1. Introduction
Hemorrhagic stroke (HS) is a life-threatening condition which accounts for 10–40% of all strokes across the Middle East [
1]. Although its overall incidence is lower than that of acute ischemic stroke, HS usually exhibits greater baseline severity and poorer outcomes [
2]. Research on intracranial hemorrhage (ICH) leads to controversial findings regarding the enhanced therapeutic response in the early phase of HS [
2,
3]. The Coronavirus disease (COVID-2019) pandemic has added to this burden. However, COVID-2019-associated HS does not differ from other forms in age distribution, sex ratio, strength of vascular risk factors, severity or outcome [
4].
Researchers have not yet built reliable predictive models for HS outcomes because HS types differ regarding anatomic localization, incidence, etiology, signs and symptoms, prognosis, and outcome [
5]. Risk factors are not well-established, and interventions to reduce risk are to be determined [
6]. Age, sex and ethnicity may serve as risk factors [
7,
8,
9,
10,
11,
12,
13]. The modifiable risk factors for ICH include excessive body weight and arterial hypertension [
14,
15]. In contrast, the non-modifiable risk factors are male sex, older age, and Asian ethnicity [
16,
17]. Some studies justify the environmental risks that increase predisposition to intracranial bleeding. For instance, the ICH incidence increases with a rise in atmospheric pressure (AP) the previous day, whereas a fall in AP does not affect the overall stroke incidence [
18]. A research found a modest correlation between AP and the number of subarachnoid hemorrhage (SAH) per day, and a stronger correlation between daily change in AP and SAH occurrence [
19]. A multicenter worldwide study reported an increased risk of intraparenchymal hemorrhage (IPH) within a few hours of exposure to a very low ambient temperature (AT) [
20]. To advance the forecast the disease course, various risk factors should be analyzed in combination with medical findings using multimodal machine learning algorithms [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
Different studies have not yet been translated into stroke reduction strategies, partly due to inconsistent conclusions which stem from a lack of precise stroke classification into ischemic vs. hemorrhagic cases. Also, the inherent complexity of weather and the need to examine the combined effects of multiple weather and clinical factors have hampered the development of robust predictive algorithms and guidelines. Further, it is unclear if absolute values or relative changes in weather metrics over time can offer better prediction. The development of the disease model serves the idea of personalized treatment when individual parameters are considered together with acute and delayed environmental effects. Few studies have considered individual variables and large air volumes. One of them reported an increased risk of intracerebral bleeding when dry polar air masses appeared, and a reduced HS risk 5-days later. This suggests that the delayed effects on stroke incidence are dependent on the cumulative effects of air masses and temperature changes [
31]. Novel studies should overcome recent research limitations by texting the entire range of relevant individual risks and weather elements.
2. Objectives
The aim is to stratify the risks of HS in the United Arab Emirates (UAE). The hot dry climate of the region imposes a substantial burden on society. The central hypothesis of the study is that atmospheric conditions in the desert climate are reliable predictors of HS risk, and it is essential to identify the strongest one. The primary objective is to elucidate the associations of weather parameters and clinicodemographic variables with HS incidence. We also aim to look at how atmospheric conditions interact with clinical risk factors to influence HS severity (second objective) and early outcomes (third objective).
3. Materials and Methods
3.1. Dataset Description
We collected a dataset of de-identified HS cases from the Al Ain Hospital (Al Ain, Abu Dhabi, UAE) information system. The dataset was labeled PRAS after the project “Prognostication of Recovery from Acute Stroke.” The weather parameters for Al Ain city were obtained from the National Oceanic and Atmospheric Administration website. We also collected and analyzed the following clinicodemographic predictors of stroke incidence and clinical outcomes: age (DEMOGRAPHY_age), sex (DEMOGRAPHY_sex), ethnicity (DEMOGRAPHY_ethnicity), body mass index (BMI), history of stroke (History_OldStroke), history of smoking (History_Smoking), current diabetes mellitus (History_DM), arterial hypertension (History_HyperTension), ischemic heart disease (History_IschemicHeartDisease), arterial hypertension (History_ArterFibrillation), and hyperlipidemia (History_HyperLipidaemia), year of HS onset (year), day of onset (ONSET_Date), time of day at onset (ONSET_LKW_time), National Institutes of Health Stroke Scale (NIHSS) score at hospital admission (Screening_tools_NIHSS), final diagnosis (Diagnosis_Final), and e.g., in-hospital mortality, modified Rankin Score (mRS) at discharge (Discharge_Plan_Modified_Rankin_Score). We added the following derivative qualitative variables to categorize the cases for analysis: age group (DEMOGRAPHY_agerange), with categories of 18–44, 45–59, 60–74, 75–89, and >90 years, and HS time of day at onset group (Day_Time), with the categories of morning (06:00–12:00), afternoon (12:00–18:00), evening (18:00–24:00), and night (00:00–06:00).
