1. Introduction
The demineralization of crustacean shells has wide applicability not only in materials science but also in aquatic research and environmental studies, as well as in the currently increasing demand of the bioeconomy sector. The main objective of the blue bioeconomy is to obtain new and valuable products from aquatic waste, making it one of the rapidly expanding research fields in the context of current priorities [
1].
The abundant aquatic waste material in focus here is the biogenic calcium carbonate originating from crustacean or gastropod shells. Among these, the Atlantic blue crab
Callinectes sapidus (Rathbun, 1896) and whelk
Rapana venosa (Valenciennes, 1846) are ranked as some of the most invasive species in the Mediterranean and Black Sea basins. There are many studies related to the increased awareness regarding the most invasive species in the Mediterranean Sea such as the one conducted by Marchessaux et al. who advocate the resilient idea of turning the threat into new opportunities [
2,
3,
4].
The Atlantic blue crab C. sapidus and the whelk R. venosa are both ranked among the 100 worst invasive species, with a negative impact on the ecology of invaded areas, seashore ecosystems, touristic areas, as well as the aquaculture exploitation of the local bivalve products. These species have raised special attention not only from environmental scientists, but also from the blue bioeconomy research field due to their potential for multidisciplinary approaches. Thus, aquatic resources can be sustainably exploited to produce value-added compounds or innovative products from aquatic waste.
We demonstrated in several recently published papers that the highly ordered 3D-nanostructure of the
C. sapidus shells, comprising mineral and organic components [
5], possesses an intricate porosity, which could be exploited for various applications [
6]. These include serving as an efficient material for solutions loading and slow release [
7,
8], a drug carrier, or an efficient absorbent of pollutants [
9], as well as a new biostimulant [
10], while being compliant with the regulations regarding their heavy metal content [
11].
The complex scaffold of chitin-protein fibrils that supports biomineralization has never been considered with respect to its potential utility as a chitin–based polymer foil. This green product might be available as a subject of the intact raw material demineralized, without powdering. As recently reviewed, numerous studies reporting chitin or chitosan production from crustaceans [
12] have used powdering as a main step in the production process, where understanding dependencies for efficient extraction is crucial. To speed up the process, the energy consumption and the workload for the multi-step preparation of powders are high, while a slower process could eliminate all these steps. The demineralization process, however, can be considered without powdering, heating, and stirring of the demineralization bath, conducting to a resulted material that can be further tailored according to the desired products.
Chitin, the second most abundant polymer after cellulose, is widely produced from the primary source of aquatic waste derived from crab and shrimp processing waste bioeconomy. Industrially, chitin is produced throughout acid treatment, hydrochloric acid being the preferred demineralization agent, even though it may negatively impact the molecular mass and the degree of deacetylation of the resulted polymer. Therefore, it may impair the purified chitin’s inherent qualities [
13]. The demineralization process is followed by deproteinization and decolorization to obtain pure, colorless chitin, without any residues, reaching the required quality for specific applications of many fields, such as drug delivery, tissue engineering, agriculture, food industry and others [
12]. Due to the variability of the chitin source, the entire production process requires optimization.
To obtain chitin from various crustacean species, the demineralization process may depend on the structural and morphological characteristics of each species, thus knowledge-based decisions on the most convenient process steps are required. Additionally, during the demineralization process, tools needed for informed decisions (to continue, to modify conditions or to stop) are scant. Most of the studies rely on obtaining the “final product” under certain conditions and consider the necessary repeating operations or improved conditions to achieve the optimal processing. This is done with the aim of ensuring compatibility with the transition to the industrial environment to the highest technology readiness level (TRL), while complying with low cost, high-quality final product, minimal workload and an environmentally friendly chemical consumption [
14].
When exposed to acid treatment (usually hydrochloric acid), the biogenic carbonate reacts to yield the secondary product, calcium acetate, besides CO
2 and water. Calcium acetate, approved by the regulatory bodies [
15], is widely used as a food additive, an acidity regulator, a preservative and stabilizing agent for nutraceuticals, a calcium supplement, and as a medication for patients with kidney disease undergoing dialysis, to control hyperphosphatemia. There are two widely used industrial methods to obtain calcium acetate. One involves the reaction between calcium carbonate and acetic acid, often using natural limestones or marble as the starting material, resulting calcium acetate, carbon dioxide, and water. The other method employs using calcium hydroxide and acetic acid, yielding calcium acetate and water. The biogenic carbonate waste material, typically referring to calcium carbonate derived from the crustaceans’ waste, bivalve, or molluscs shells, is not usually considered, as it is often blamed for potential impurity residues that may alter the quality of the calcium acetate product.
To the best of our knowledge, there are neither reported studies on obtaining transparent polymeric foils of chitin from unground, unpowdered biogenic materials derived from
C. sapidus, the mantis shrimp
Squilla mantis (Linnaeus, 1758), and the European spider crab
Maja squinado (Herbst, 1788), nor reports on calcium acetate obtained from these crustaceans. Md. Iftekhar Shams et al. noted the preparation of an optically transparent crab-shell by removing non-chitin components (e.g. calcium carbonate, proteins, lipids, pigments) to create transparent nanocomposites with improved properties. The species considered was
Chionoecetes opilio (Fabricius, 1788), and the demineralization process occurred under hydrochloric acid treatment [
16].
In this paper we demonstrate the usefulness of the Raman spectroscopy techniques to assist the demineralization process of the biogenic carbonates derived from three distinct crustacean species to obtain chitin, or derived from R. venosa shells, to valorize its abundant calcium content for calcium acetate production, as this gastropod shell does not comprise chitin. We compared the in-line process control results in terms of Raman spectroscopy signal, to timely check the appearance of the chitin signal in the resulting demineralized biological samples using both the lab-based Raman system and the hand-held Raman instrument. We further comparatively evaluate the calcium acetate by-products resulted after applying a ‘green’ method, using the acetic acid reaction on the biogenic waste at room temperature, without powdering or heating. Finally, we evaluate the quality of the calcium acetate resulted from the crustaceans and snail shells. Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and scanning electron microscopy combined with energy dispersive X-ray spectroscopy (SEM-EDX) have been employed as cross validation methods to confirm the identity and the morphology of the final bio-products.
Considering the time, cost, effort and chemicals required for such a crucial industrial approach, here we propose the implementation of Raman technology as an effective tool to assist every step of this economically important activity.
