PIDM: 5.4% versus 5.9%).
Where did Omicron acquire its harder outer shell? A likely scenario is that it has been hiding in a burrowing animal such as pangolin. Viruses of a burrowing animal host need a harder outer shell to last a longer time in buried feces in order to be of a viable spread. It has also been known from other virus that while not necessary, a harder inner shell also helps protect the virus from the environmental damage. For these reasons, it is therefore not surprising to observe that Omicron has also lower PIDN values (PIDN: 44.7%, BA.1, 44.5%, XBB.1.16, 48.2%, Wuhan-Hu-1) than the other variants. Again, pangolin is likely to create a better environment for the evolutionary hardening of both M and N as a burrowing animal, which is the reason that pangolin-CoVs have a greater range of PIDN values that matches those of SARS-CoV-2). A lower PIDN, however, has important implications. Like many viral inner shell proteins, it is intimately associated with the replication process and a lower N disorder of pang 2017 and Omicron allows SDMs to predict attenuation in both viruses, which have similar PIDN values (pang2017: 44.8%, XBB.1.16: 44.5%). Our experimental study shows that both viruses exhibit similar properties such as growth rates in viral titration and plaque sizes. There are, of course, subtle differences but these subtle differences can be traced to the differences in the localities of the disordered regions of the two viruses and the presence of FCS in Omicron. The molecular analysis of N using disorder that is able to account for that the small discrepancies can be further utilized. The molecular analytical methodology using disorder that is able to detect the presences greater disorder at the NTD RNA-Binding region of XBB.1.16 responsible for its greater proliferation at a later stage can be used to design further attenuation in future vaccine development.
Author Contributions
Conceptualization, G.K.-M.G., L.S., L.W..; data curation, G.K.-M.G., V.N.U., L.S., L.W..; formal analysis, G.K.-M.G, L.S., L.W.; investigation, G.K.-M.G., L.S., L.W.. ; methodology, A.K.D., G.K.-M.G., LS., L.W.; resources, A.K.D. and J.A.F., L.S.; software, G.K.-M.G., L.S.; validation, G.K.-M.G., L.S.; visualization, V.N.U.; writing—original draft, G.K.-M.G., L.S., L.W. ; writing—review and editing, V.N.U, G.K.-M.G.; bioinformatics analysis, G. K-M,G., V.N.U., A.K.D., J.A.F.; wet lab experimental, L.S., L.W.
Funding
This research was supported by NSFC-MFST project (China–Mongolia) (grant number 32161143027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
G.K.M.G. is the owner of Goh’s BioComputing, Singapore. He has written a book, “The Viral Shapeshifters: Strange Behaviors of HIV and Other Viruses”. The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Worobey, M.; Levy, J.I.; Malpica Serrano, L.; Crits-Christoph, A.; Pekar, J.E.; Goldstein, S.A.; Rasmussen, A.L.; Kraemer, M.U.G.; Newman, C.; Koopmans, M.P.G.; et al. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 2022, 377, 951–959. [Google Scholar] [CrossRef]
- Pekar, J.E.; Magee, A.; Parker, E.; Moshiri, N.; Izhikevich, K.; Havens, J.L.; Gangavarapu, K.; Malpica Serrano, L.M.; Crits-Christoph, A.; Matteson, N.L.; et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science 2022, 377, 960–966. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus disease (COVID-19) pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zai, J.; Zhao, Q.; Nie, Q.; Li, Y.; Foley, B.T.; Chaillon, A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol 2020, 92, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.Y.; Yip, A.J.W.; Sharma, A.; Lal, S.K. SARS coronavirus outbreaks past and present-a comparative analysis of SARS-CoV-2 and its predecessors. Virus Genes 2021, 57, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature 2020, 583, 286–289. [Google Scholar] [CrossRef]
- Fan, H.H.; Wang, L.Q.; Liu, W.L.; An, X.P.; Liu, Z.D.; He, X.Q.; Song, L.H.; Tong, Y.G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020, 133, 1051–1056. [Google Scholar] [CrossRef]