Atmospheric features. The following data were collected from the Al Ain meteorological station: daily AT, relative humidity (RH), wind speed (WS), and AP. The number of days between stroke onset and a given weather event was expressed by a number after the acronym (e.g., WS7 is the wind speed 7-days before the hemorrhage onset). We also calculated the humidity index (humidex) from AT and RH. The daily change and associated lag (up to 7-days before HS onset) were calculated for air temperature (TDIF), pressure (PDIF), wind speed (WDIF), relative humidity (RHDIF), and humidex (HDIF).
3.2. Study Design and Patients
The primary outcome was the number of daily emergency hospital admissions for HS. We retrospectively analyzed the records of all patients (n=160) with nontraumatic intracranial bleeding who were admitted to the Stroke Unit of Al Ain Hospital from January 1, 2016 to December 31, 2019 (see
Table 1). In accordance with the national healthcare standards, all the patients were examined by a neurologist and underwent a brain computerized tomography (CT) scan, a complete etiologic review, and other essential tests to fulfill the HS diagnostic criteria. The inclusion criterion was CT conformation of nontraumatic SAH [I60 in International Classification of Diseases (ICD-10)], nontraumatic intracerebral hemorrhage (I61 in ICD-10; most cases of 431 in ICD-9), or unspecified nontraumatic ICH (I62.9 in ICD-10; 432.9 in ICD-9). The study was reviewed by the Al Ain Hospital Research Ethics Governance Committee (reference number AAHEC-12-19-033) and approved for the retrospective analysis of the data obtained as the standard of care; the procedures followed were in accordance with institutional guidelines.
4. Calculation
To address the associations of desert weather parameters and clinicodemographic variables with HS incidence (first objective), we conducted a comparative analysis of groups divided by age (