2. Materials and Methods
2.1. Biogenic Material Selection and Processing
Biological samples from three crustacean species – C. sapidus, S. mantis and M. squinado - were acquired through a collaboration between the Babeș-Bolyai University and the University of Dubrovnik, originating from the Neretva River Delta (South-Easten Adriatic Sea). The specimens of S. mantis and M. squinado were caught and maintained in frozen conditions, while the shells from C. sapidus represented food waste from cooked crabs. One specimen from each S. mantis and M. squinado were eviscerated. We considered waste biogenic material from cooked carapace fragments of C. sapidus, cuticle segments of abdomen, telson cuticle from S. mantis and the whole, raw carapace of M. squinado for the experimental studies. Fresh specimens of the R. venosa snail have been gathered from a cluster of individuals along the Romanian shores of the Black Sea, specifically Năvodari, at the geographical coordinates 44°18’07.4"N, 28°37’38.4"E. The R. venosa shells were randomly selected from a large stock comprising both adult specimens with intensive pink-orange pigmentation and juvenile specimens with pronounced blue pigmentation.
The selected crustacean shells were cleaned from adherent aquatic materials, degreased, washed abundantly with deionized pure water (resistivity 18.2 MΩ × cm at 22°C) and immersed in pure glacial acetic acid. R. venosa specimens were immersed in a vinegar bath (acetic acid 9%), after undergoing a thorough cleaning process with the removal of soft tissue from the shells. Demineralization occurred at room temperature, with the process systematically monitored through periodic analysis of the biological samples by Raman techniques.
2.2. Chemicals
Glacial acetic acid was provided by Sigma Aldrich, while geogenic calcium carbonate was purchased from CHIMREACTIV S.R.L, both substances being used without any further purification.
2.3. Demineralization By-Products and Reference Calcium Acetate Synthesis
The immersion bathing solutions of the biological samples were evaporated by exposure to controlled heat to fully investigate the demineralization process. Given that calcium carbonate is the main mineral of the biological samples considered, the reaction between this compound and acetic acid was observed to obtain geogenic calcium acetate as a reference material of the demineralization by-products. Geogenic calcium acetate was synthetized using 1 mg of standard geogenic calcium carbonate dissolved in a mixture solution of 2 ml pure glacial acetic acid and 3 ml acetic acid aqueous solution 10%. The obtained mixture was prepared under magnetic stirring at controlled temperature for 60 minutes, the resulted solution being evaporated under controlled heat. All powders obtained were dried in the oven at 60°C for 24 hours and investigated through Raman spectroscopy and X-ay diffraction.
2.4. Instrumentation
As a lab-based instrument to validate the hand-held Raman system, we employed the Renishaw InVia Reflex Raman system (Renishaw, UK) with a Leica confocal microscope. For Raman excitation, a laser diode emitting at 785 nm has been employed to characterize the starting materials and the ones during the demineralization progress. An additional Cobolt diode pumped solid state laser emitting at 532 nm has been employed to control the presence of native carotenoids in biogenic shells, exploiting their selective signal under resonance Raman conditions, and a He-Ne- laser providing the 632.8 nm excitation line has been used for detecting the carotenoproteins resonant signal. The instrument calibration was achieved with the internal silicon providing the band centred at 520 cm-1. WiRETM 3.4 Software (Renishaw, United Kingdom) was used for data acquisition. The spectral resolution was 1 cm-1 in NIR and 0.5 cm-1 for the visible range excitation.
A hand-held TacticID® Mobile Raman system model BWS493TSII (BWTEK, a Metrohm Group Company) with a NIR-laser emitting at 1064 nm, 220 mW with a TE-Cooled InGaAs Array detector, has been used to record spectra during process in the 176 - 2000 cm
-1 spectral range, with a spectral resolution of 11 cm
-1. The system is equipped with a database of 1200 spectra of synthetic chemicals, narcotics, drugs, explosives, cutting agents, precursors, and solvents [
17].
A Shimadzu FT-IR IRSPIRIT with an QATR-S accessory, holding a single-reflection integration-type ATR module, with a diamond prism, has been employed to record the FT-IR spectra of the demineralized fragments in the 650-4000 cm-1 spectral range, setting 50 accumulations per spectrum, with 8 cm-1 spectral resolution selected in the LabSolutions IR software.
X-ray powder diffraction (XRD) analyses were achieved using a Bruker D8 Advance diffractometer in Bragg-Brentano geometry, possessing a Cu tube with = 0.15418 nm, a Ni filter and a LynxEye detector. Corundum (NIST SRM1976a) was used as an internal standard. The data were collected in the 3.8 – 64° 2θ interval at a 0.02° 2θ step, measuring each step for 0.2 seconds. Acetate precipitates were ground in an agate mortar and placed in Bruker PMMA sample holders. In the case of the demineralized foils, surface XRD was performed on foil fragments, with the surface of the fragments aligned to the X-ray beam. The identification of mineral phases was performed with the Diffrac.Eva 2.1 software (Bruker AXS) using the PDF2 (2023) database from the ICDD (International Centre for Diffraction Data).
Scanning electron microscopy and energy-dispersive X-ray spectroscopy (SEM-EDX) analyses have been achieved using a Hitachi SU8320 ultra-high resolution cold field emission scanning electron microscope (Hitachi, Japan) with a Quorum Q150T gold sputtering coater of controlled thickness of 11 nm at a rate of 14 nm/min and evaporating carbon for EDX analysis using an Oxford energy-dispersive X-ray module (Oxford, UK) for semiquantitative elemental analysis of the demineralized shell fragments.
The dataset underwent comprehensive processing and analysing using OriginPro 2021b, OriginLab Corporation, Northampton, MA, USA. The data processing steps are illustrated in the supplementary figure (
Figure S1) which includes recording multiple spectra, calculating averaged signal, background subtraction, and comparison with the reference spectral data of α-chitin.
Author Contributions
Conceptualization, S.C.P.; methodology, S.C.P., I.C.P., K. M., D.A.D.; validation, S.C.P., I.C.P., K.M., T.T., D.A.D., N.B.; formal analysis, S.C.P., K.M., I.C.P.; investigation, S.C.P., I.C.P., K.M., D.A.D, F.N., T.T., L.B., B.N.; resources, S.C.P, F.N., D.A.D, B.N.; data curation, S.C. P., K. M., I. P., L.B., B.N.; writing—original draft preparation, I.C.P., K.M.; writing—review and editing, S.C.P., I.C.P., K.M., D.A.D, T.T., B.N.; visualization, S.C.P., I.C.P., K.M., D.A.D., L.B-T., T.T., F.N., N.B.; supervision, S.C.P.; All authors have read and agreed to the published version of the manuscript.
Figure 1.
(A) Untreated anatomical parts as following: carapace fragments (a) and ventral fragment (b) of C. sapidus; cuticle segments of abdomen (c), uropod (d) and telson cuticle (e) from S. mantis; carapace of M. squinado (f); (B) Crustacean anatomical shell fragments after exposure to acetic acid for 65 days as following: carapace fragment (a) and ventral fragment (b) of C. sapidus; cuticle segment of abdomen (c), uropod (d) and telson cuticle (e) from S. mantis; carapace of M. squinado (f); (C) Adult R. venosa shell during vinegar demineralization (a) and after 14 days of treatment in ventral (b) and lateral (c) view.