- Lam, T.T.; Jia, N.; Zhang, Y.W.; Shum, M.H.; Jiang, J.F.; Zhu, H.C.; Tong, Y.G.; Shi, Y.X.; Ni, X.B.; Liao, Y.S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Temmam, S.; Vongphayloth, K.; Baquero, E.; Munier, S.; Bonomi, M.; Regnault, B.; Douangboubpha, B.; Karami, Y.; Chretien, D.; Sanamxay, D.; et al. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature 2022, 604, 330–336. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Latinne, A.; Thuy, H.B.; Long, N.V.; Ngoc, P.T.B.; Anh, N.T.L.; Thai, N.V.; Phuong, T.Q.; Thai, H.V.; Hai, L.K.; et al. Evidence of SARS-CoV-2 Related Coronaviruses Circulating in Sunda pangolins (Manis javanica) Confiscated From the Illegal Wildlife Trade in Viet Nam. Front Public Health 2022, 10, 826116. [Google Scholar] [CrossRef]
- Garner, E.; Romero, P.; Dunker, A.K.; Brown, C.; Obradovic, Z. Predicting Binding Regions within Disordered Proteins. Genome Inform Ser Workshop Genome Inform 1999, 10, 41–50. [Google Scholar] [PubMed]
- Li, X.; Romero, P.; Rani, M.; Dunker, A.K.; Obradovic, Z. Predicting Protein Disorder for N-, C-, and Internal Regions. Genome Inform Ser Workshop Genome Inform 1999, 10, 30–40. [Google Scholar] [PubMed]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins 2001, 42, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. Protein intrinsic disorder toolbox for comparative analysis of viral proteins. BMC Genomics 2008, 9 (Suppl. S2), S4. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K. Viral shapeshifters: strange behaviors of HIV and other viruses; Simplicity Research Institute: 2017.
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Shell Disorder Analysis Suggests That Pangolins Offered a Window for a Silent Spread of an Attenuated SARS-CoV-2 Precursor among Humans. J Proteome Res 2020, 19, 4543–4552. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Shell disorder analysis predicts greater resilience of the SARS-CoV-2 (COVID-19) outside the body and in body fluids. Microb Pathog 2020, 144, 104177. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Rigidity of the Outer Shell Predicted by a Protein Intrinsic Disorder Model Sheds Light on the COVID-19 (Wuhan-2019-nCoV) Infectivity. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. A comparative analysis of viral matrix proteins using disorder predictors. Virol J 2008, 5, 126. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Zika and Flavivirus Shell Disorder: Virulence and Fetal Morbidity. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. HIV Vaccine Mystery and Viral Shell Disorder. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. Correlating Flavivirus virulence and levels of intrinsic disorder in shell proteins: protective roles vs. immune evasion. Mol Biosyst 2016, 12, 1881–1891. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Computational, Experimental, and Clinical Evidence of a Specific but Peculiar Evolutionary Nature of (COVID-19) SARS-CoV-2. J Proteome Res 2022, 21, 874–890. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Shell Disorder Models Detect That Omicron Has Harder Shells with Attenuation but Is Not a Descendant of the Wuhan-Hu-1 SARS-CoV-2. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. Detection of links between Ebola nucleocapsid and virulence using disorder analysis. Mol Biosyst 2015, 11, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, C.; Zhang, C.; Cui, H.; Chen, Z.; Jiang, X.; Wang, T.; Li, Y.; Liu, J.; Wan, Z.; et al. SARS-CoV-2-related pangolin coronavirus exhibits similar infection characteristics to SARS-CoV-2 and direct contact transmissibility in hamsters. iScience 2022, 25, 104350. [Google Scholar] [CrossRef]
- Lu, S.; Luo, S.; Liu, C.; Li, M.; An, X.; Li, M.; Hou, J.; Fan, H.; Mao, P.; Tong, Y.; et al. Induction of significant neutralizing antibodies against SARS-CoV-2 by a highly attenuated pangolin coronavirus variant with a 104nt deletion at the 3'-UTR. Emerg Microbes Infect 2023, 12, 2151383. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. A Study on the Nature of SARS-CoV-2 Using the Shell Disorder Models: Reproducibility, Evolution, Spread, and Attenuation. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.C.; Ng, K.C.; Ching, R.H.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Sigal, A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol 2022, 22, 69–71. [Google Scholar] [CrossRef]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Hou, Y.J.; Chiba, S.; Leist, S.R.; Meganck, R.M.; Martinez, D.R.; Schafer, A.; Catanzaro, N.J.; Sontake, V.; West, A.; Edwards, C.E.; et al. Host range, transmissibility and antigenicity of a pangolin coronavirus. Nat Microbiol 2023, 8, 1820–1833. [Google Scholar] [CrossRef]