Table 2), sex, and ethnicity (
Table 3). As the variables had non-normal distributions, we utilized nonparametric tests for the analysis. Differences in continuous variables between sexes and ethnic groups were assessed either by Mann–Whitney U test or Kruskal–Wallis test. Differences between categorical variables were assessed using the Fisher’s exact test or the Chi-square test. Associations between ICH incidence and weather parameters (
Table 3), ethnicity, and sex (
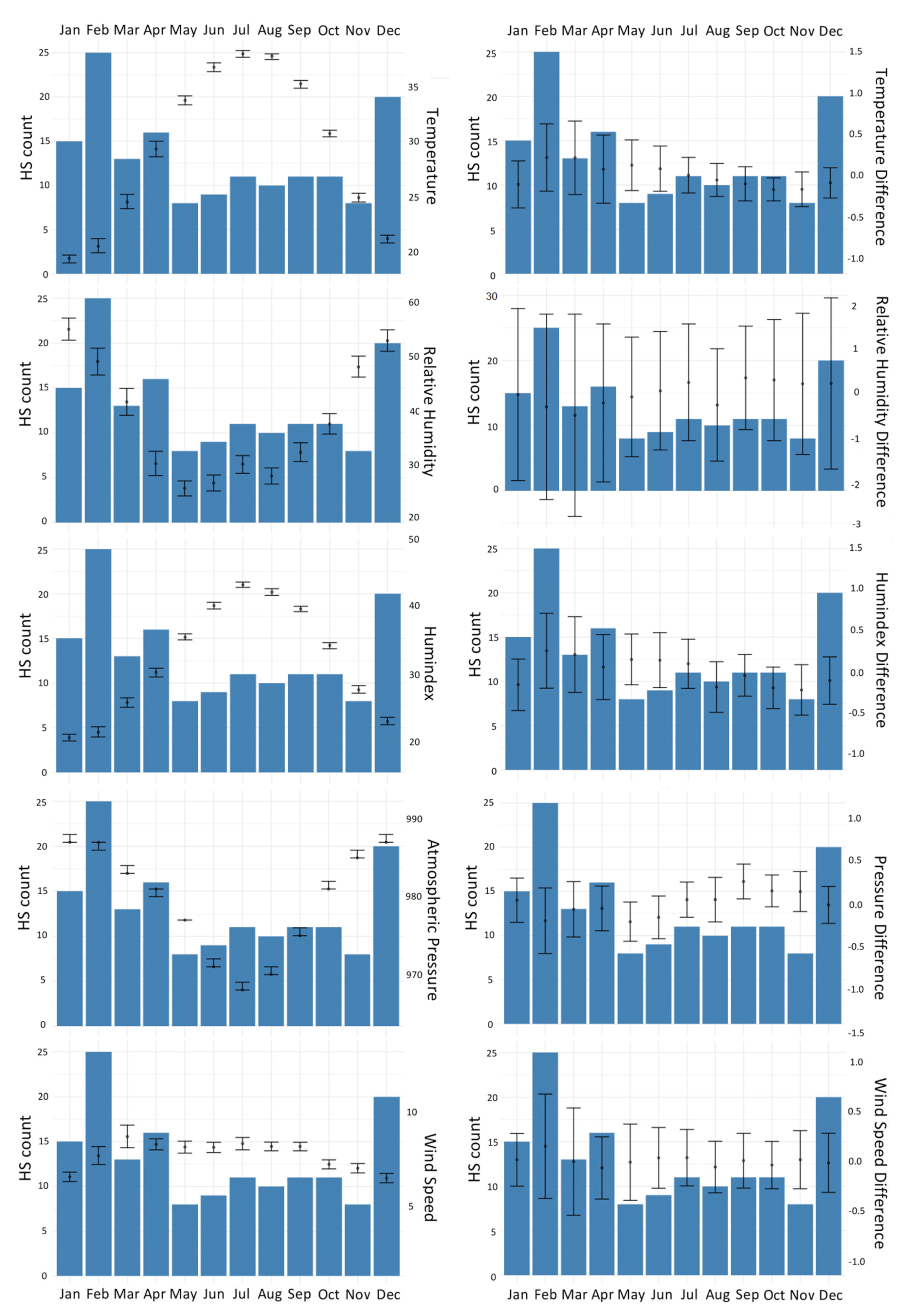
Table 4) were described with the point biserial correlation coefficients between the daily changes in meteorological factors and HS incidence. We also constructed barplots (
Figure 1) representing regional circannual weather changes and HS morbidity rates. We studied the immediate and delayed effects of weather on HS incidence with distributed lag nonlinear model analysis (
Figure 1).
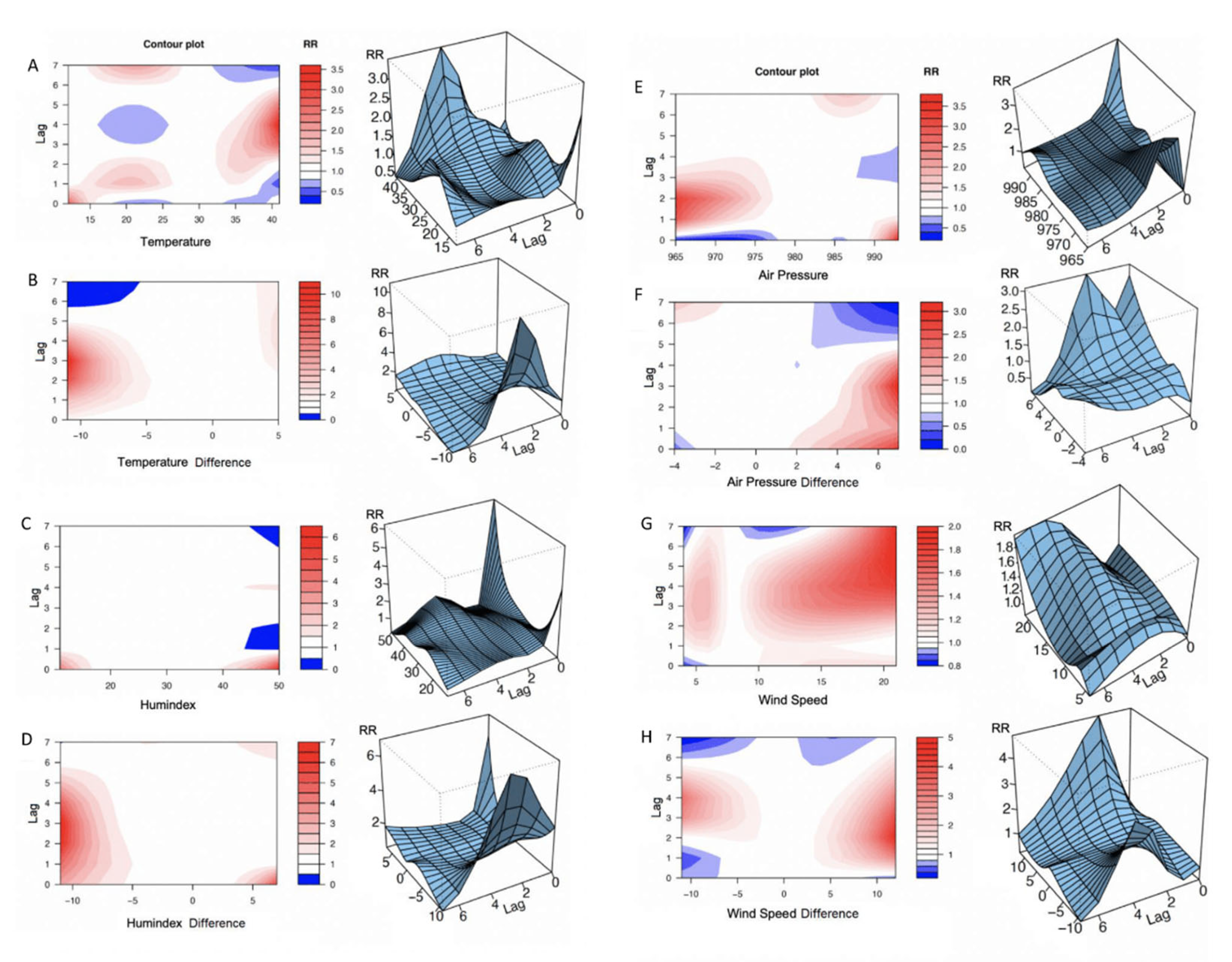
Figure 1A-H present the models of the relative risk (RR). The reference values used to calculate RR were the point of overall minimum HS incidence [
32].
To address the second objective, we used both descriptive statistics and machine learning (ML) approaches. Stroke severity was stratified into 38 cases with NIHSS scores of ≤ 4 and 47 cases with NIHSS scores of >4. Cases with missing NIHSS scores were excluded from the analysis. For both classes, we built barplots for the weather parameters and clinicodemographic factors to check the separability of data between cohorts. We also analyzed the variance of HS onset time according to severity. Then we constructed a model for predicting NIHSS score at admission from the set of clinicodemographic and weather-related parameters. To prepare the models, we used the following pipeline. First, we ranked the