Figure 1.
(A) Untreated anatomical parts as following: carapace fragments (a) and ventral fragment (b) of C. sapidus; cuticle segments of abdomen (c), uropod (d) and telson cuticle (e) from S. mantis; carapace of M. squinado (f); (B) Crustacean anatomical shell fragments after exposure to acetic acid for 65 days as following: carapace fragment (a) and ventral fragment (b) of C. sapidus; cuticle segment of abdomen (c), uropod (d) and telson cuticle (e) from S. mantis; carapace of M. squinado (f); (C) Adult R. venosa shell during vinegar demineralization (a) and after 14 days of treatment in ventral (b) and lateral (c) view.
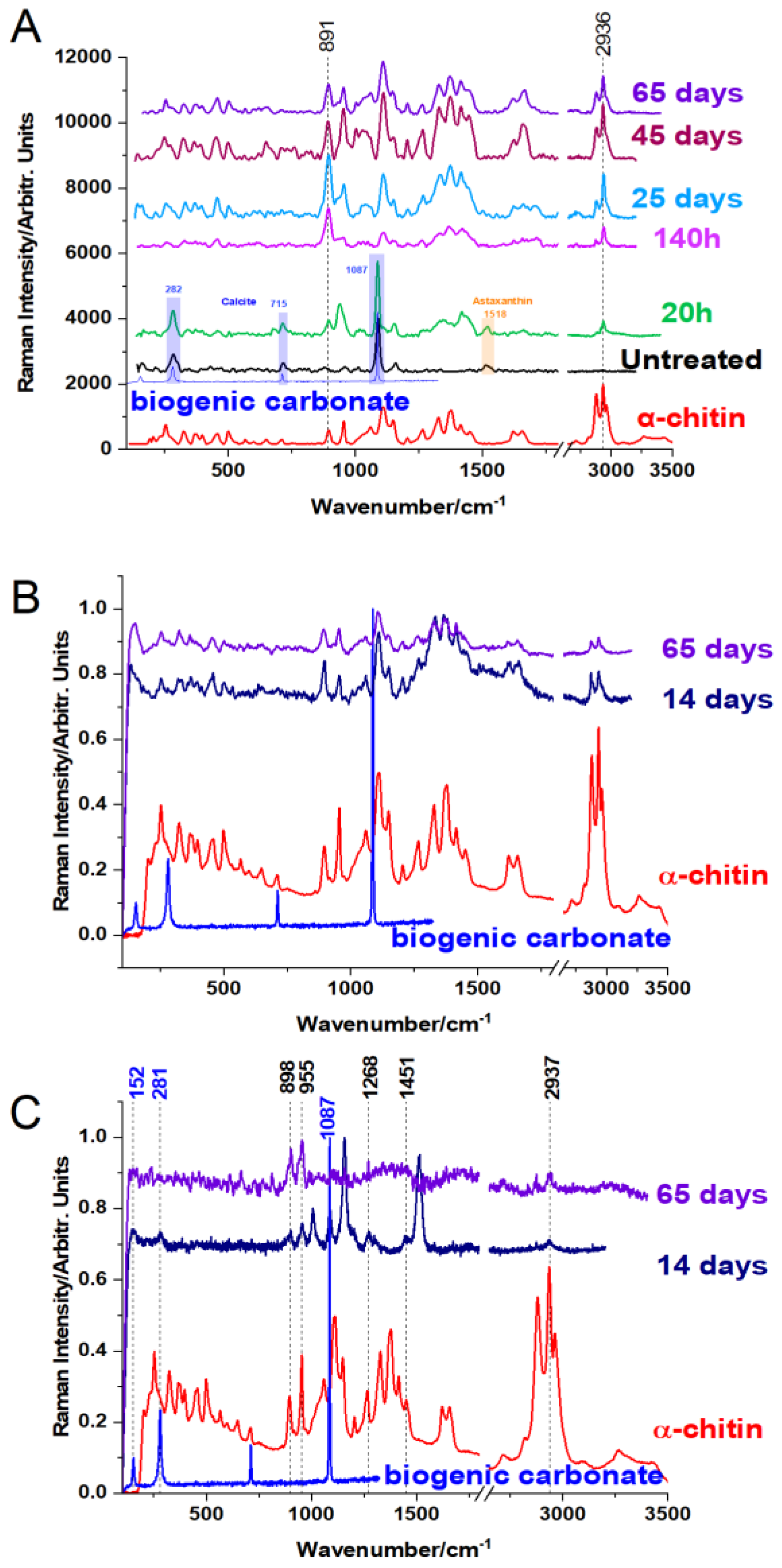
Figure 2.
Raman spectra evolution of the biogenic material from C. Sapidus (A), S. mantis (B), and M. squinado (C) at various times (indicated as hours or days) during the acetic acid demineralization process, using 785 nm laser line, compared to the reference spectrum of α-chitin, as indicated. The blue spectra show the reference signal of calcium carbonate to highlight its disappearance in the final products.
Figure 2.
Raman spectra evolution of the biogenic material from C. Sapidus (A), S. mantis (B), and M. squinado (C) at various times (indicated as hours or days) during the acetic acid demineralization process, using 785 nm laser line, compared to the reference spectrum of α-chitin, as indicated. The blue spectra show the reference signal of calcium carbonate to highlight its disappearance in the final products.