- European_CDC. European Center for Disease Prevention and Control, Weekly epidemilogical update: Omicron variant of concern (VOC). Available online: https://www.ecdc.europa.eu/en/news-events/weekly-epidemiological-update-omicron-variant-concern-voc-week-2-data-20-january-2022 (accessed on Augusr=t 14).
- DeGrasse, D.C.; Black, S.D. The Rise of SARS-CoV-2 (COVID-19) Omicron Subvariant Pathogenicity. Cureus 2023, 15, e40148. [Google Scholar] [CrossRef]
- Kupferschmidt, K. Where did 'weird' Omicron come from? Science 2021, 374, 1179. [Google Scholar] [CrossRef] [PubMed]
- Bondos, S.E.; Dunker, A.K.; Uversky, V.N. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Commun Signal 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Oldfield, C.J.; Meng, J.; Romero, P.; Uversky, V.N.; Dunker, A.K. Mining alpha-helix-forming molecular recognition features with cross species sequence alignments. Biochemistry 2007, 46, 13468–13477. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Brown, C.J.; Lawson, C.J.D.; Iakoucheva-Sebat, L.M.; Vucetic, S.; Obradovic, Z. The Protein Trinity: Structure/Function Relationships That Include Intrinsic Disorder. ScientificWorldJournal 2002, 2, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Brown, C.J.; Lawson, J.D.; Iakoucheva, L.M.; Obradovic, Z. Intrinsic disorder and protein function. Biochemistry 2002, 41, 6573–6582. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J Mol Graph Model 2001, 19, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Oldfield, C.J.; Radivojac, P.; Vacic, V.; Cortese, M.S.; Dunker, A.K.; Uversky, V.N. Analysis of molecular recognition features (MoRFs). J Mol Biol 2006, 362, 1043–1059. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Cheng, Y.; Cortese, M.S.; Romero, P.; Uversky, V.N.; Dunker, A.K. Coupled folding and binding with alpha-helix-forming molecular recognition elements. Biochemistry 2005, 44, 12454–12470. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Dunker, A.K. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem 2014, 83, 553–584. [Google Scholar] [CrossRef]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem Sci 2002, 27, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: a point where biology waits for physics. Protein Sci 2002, 11, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. What does it mean to be natively unfolded? Eur J Biochem 2002, 269, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Unusual biophysics of intrinsically disordered proteins. Biochim Biophys Acta 2013, 1834, 932–951. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim Biophys Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are "natively unfolded" proteins unstructured under physiologic conditions? Proteins 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Vacic, V.; Oldfield, C.J.; Mohan, A.; Radivojac, P.; Cortese, M.S.; Uversky, V.N.; Dunker, A.K. Characterization of molecular recognition features, MoRFs, and their binding partners. J Proteome Res 2007, 6, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem Rev 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 1999, 293, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. Protein intrinsic disorder and influenza virulence: the 1918 H1N1 and H5N1 viruses. Virol J 2009, 6, 69. [Google Scholar] [CrossRef]
- Xue, B.; Williams, R.W.; Oldfield, C.J.; Goh, G.K.; Dunker, A.K.; Uversky, V.N. Viral disorder or disordered viruses: do viral proteins possess unique features? Protein Pept Lett 2010, 17, 932–951. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Uversky, V.N. Understanding Viral Transmission Behavior via Protein Intrinsic Disorder Prediction: Coronaviruses. J Pathog 2012, 2012, 738590. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Uversky, V. Prediction of Intrinsic Disorder in MERS-CoV/HCoV-EMC Supports a High Oral-Fecal Transmission. PLoS Curr 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. A Novel Strategy for the Development of Vaccines for SARS-CoV-2 (COVID-19) and Other Viruses Using AI and Viral Shell Disorder. J Proteome Res 2020, 19, 4355–4363. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Feasibility of the vaccine development for SARS-CoV-2 and other viruses using the shell disorder analysis. Pac Symp Biocomput 2021, 26, 143–153. [Google Scholar] [PubMed]
- UniProt_Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- MySQL. Available online: https://www.mysql.com (accessed on August 14).