relative importance of clinicodemographic risk factors according to their impurity-based predictive potential. For ranking, we utilized a set of tree-based classifiers and then averaged all the received scores. We performed single imputation procedures by replacing missing continuous parameters (predictors) with mean values and qualitative features – with median values. To standardize features, we used a standard scalar.
Next, we analyzed the informative value of both clinicodemographic predictors and weather parameters to forecast HS severity. We selected top informative predictors and built the ML models forecasting NIHSS score class to compare the informative values of clinicodemographic risk factors taken separately and in combination with weather parameters. A stratified tenfold cross-validation technique was used to train several ML classification models to evaluate the classifier output quality. We used 90% of the data for each fold to train the model and the other 10% for testing. The decision matrices built on the test dataset for all iterations were combined and used to calculate the performance metrics. We assessed model performance by calculating the area under the receiver operating characteristic curve (AUC), precision-recall metrics, F1-scores, specificity, and sensitivity.
To address the third objective, we divided all the cases into two classes according to the early outcomes, 36 with mild or moderate disability (mRS ≤ 3) and 41 cases with severe disability (mRS > 3). This reduced the study sample size because cases without mRS values were excluded. The data processing algorithm was similar to that used for the second objective. We first prepared barplots to assess the separability in clinicodemographic risk factor values between the groups. Then, we analyzed the variance of HS onset time according to the early outcomes.