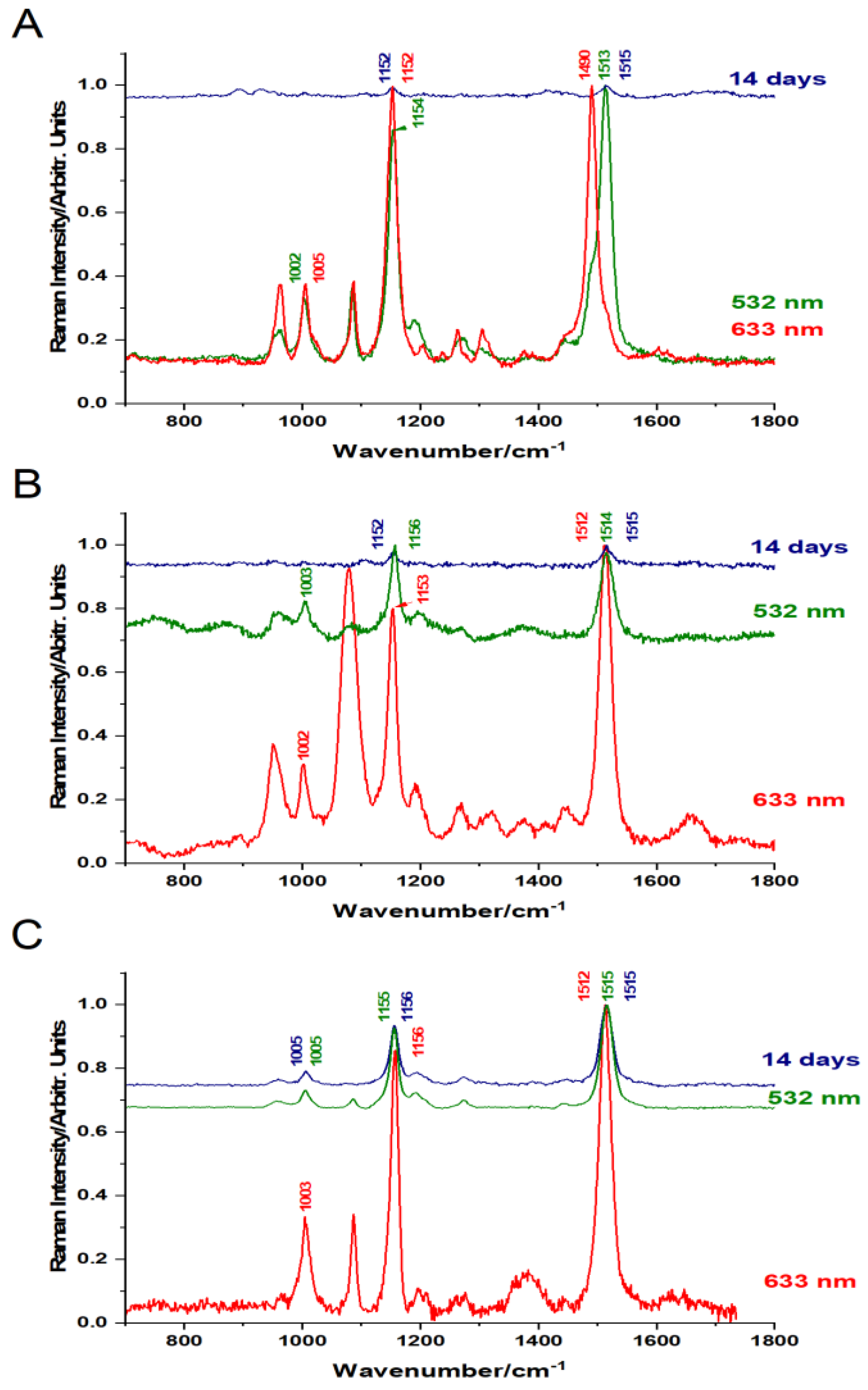
Figure 3.
Pigments detected in the raw crustacean shells of C. sapidus (A), S. mantis (B), and M. squinado (C), under resonant Raman excitation with 532 nm for carotenoids (green line) and 633 nm for carotenoproteins (red line) before demineralization, and after 14 days of exposure to the acetic acid bath solution (top spectra, navy - blue line in each case).
Figure 3.
Pigments detected in the raw crustacean shells of C. sapidus (A), S. mantis (B), and M. squinado (C), under resonant Raman excitation with 532 nm for carotenoids (green line) and 633 nm for carotenoproteins (red line) before demineralization, and after 14 days of exposure to the acetic acid bath solution (top spectra, navy - blue line in each case).
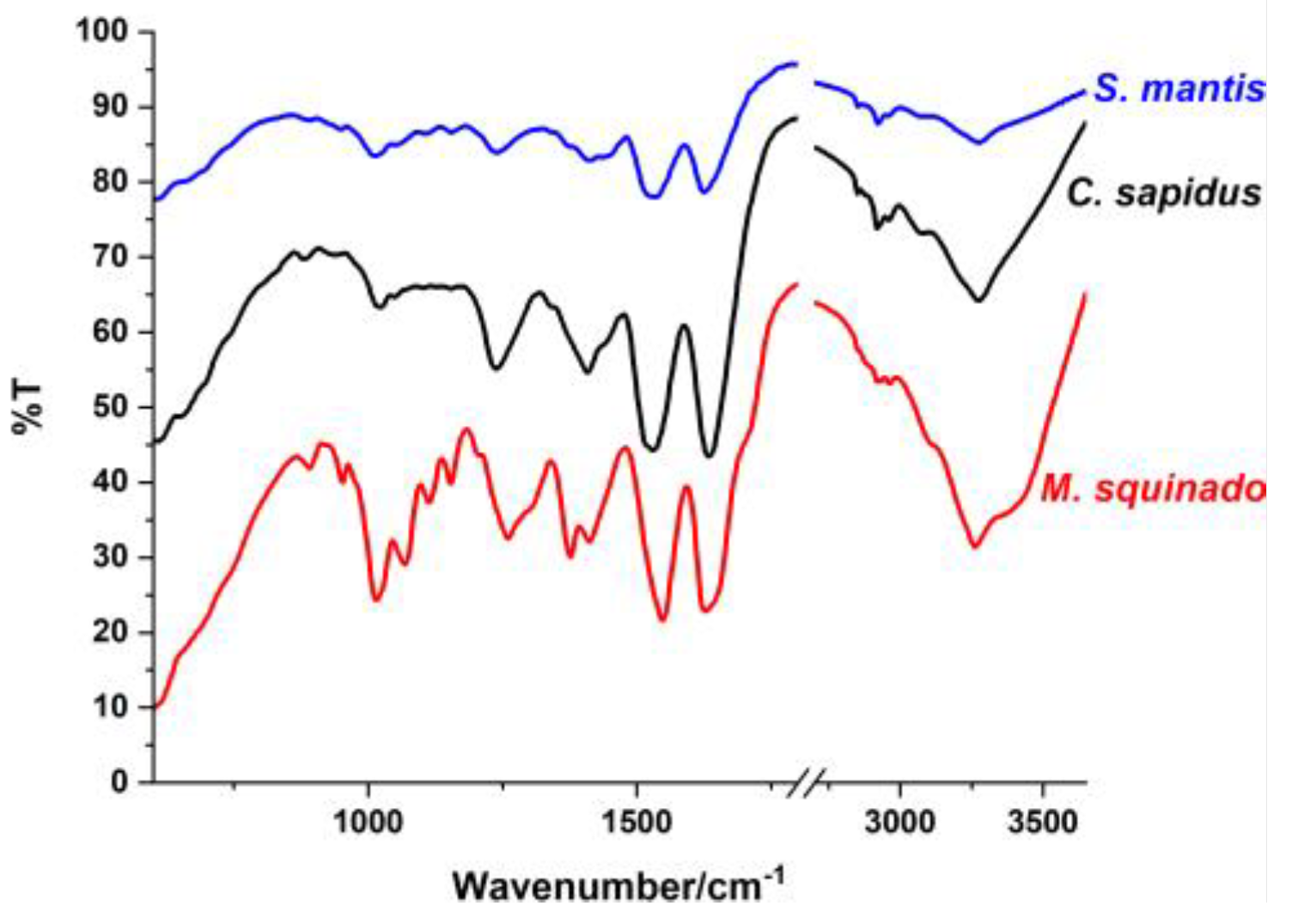
Figure 4.