- JAVA: JAVA Programming Language. Available online: https://www.java.com (accessed on August, 14).
- Python: Python Programming Language. Available online: https://www.python.org (accessed on 22 December ).
- TREX: CLUSTALW. Available online: http://www.trex.uqam.ca/index.php?action=align&project=trex (accessed on August, 14).
- OpenOffice. Available online: https://www.openoffice.org/ (accessed on August, 14).
- GIMP: GNU Image Manipulator Program. Available online: https://www.gimp.org (accessed on August, 14).
- R_Core_Team. R: A language and environment for statistical computing. 2013.
- NCBI-BLASTP. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on August, 14). Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on August, 14).
- Lu, S.; Luo, S.; Bai, B.; Fan, Z.; Hong, B.; Xu, W.; Ma, H.; Chen, W.; Fan, H.; Tong, Y.; et al. Omicron breakthrough infections in wild-type SARS-CoV-2 vaccinees elicit high levels of neutralizing antibodies against pangolin coronavirus GX_P2V. J Med Virol 2023, 95, e29031. [Google Scholar] [CrossRef]
- Goh, G.K.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Nipah shell disorder, modes of infection, and virulence. Microb Pathog 2020, 141, 103976. [Google Scholar] [CrossRef] [PubMed]
- Longhi, S.; Bloyet, L.M.; Gianni, S.; Gerlier, D. How order and disorder within paramyxoviral nucleoproteins and phosphoproteins orchestrate the molecular interplay of transcription and replication. Cell Mol Life Sci 2017, 74, 3091–3118. [Google Scholar] [CrossRef] [PubMed]
- Malamud, D.; Abrams, W.R.; Barber, C.A.; Weissman, D.; Rehtanz, M.; Golub, E. Antiviral activities in human saliva. Adv Dent Res 2011, 23, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest 2002, 109, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N Engl J Med 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.E.; Nicholas, T.E. Alveolar type I and type II cells. Aust N Z J Med 1984, 14, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, P., (Ed.) How the Body Works: A Comprehensive Illustrated Encyclopedia of Anatomy. Amber Books Limited: 2016.