Using a similar pipeline, we constructed models for predicting mRS score at discharge from clinicodemographic risk factors and weather-related parameters. First, we ranked clinicodemographic risk factors according to classification strength. In addition to the predictors used in the second objective, we included NIHSS score at admission. Then, we analyzed the informative value of the clinicodemographic predictors and weather parameters for forecasting HS severity. We built classifying ML models to predict the early stroke outcome from the clinicodemographic risk factors and in combination with weather parameters. Additionally, we looked for possible options to reduce the amount of input data without reducing model performance. Moreover, parsing input may reduce “noise” from useless variables, thereby improving model outcome metrics. For this purpose, we included each significant variable one by one as a single predictor in a binary classification model and selected those with the highest AUC (i.e., the most informative variables). For evaluating classifier performance, we used F1-scores (the harmonic mean of precision and recall, as both measures are of equal importance). Finally, we compared prediction accuracy from all significant features and the most informative ones.
5. Results
5.1. Effects of Weather Parameters on Hemorrhagic Stroke Incidence by Age, Sex, and Ethnicity
Table 1 and
Table 2 summarize the incidence of ICH in Al Ain as estimated by the stroke unit admissions. The mean number of HS cases per annum was 43 (7.60 per 100,000 people), with a twice higher incidence among males (30.5, 9.67 per 100,000) than females (12.5, 5.00 per 100,000). The incidence of HS doubled after 45 years of age and tripled in individuals aged 75 and above.
Table 3 compares the clinicodemographic features of the study cohort by sex and ethnicity.
Table 4 illustrates the basic distribution of meteorological factors and correlations with HS incidence. Daily changes in the mean values of temperature, AP, RH, and humidex were significantly correlated with HS incidence (all p < 0.05). Furthermore, HS incidence had a positive associations with AP (r = 0.089) and RH (r = 0.075), and a negative associations with air temperature (r = -0.112) and humidex (r = -0.108). In contrast, neither daily change in average wind speed (WS) nor the average daily change in any of these four meteorological factors was associated with HS incidence. The barplots in
Figure 4 further support the results from
Table 4 by demonstrating the correlations of daily changes in meteorological factors with HS incidence. Again, average daily changes in temperature and humidex were positively correlated with HS incidence. In contrast, AP and RH were negatively correlated with HS incidence. Furthermore, neither WS nor average daily change in any metrological factor was significantly associated with HS incidence.
We also examined if the associations between weather parameters and HS susceptibility differed according to ethnicity and sex.
Table 5 shows the variation in mean annual weather estimates throughout the studied period and the correlation coefficients (with p-values) for HS incidence versus weather parameters stratified by age and ethnicity. The associations of monthly HS rate with corresponding meteorological data were stronger in ethnic Arabs than Asians and were more pronounced in females than males.
The contour plots in
Figure 1 illustrate the immediate and delayed effects of AT, perceived temperature (humidex) and the daily changes in these values on HS risk. There was an increase in risk at higher temperatures with a 2- to 6-day lag, with the most substantial effect on the fourth day after the weather event (
Figure 1a-b). Moderately elevated risk was also found on days when the temperature dropped below 20°C. Ambient temperature change to lower values increased the risk of ICH, the tendency was more pronounced from the second to the fourth day after the weather event. After 1-week, HS risk returned to baseline for all weather events. A rise in humidex above 40°C and a drop below 15°C increased the relative stroke risk on the day of the change (
Figure 1c-d). High humidex values also reduced relative risk on the second and the seventh days after the weather event. A decrease in humidex by >5°C resulted in a higher risk of ICH in the period between the first and the fifth day. Similarly, an increased risk was associated with humidex increases of >5°C.
High AP and greater daily changes were associated with higher HS risk. However, the elevation of AP above 990 mbar resulted in a one-day increase in relative risk and a subsequent decrease on the fourth day after the weather event (
Figure 1e). AP changes above 6 mbar resulted in a prolonged increase in stroke incidence up to the fourth day after the weather event but a reduced incidence on the sixth day after the weather event (
Figure 1f). A WS increase demonstrated a longer-lasting effect on HS risk, predicting high incidence from the second day to the end of the seventh day after the weather event (
Figure 1g-1h).