FT-IR spectra of the intact, transparent, demineralized foil products resulted from the three crustacean species after 65 days of acetic acid treatment: S. mantis (blue line), C. sapidus (black line), and M. squinado (red line).
Figure 4.
FT-IR spectra of the intact, transparent, demineralized foil products resulted from the three crustacean species after 65 days of acetic acid treatment: S. mantis (blue line), C. sapidus (black line), and M. squinado (red line).
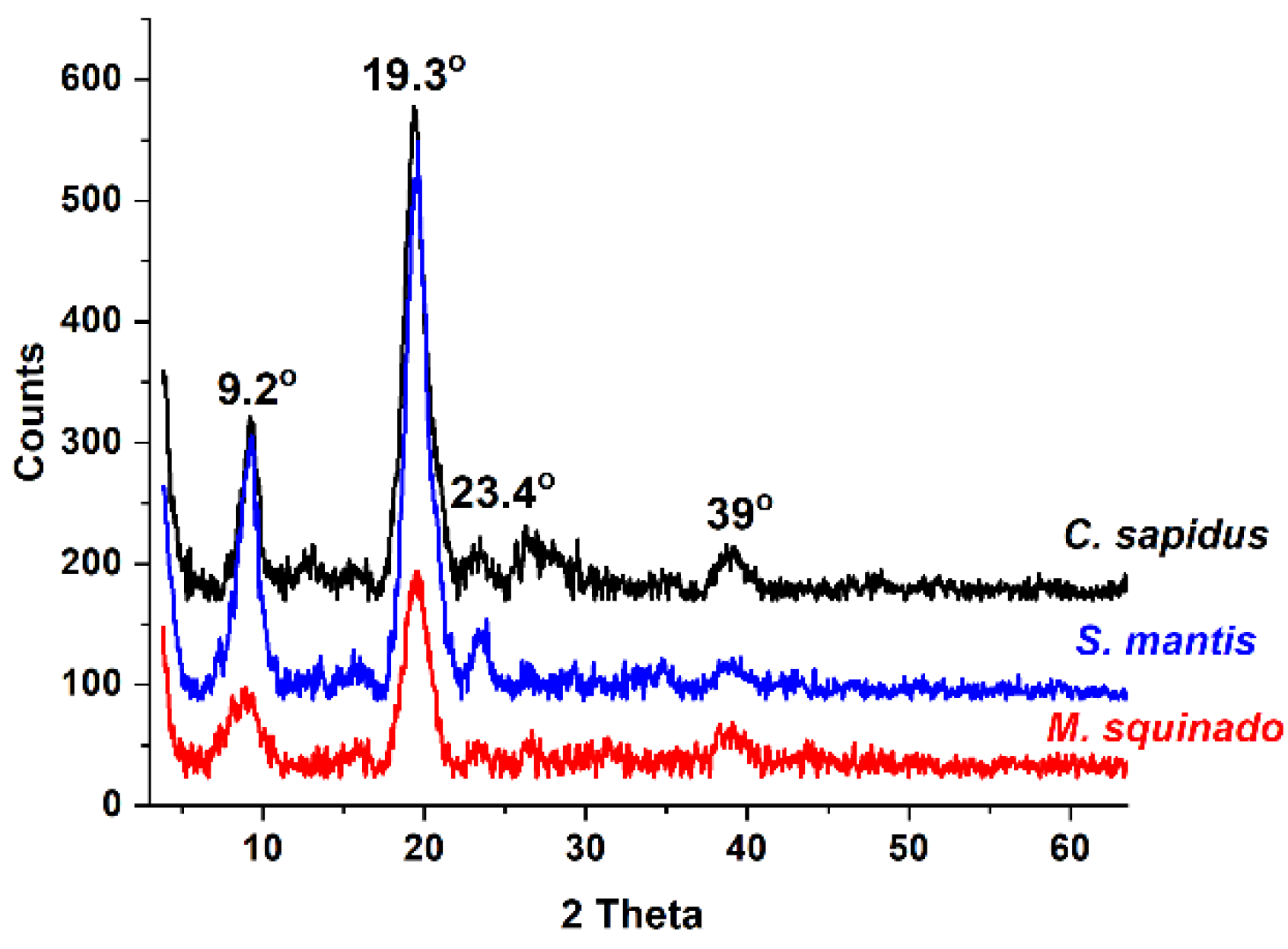
Figure 5.
XRD diffraction patterns of the intact, transparent, demineralized foil products resulted from the three crustacean species after 65 days of acetic acid treatment: C. sapidus (black line), S. mantis (blue line), M. squinado (red line).
Figure 5.
XRD diffraction patterns of the intact, transparent, demineralized foil products resulted from the three crustacean species after 65 days of acetic acid treatment: C. sapidus (black line), S. mantis (blue line), M. squinado (red line).