- Mire, C.E.; Satterfield, B.A.; Geisbert, J.B.; Agans, K.N.; Borisevich, V.; Yan, L.; Chan, Y.P.; Cross, R.W.; Fenton, K.A.; Broder, C.C.; et al. Pathogenic Differences between Nipah Virus Bangladesh and Malaysia Strains in Primates: Implications for Antibody Therapy. Sci Rep 2016, 6, 30916. [Google Scholar] [CrossRef]
- Santaniello, A.; Perruolo, G.; Cristiano, S.; Agognon, A.L.; Cabaro, S.; Amato, A.; Dipineto, L.; Borrelli, L.; Formisano, P.; Fioretti, A.; et al. SARS-CoV-2 Affects Both Humans and Animals: What Is the Potential Transmission Risk? A Literature Review. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Uversky, V.N.; Elrashdy, F.; Aljadawi, A.; Redwan, E.M. Household pets and SARS-CoV2 transmissibility in the light of the ACE2 intrinsic disorder status. J Biomol Struct Dyn 2022, 40, 1441–1444. [Google Scholar] [CrossRef]
- Lehmann, D.; Halbwax, M.L.; Makaga, L.; Whytock, R.; Ndindiwe Malata, L.L.; Bombenda Mouele, W.; Momboua, B.R.; Koumba Pambo, A.F.; White, L.J.T. Pangolins and bats living together in underground burrows in Lope National Park, Gabon. Afr J Ecol 2020, 58, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. How does recombination affect phylogeny estimation? Trends Ecol Evol 2000, 15, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol 2020, 30, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J Gen Virol 2020, 101, 925–940. [Google Scholar] [CrossRef]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol J 2020, 17, 145. [Google Scholar] [CrossRef]
- Wei, C.; Shan, K.J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a mouse origin of the SARS-CoV-2 Omicron variant. J Genet Genomics 2021, 48, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Holmes, S.; Drickamer, L.C.; Robinson, A.S.; Gillie, L.L. Burrows and burrow-cleaning behavior of house mice (Mus musculus domesticus). The American Midland Naturalist 2001, 146, 53–62. [Google Scholar] [CrossRef]
Figure 1.
CoV Transmission SDM. Groups A-C are heavily dependent on PIDN, whereas CoVs in group D have abnormally low M disorder (PIDM) that includes all COVID-19-related viruses.
Figure 1.
CoV Transmission SDM. Groups A-C are heavily dependent on PIDN, whereas CoVs in group D have abnormally low M disorder (PIDM) that includes all COVID-19-related viruses.
Figure 2.
SDMs: Effects of Evolutionary Mutations on the Shell Proteins. A. A harder M protects the virion from damage incurred from antimicrobial enzymes in the saliva and mucus as in the case of SARS-CoV-2 even if a more disordered N allows replication of more infectious particles, especially in vital organs which is the case in SARS-CoV-1. B. Attenuation is likely to occur when the virus enters animal hosts like pigs or pangolins, where fecal-oral transmission is an important route. It is believed that SARS-CoV-2 entered and re-entered pangolins multiple times before it appeared as Wuhan-Hu-1 or Omicron.
Figure 2.
SDMs: Effects of Evolutionary Mutations on the Shell Proteins. A. A harder M protects the virion from damage incurred from antimicrobial enzymes in the saliva and mucus as in the case of SARS-CoV-2 even if a more disordered N allows replication of more infectious particles, especially in vital organs which is the case in SARS-CoV-1. B. Attenuation is likely to occur when the virus enters animal hosts like pigs or pangolins, where fecal-oral transmission is an important route. It is believed that SARS-CoV-2 entered and re-entered pangolins multiple times before it appeared as Wuhan-Hu-1 or Omicron.
Figure 3.
CoV Phylogenetic Trees Using M protein. Omicron (XBB.1.16 and BA.1) is clustered alongside pangolin-CoVs and bat-CoVs, not SARS-CoV-2. Both trees were generated using Clustal Omega. A similar tree using M but utilizing ClustalW with distance optimization placed Omicron even closer to pangolin-CoV.
Figure 3.
CoV Phylogenetic Trees Using M protein. Omicron (XBB.1.16 and BA.1) is clustered alongside pangolin-CoVs and bat-CoVs, not SARS-CoV-2. Both trees were generated using Clustal Omega. A similar tree using M but utilizing ClustalW with distance optimization placed Omicron even closer to pangolin-CoV.
Figure 4.
Virulence-Inner Shell Disorder Model as Applied to COVID-19-Related Viruses. Strong correlation (r=0.8) between SARS-CoV-2 and Omicron PIDN and CFR has been found. CFRs of SARS-CoV-2 (SARS2, non-Omicron) have been estimated to be 2.0-0.5%.