5.2. Effects of Weather Parameters on Hemorrhagic Stroke Severity at Presentation
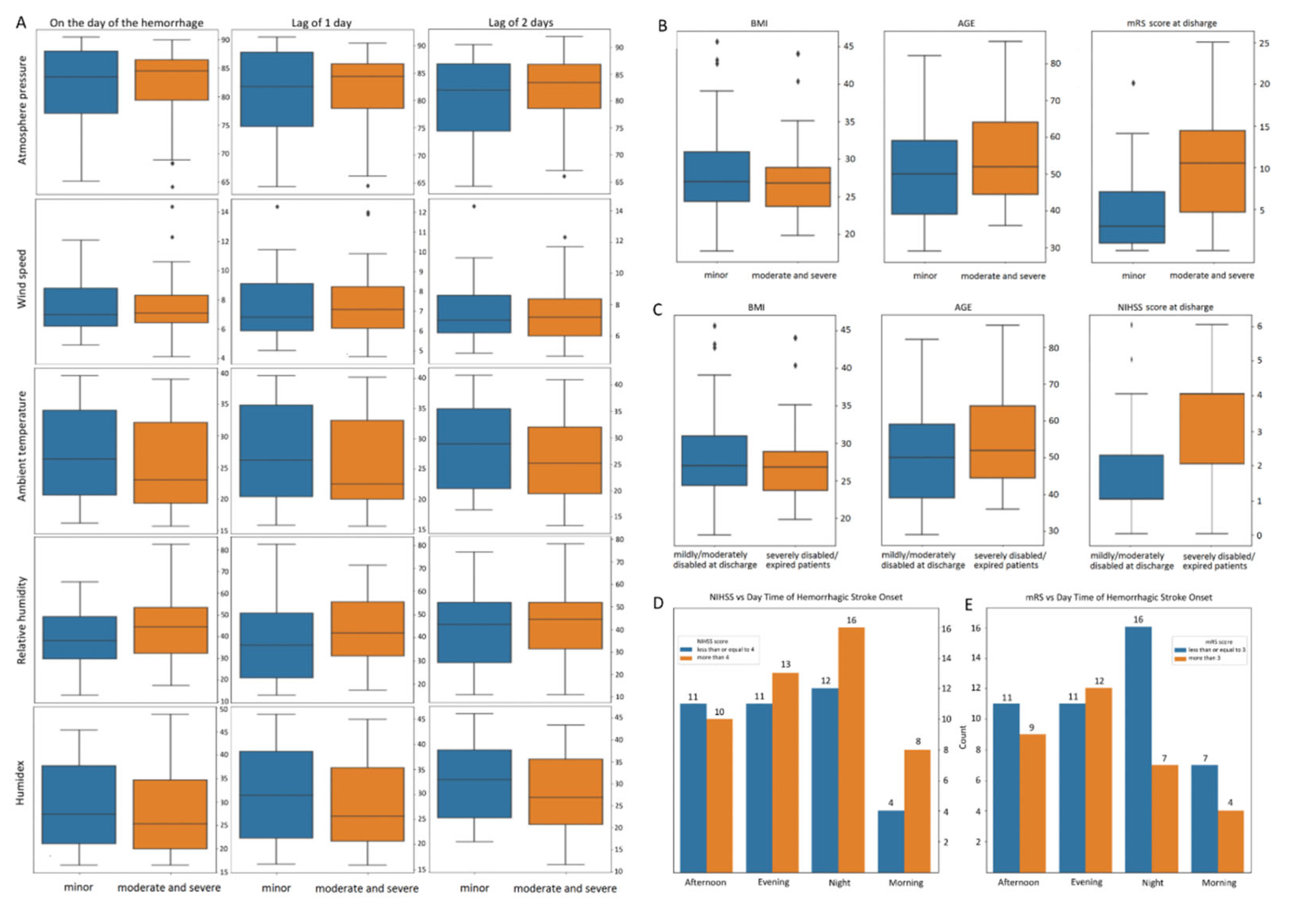
The weather parameters (
Figure 2a) and individual clinicodemographic features (
Figure 2b-c) barplots illustrate specific separability of cohorts according to NIHSS scores (>4 or
≤4). The comparison of these NIHSS score groups revealed that the strokes occurring in the morning or at night were more