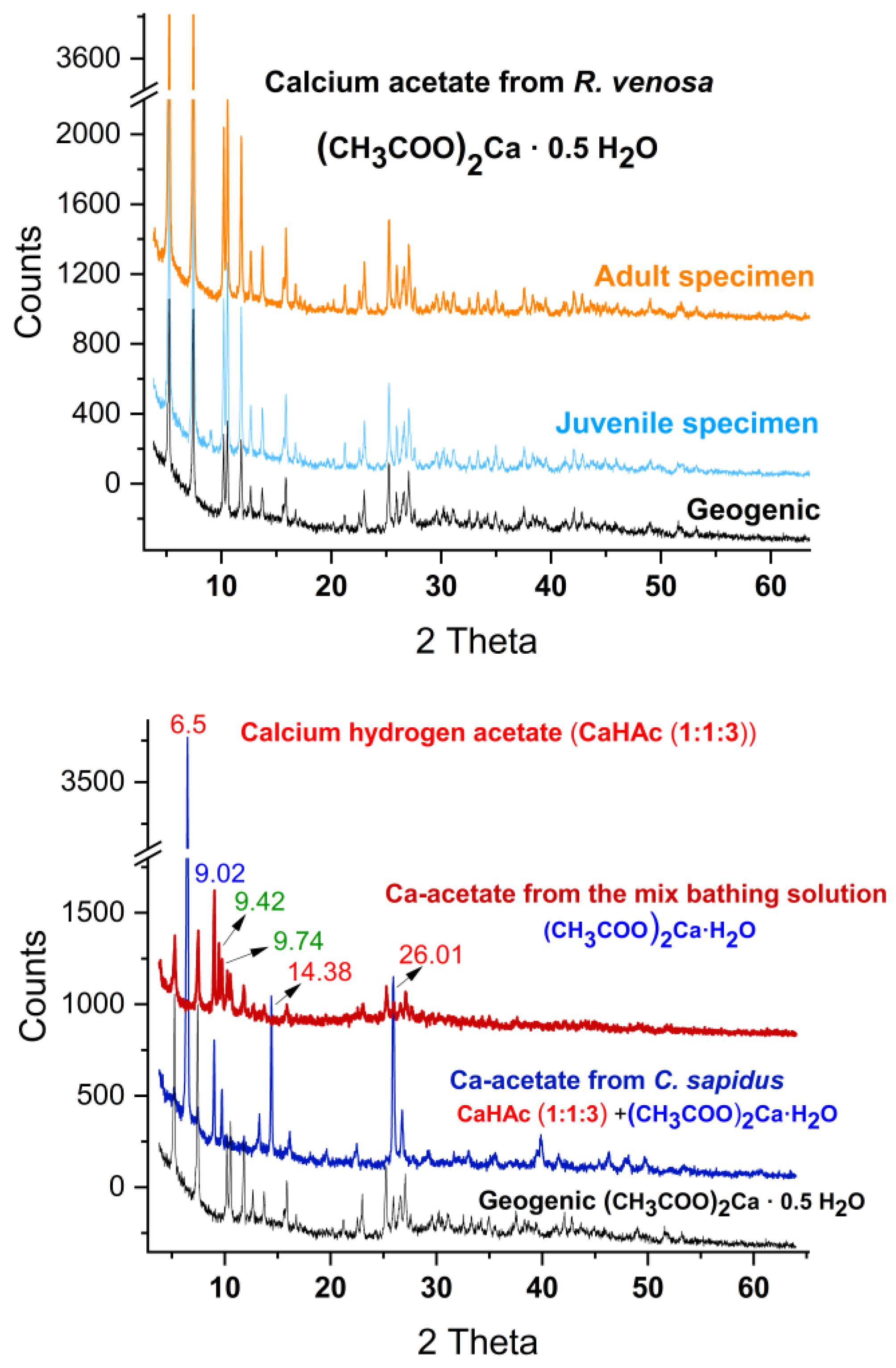
Figure 6.
XRD pattern of the calcium acetate hemihydrate by-products from R. venosa snail (top panel): from adult pink shells (orange line), and juvenile blue pigmented shells (light-blue line). Calcium acetate by-products from crustaceans (bottom panel) shows different hydrated forms Ca-acetate from bathing solution of mixed crustacean shells (dark-red line), Ca-acetate from C. sapidus as a mixture of Ca-hydrogen acetate and hydrate (navy-blue line), and geogenic Ca-acetate hemihydrate (black line), as indicated on each pattern. Additional peaks in the Ca-acetate from bathing solution of mixed crustacean shells (dark-red line) plotted in green indicate other contributions.
Figure 6.
XRD pattern of the calcium acetate hemihydrate by-products from R. venosa snail (top panel): from adult pink shells (orange line), and juvenile blue pigmented shells (light-blue line). Calcium acetate by-products from crustaceans (bottom panel) shows different hydrated forms Ca-acetate from bathing solution of mixed crustacean shells (dark-red line), Ca-acetate from C. sapidus as a mixture of Ca-hydrogen acetate and hydrate (navy-blue line), and geogenic Ca-acetate hemihydrate (black line), as indicated on each pattern. Additional peaks in the Ca-acetate from bathing solution of mixed crustacean shells (dark-red line) plotted in green indicate other contributions.
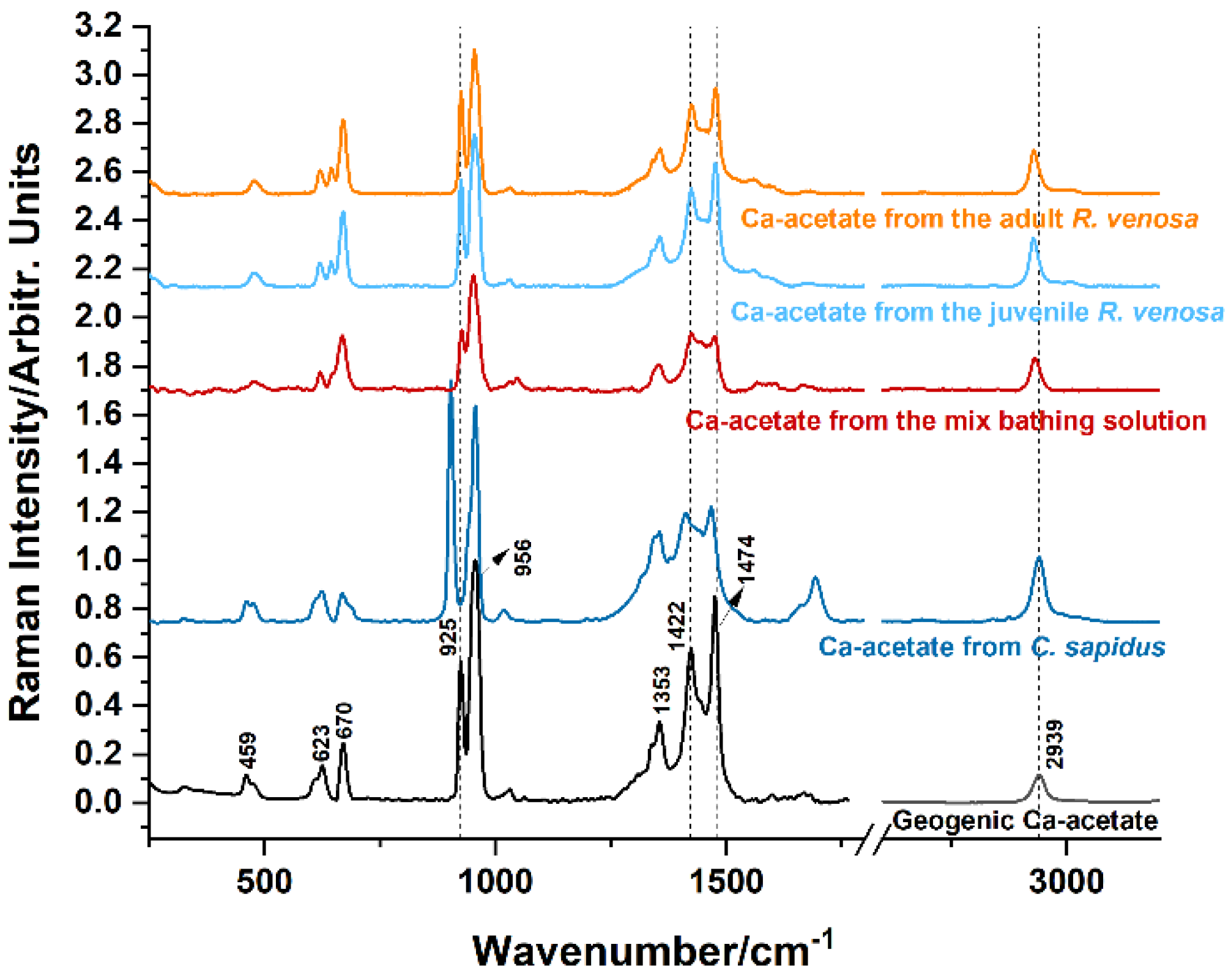
Figure 7.