Figure 4.
Virulence-Inner Shell Disorder Model as Applied to COVID-19-Related Viruses. Strong correlation (r=0.8) between SARS-CoV-2 and Omicron PIDN and CFR has been found. CFRs of SARS-CoV-2 (SARS2, non-Omicron) have been estimated to be 2.0-0.5%.
Figure 5.
The PONDR®-VLXT Plot and Sequence Comparison.
Figure 5.
The PONDR®-VLXT Plot and Sequence Comparison.
Figure 6.
Comparative Analysis of Pangolin Coronavirus Pang2017 and SARS-CoV-2 XBB.1.16 Growth Characteristics in Vero Cells. (a) Microscopic evaluation of cytopathic effects in Vero cells post infection with Pang2017 and XBB.1.16 reveals analogous cellular morphological changes, including cell degeneration and rounding. By 48 hours, XBB.1.16-infected cells display pronounced monolayer disruption; (b) One-step growth kinetics of Pang2017 and XBB.1.16 in Vero cells over time. Pang2017's viral genome exhibited an initial increase between 8-10 hours post-infection, with a consistently higher concentration relative to XBB.1.16 from 10-36 hours. Following 48 hours post-infection, Pang2017's proliferation plateaued, lagging behind XBB.1.16; (c) At the 120-hour mark, plaque assays demonstrated that Pang2017 (149.4±11.85 μm, n=30) forms significantly smaller plaques than XBB.1.16 (242.7±11.14 μm, n=30) in Vero cells. Data are presented as Means ± SD. P****<0.0001, determined using a two-tailed Student's t-test.
Figure 6.
Comparative Analysis of Pangolin Coronavirus Pang2017 and SARS-CoV-2 XBB.1.16 Growth Characteristics in Vero Cells. (a) Microscopic evaluation of cytopathic effects in Vero cells post infection with Pang2017 and XBB.1.16 reveals analogous cellular morphological changes, including cell degeneration and rounding. By 48 hours, XBB.1.16-infected cells display pronounced monolayer disruption; (b) One-step growth kinetics of Pang2017 and XBB.1.16 in Vero cells over time. Pang2017's viral genome exhibited an initial increase between 8-10 hours post-infection, with a consistently higher concentration relative to XBB.1.16 from 10-36 hours. Following 48 hours post-infection, Pang2017's proliferation plateaued, lagging behind XBB.1.16; (c) At the 120-hour mark, plaque assays demonstrated that Pang2017 (149.4±11.85 μm, n=30) forms significantly smaller plaques than XBB.1.16 (242.7±11.14 μm, n=30) in Vero cells. Data are presented as Means ± SD. P****<0.0001, determined using a two-tailed Student's t-test.