severe at presentation than HS occurring at any other time of the day (
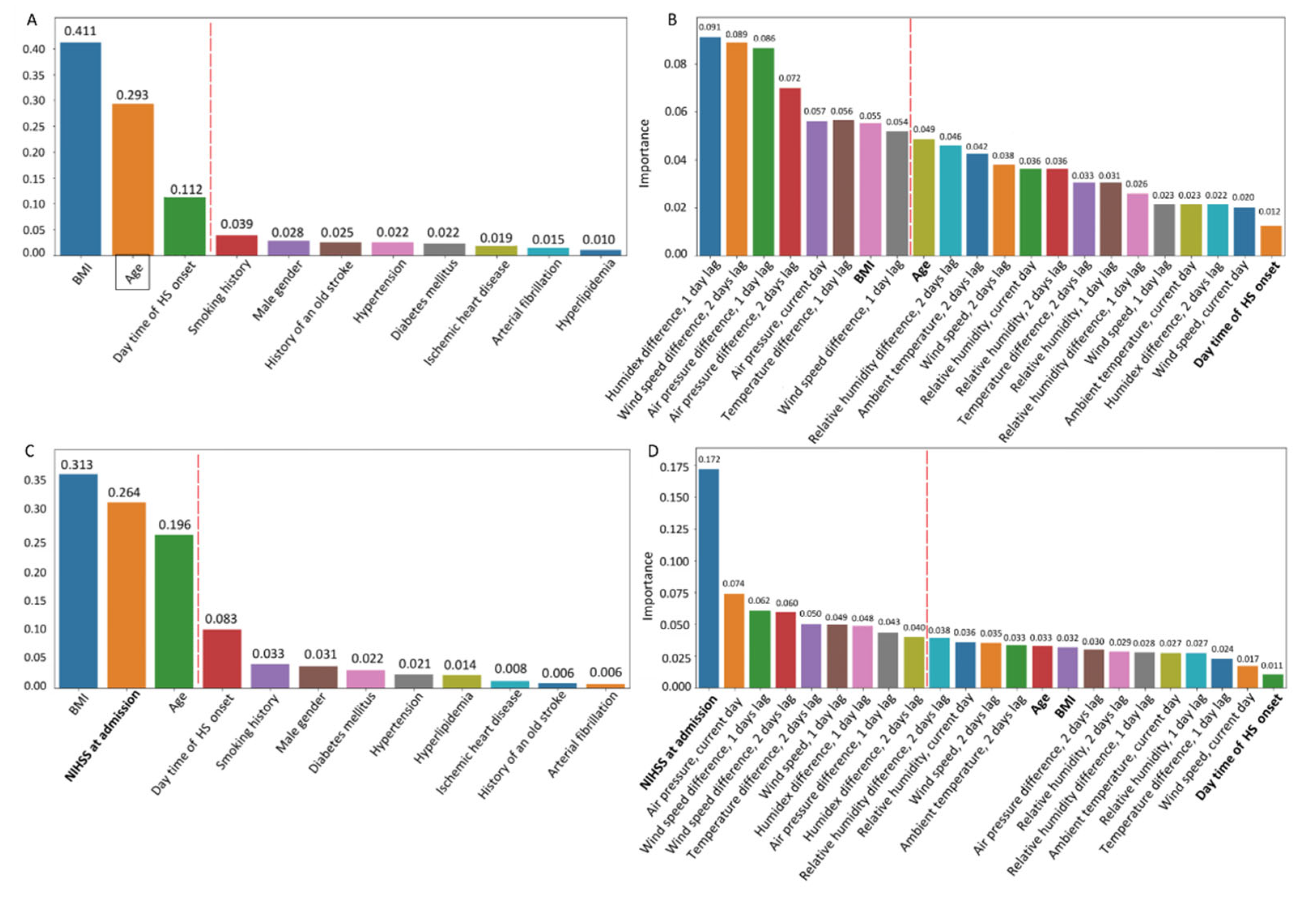
Figure 2d-e). Feature selection was performed to build a predictive model for NIHSS score at admission. The importance of risk factors was ranked according to their impurity-based predictive potential (
Figure 3). In
Figure 3a,b, the red dashed line separates the high-value parameters from the nonsignificant ones.