Calcium acetate hydrates formed in the demineralization bath solutions of adult (orange line) and juvenile R. venosa (light-blue line) specimens, mixed crustacean specimens (dark-red line), and C. sapidus (navy-blue line), compared to the geogenic calcium acetate (black line).
Figure 7.
Calcium acetate hydrates formed in the demineralization bath solutions of adult (orange line) and juvenile R. venosa (light-blue line) specimens, mixed crustacean specimens (dark-red line), and C. sapidus (navy-blue line), compared to the geogenic calcium acetate (black line).
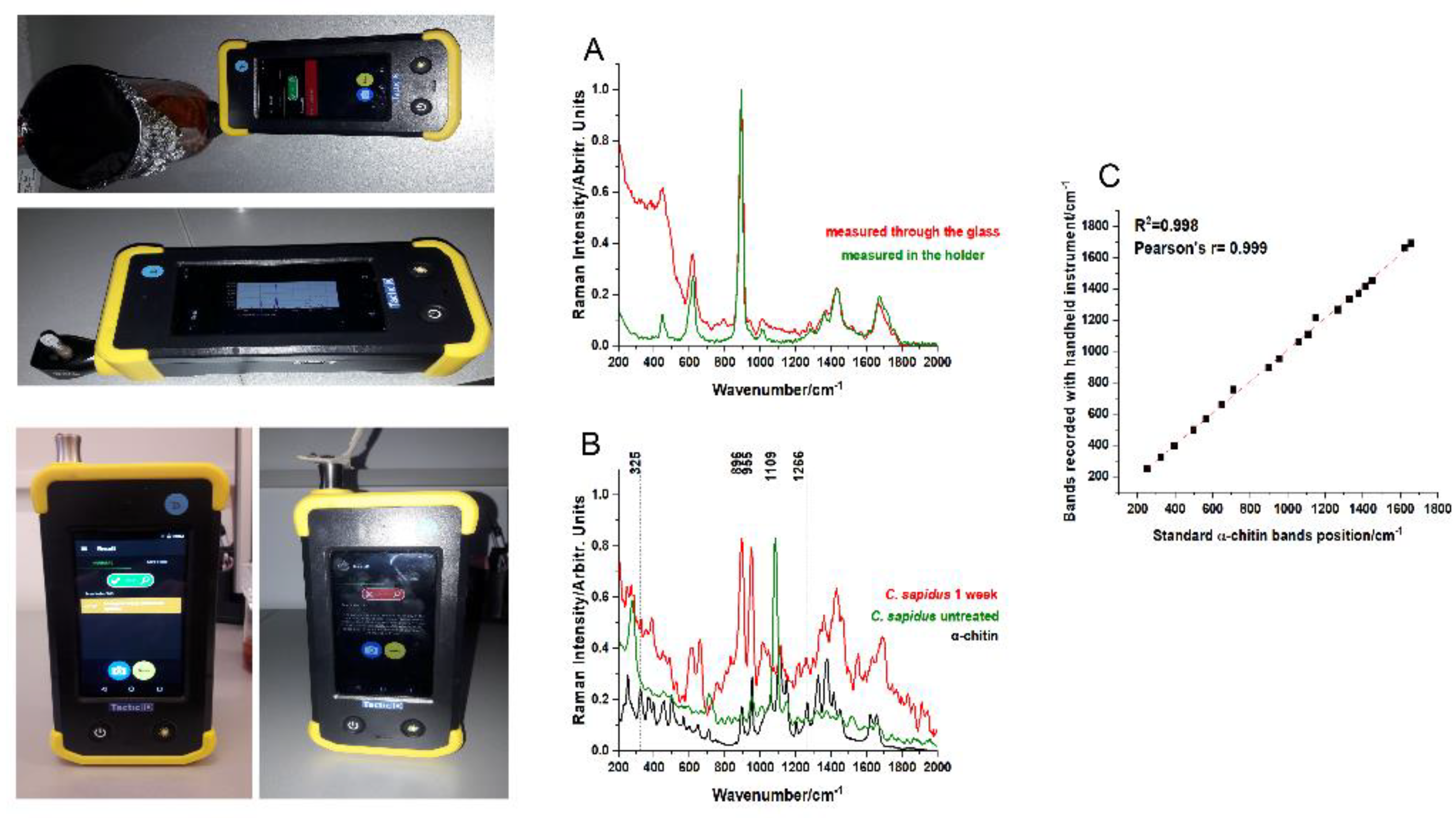
Figure 8.
Using a hand-held TacticID Raman handheld instrument to control the demineralization process: images of the instrument at work and the displayed Raman spectra of the acetic acid bath solution (A); measured through the demineralization glass container (upper image, red line in the graph) and through its holder accessory for liquids (lower image, green line in the graph). (B) Setup of measurement of C. sapidus fragments: untreated (left image, green line in the graph) showing the strong calcium carbonate signal of raw shells and their disappearance after acetic acid treatment (right image, red line in the graph). The chitin reference signal is shown for comparison. Excitation: 1064 nm. (C) Linear correlation (R2= 0.998, Pearson’s r=0.998) of the chitin bands recorded with TacticID Raman handheld instrument from the final foil product, with the standard α-chitin bands recorded with a lab-based instrument.
Figure 8.
Using a hand-held TacticID Raman handheld instrument to control the demineralization process: images of the instrument at work and the displayed Raman spectra of the acetic acid bath solution (A); measured through the demineralization glass container (upper image, red line in the graph) and through its holder accessory for liquids (lower image, green line in the graph). (B) Setup of measurement of C. sapidus fragments: untreated (left image, green line in the graph) showing the strong calcium carbonate signal of raw shells and their disappearance after acetic acid treatment (right image, red line in the graph). The chitin reference signal is shown for comparison. Excitation: 1064 nm. (C) Linear correlation (R2= 0.998, Pearson’s r=0.998) of the chitin bands recorded with TacticID Raman handheld instrument from the final foil product, with the standard α-chitin bands recorded with a lab-based instrument.