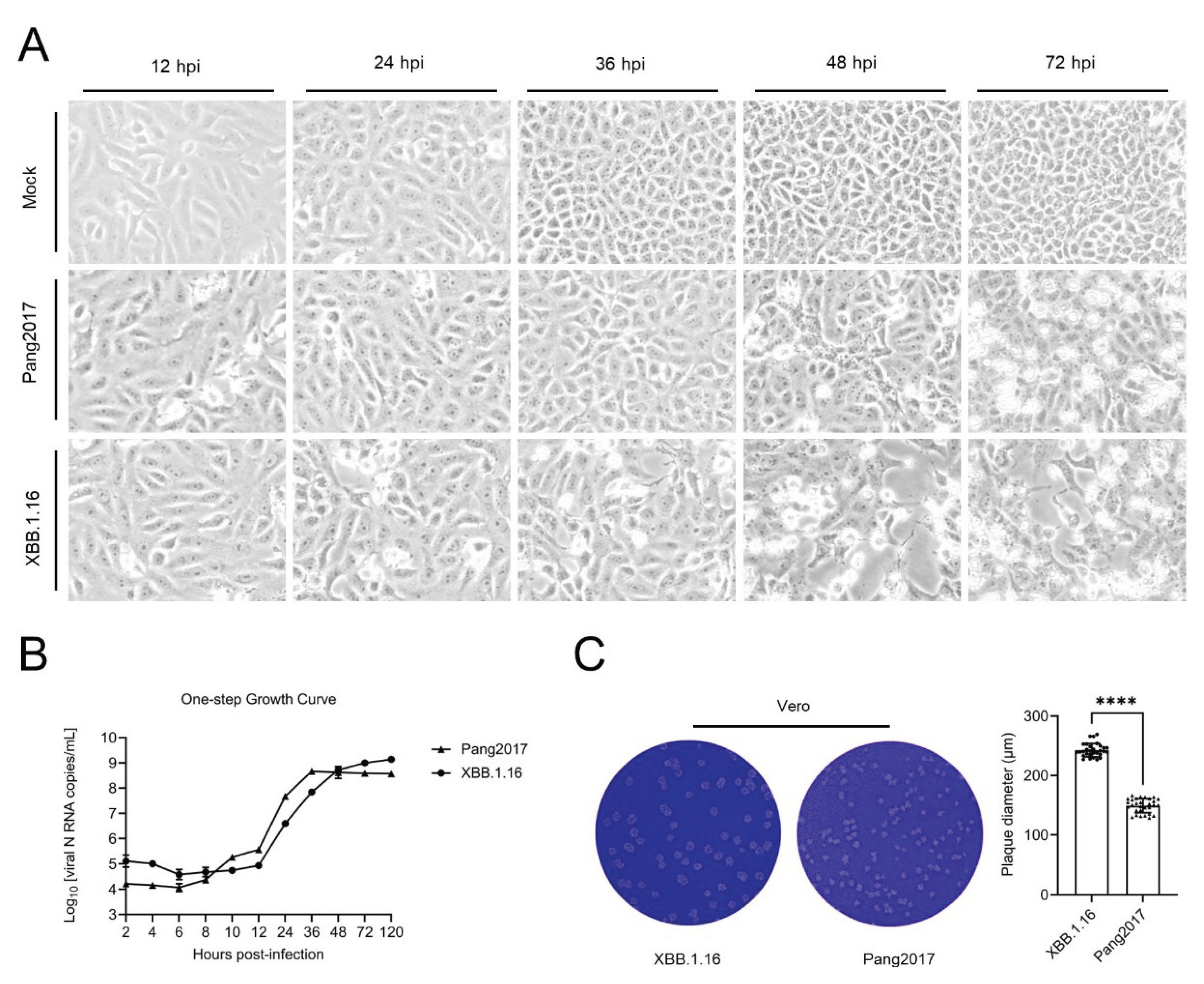
Table 1.
Shell Disorder Models (SDMs). Three closely related SDMs were built based levels of disorder of shell proteins of viruses and known characteristics of viruses.
Table 1.
Shell Disorder Models (SDMs). Three closely related SDMs were built based levels of disorder of shell proteins of viruses and known characteristics of viruses.
| Year of First Publication |
Shell Disorder Model |
Details |
| 2008 |
Parent Viral Shape-shifter Model |
Abnormally huge levels of disorder were found at the outer shell of many HIV-1 variants and may sexually transmitted viruses such as HSV and HCV. This could account for the lack of an effective HIV vaccine. |
| 2012 |
CoV Transmission SDM |
Levels of fecal-oral and respiratory CoV transmission is predicted by levels of shell disorder. |
| 2015 |
Virulence-inner Shell Disorder Model |
High correlations between the inner shell and virulence of a variety of viruses have been detected. |
Table 2.
Bat-CoV, COVID-19-Related viruses and SARS-CoV-1. All known COVID-19 viruses have abnormally hard outer shells (low PIDM values). The pattern of N PID for pangolin-CoVs is similar to that of SARS-CoV-2 and Omicron, not bat-CoVs. Non-COVID-19-related bat-CoV is listed here for comparison purposes. SDMs have detected two sub-variants of Delta, not previously observed. We named them, Delta1 and Delta2.
Table 2.
Bat-CoV, COVID-19-Related viruses and SARS-CoV-1. All known COVID-19 viruses have abnormally hard outer shells (low PIDM values). The pattern of N PID for pangolin-CoVs is similar to that of SARS-CoV-2 and Omicron, not bat-CoVs. Non-COVID-19-related bat-CoV is listed here for comparison purposes. SDMs have detected two sub-variants of Delta, not previously observed. We named them, Delta1 and Delta2.
| Coronavirus |
Se-quence
Similari-ty
M (%) |
PIDM
(%) |
Accession:
UniProt(U)
GenBank(G) |
Sequence
Similarity
N (%) |
PIDN
(%) |
Accession
UniProt(U)
GenBank(G |
SARS-CoV-1
Civet-SARS-CoV |
90.5
90.1 |
8.6
8.6 |
P59596(U)
Q3ZTE9(U) |
90.5
90.01 |
50.2
49.1 |
P59595(U)
Q3ZTE4(U) |
COVID-Related Bat-CoV
RaTG13
Laotian Bat-CoV
[Banal-52]
[Banal-103]
[Banal-236] |
99.6
98,7
98.7
99.1 |
6.0+0.2
4.1
6.3
5.9
4.1 |
QHR63303(G)
UAY13220(G)
UAY13232(G)
UAY13256(G)
|
99.1
99.3
99.1
99.3 |
48.3+0.2
48.2
48.5
48.5 |
QHR63308(G)
UAY13225.1
UAY13257.1
UAY1326.1 |
Pangolin-CoV
2019
2018
2017***
|
98.2
97.7
98.2
|
5.6+0.9a
6.3
4.5
5.9
|
QIG55948(G)
QIQ54051(G)
QIA48617(G) |
98
93.8
94
93.32 |
46.6+1.6a48.7
46.3
44.8
46.5 |
QIG55953(G)
QIQ54056(G)
QIA48630(G)
QIA48656(G) |
SARS-CoV-2
[Wuhan]
[Delta1]
[Delta2[
[Omicron]**
|
100
99.1
99.1
98.7
|
5.9
5.9
5.9
5.7+0.4
|
YP009724393(G)
QUX81285(G)
QUX81285(G)
|
100
99.3
99.1
98.6
|
48.2
46.8
47.5
44.5+0.4
|
YP009724397(G)
QYM89997(G)
QYM89845(G)
|
Omicron
BA.1.44
XBB.1.16 |
98.7
99.1 |
5.4
5.9 |
UFO69282(G) WIL50320(G) |
98.6
98.3 |
44.7
44.2 |
UFO69287(G)
WIL50325(G) |
Bat-CoV
RATG13
Bat 512
HKU3
HKU4
HKU5 |
99.6
35.5
91
42.7
44.7 |
11+15a
4.1
15.3
7.7
16.4
11.8 |
QHR63303(G)
Q0Q463(U)
Q3LZX9(U)
A3EXA0(U)
A3EXD6(U) |
99.1
29.4
89.6
51.1
47.9 |
47.7+0.9a
48.5
46.5
48
48.5
47.1 |
QHR63308(G)
Q0Q462(U)
Q3LZX4(U)
A3EXA1(U)
A3EXD7(U) |
Table 3.
The Correlation (r=0.8) Between PIDN and Viral Growth.
Table 3.
The Correlation (r=0.8) Between PIDN and Viral Growth.
| Virus/Isolate |
PIDN
|
Non-Attenuation/Aggressiveness |
| SARS-CoV-1 |
50.00% |
+++ |
| BANAL-236 |
48.5% |
++ |
| Pang2019 |
48.5 |
++ |
| Wuhan-Hu-1 |
48.20% |
+ |
| XBB.1.16 |
44.5 |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).