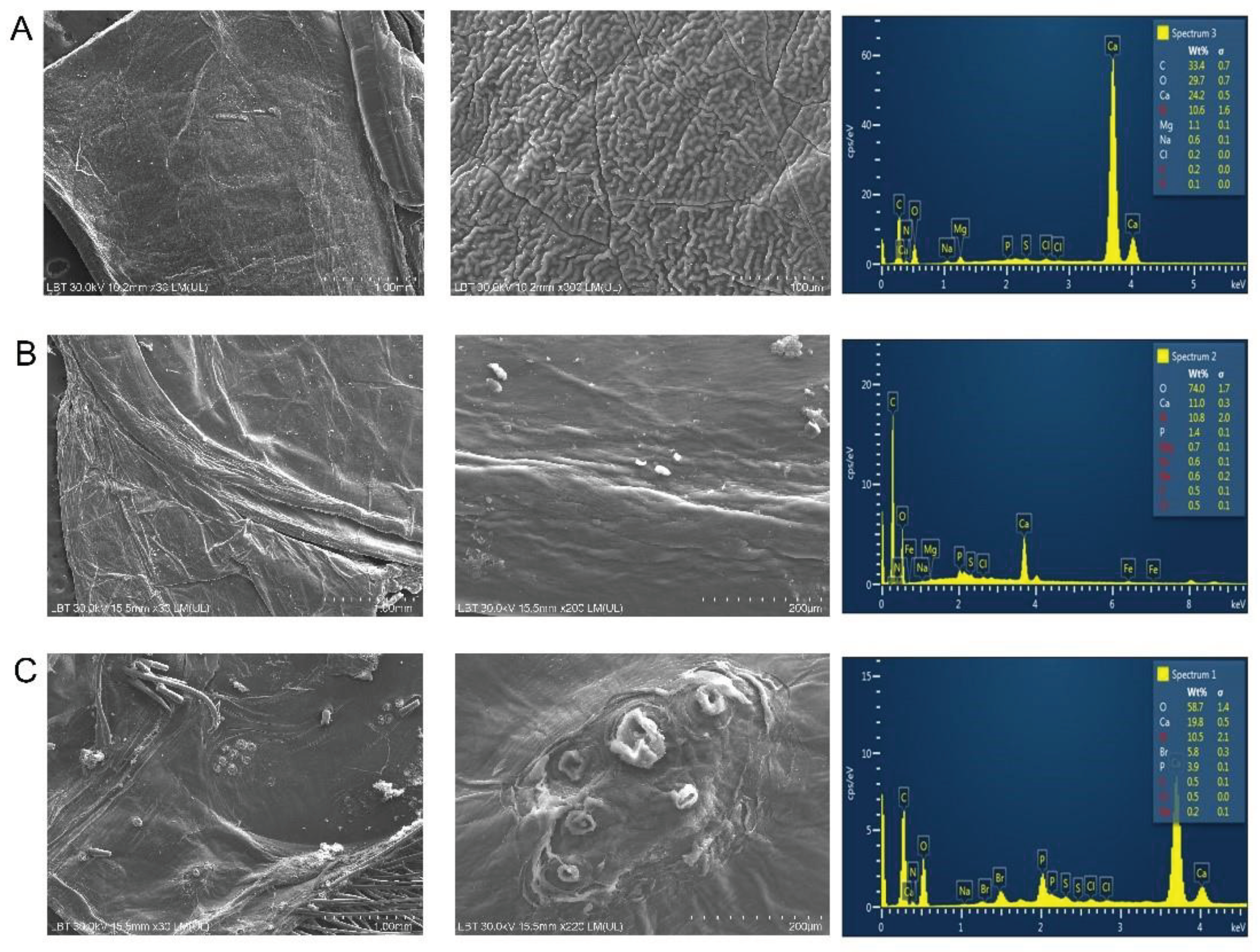
Figure 9.
SEM images of the surface morphology of the acetic acid bath treated cuticle of three species with corresponding EDX graphs; C. sapidus (A), S. mantis (B) and M. squinado (C).
Figure 9.
SEM images of the surface morphology of the acetic acid bath treated cuticle of three species with corresponding EDX graphs; C. sapidus (A), S. mantis (B) and M. squinado (C).
Table 1.
Summarized Raman bands observed in spectra of the demineralized, chitin-based foil products derived from the three crustacean species, compared to the pure α-chitin.
Table 1.
Summarized Raman bands observed in spectra of the demineralized, chitin-based foil products derived from the three crustacean species, compared to the pure α-chitin.
| Chitin-based foil from C. sapidus |
Waste shell of C. sapidus |
Waste fragment of M. squinado |
Waste shell
S. mantis (abdomen cuticle) |
α-chitin
Raman bands/cm-1 |
Hand-held
TacticID
Raman
Instrument,
1064 nm |
Renishaw InVia Reflex Raman system, 785 nm |
Renishaw |
Renishaw |
|
| 250,9 |
253 |
|
254 |
253 |
| |
|
|
|
269 |
| |
|
|
|
273 |
| 325 |
325 |
|
325 |
325 |
| |
|
|
|
366 |
| |
|
|
369 |
369 |
| |
373 |
|
|
373 |
| 395 |
395 |
|
|
397 |
| |
|
|
|
429 |
| 457 |
456 |
|
457 |
458 |
| |
|
|
|
481 |
| 499 |
501 |
|
501 |
499 |
| |
527 |
|
|
530 |
| |
|
|
|
533 |
| 567 |
565 |
|
|
566 |
| |
599 |
|
|
599 |
| 658 |
650 |
|
|
649 |
| 755 |
709 |
|
|
710 |
| 899 |
894 |
898 |
894 |
899 |
| 955 |
953 |
955 |
952 |
955 |
| |
|
|
|
1043 |
| 1059 |
1059 |
|
|
1059 |
| 1109 |
1109 |
|
1108 |
1109 |
| |
1146 |
|
1147 |
1149 |
| 1266 |
1263 |
1268 |
1265 |
1266 |
| 1337 |
1328 |
|
1330 |
1328 |
| 1373 |
1372 |
|
1374 |
1378 |
| 1416 |
1414 |
|
1415 |
1415 |
| 1451 |
1448 |
1451 |
|
1451 |
| 1629 |
1620 |
|
1621 |
1622 |
| 1663 |
1657 |
|
1658 |
1657 |
| Out of the instrument range |
2880 |
|
2882 |
2881 |
| 2913 |
|
|
2909 |
| 2937 |
2937 |
2937 |
2936 |
| 2958 |
|
|
2963 |