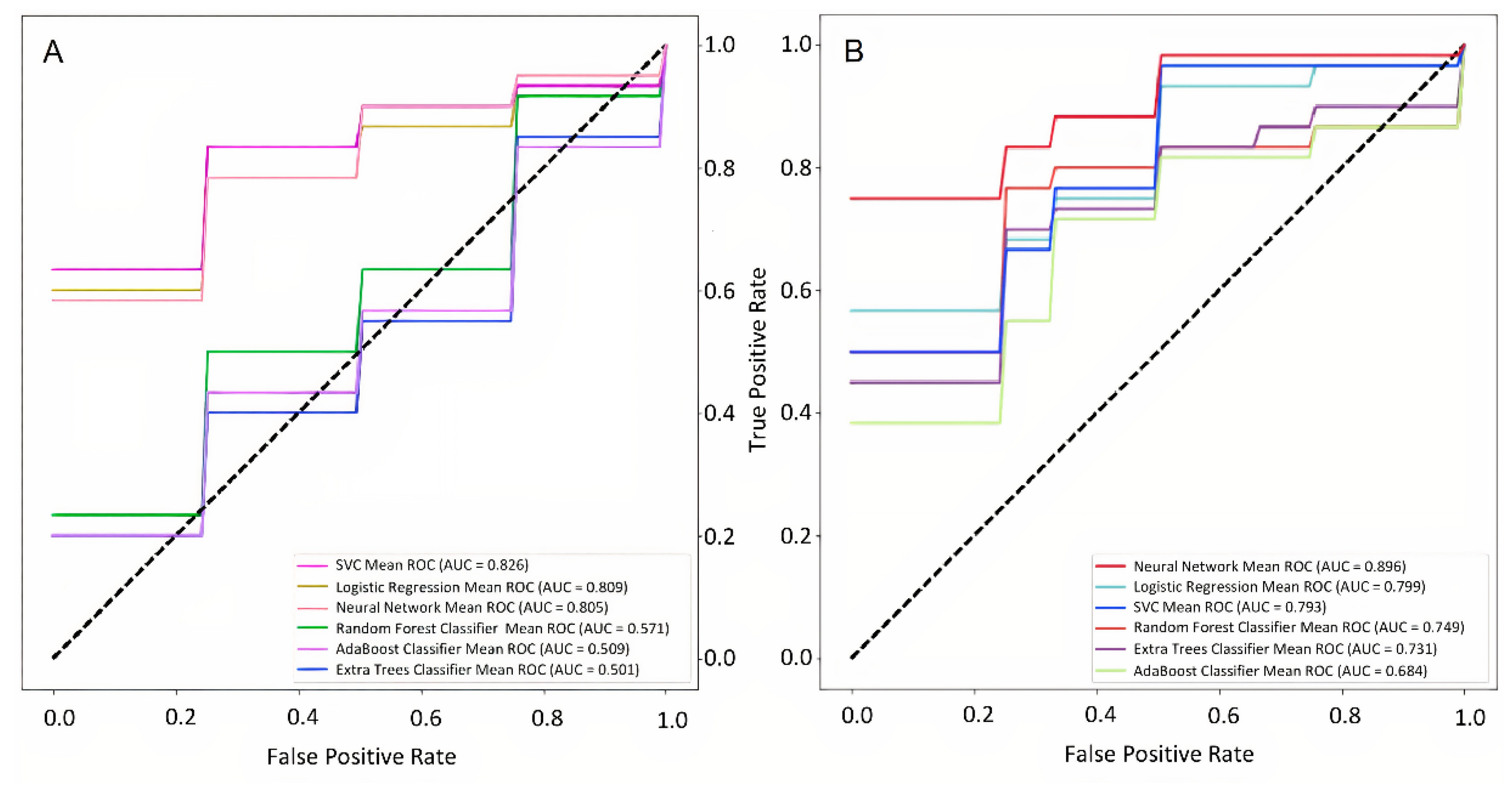
Figure 5a illustrates the performance of the model which includes the most informative clinicodemographic risk factors;
Figure 5b - all significant clinicodemographic and environmental features.
Table 6 lists the performance metrics for each model. Adding weather parameters increased NIHSS prediction model sensitivity from 75% to 87.5% and specificity from 62% to 89%.
5.3. Early Outcomes of Hemorrhagic Stroke
We also divided the study cohort into two groups according to disability level at discharge as assessed by mRS. The barplot of
Figure 2c shows the BMI, age, and NIHSS score distribution between mRS groups (
≤ 3 or > 3). We analyzed the relationship between the variance of HS onset and early outcomes (
Figure 2e).
Figure 3c presents the ranked clinicodemographic risk factors according to impurity-based predictive potential, and
Figure 3d ranks the complete list of clinicodemographic and weather-related predictors. The red dashed line demarcates the significant factors selected for modeling. We found some separability in BMI and age between patients with different levels of disability at discharge according to mRS (
Figure 2c). The predictive values of all three clinical variables were roughly equal, although BMI was the most informative (
Figure 3c). The separability between groups was noticeably more evident for NIHSS score at admission, contradicting the feature selection results. However, the NIHSS score at admission was the top feature in the complete list of clinicodemographic and weather-related predictors ranked by informative value, while BMI and age were unimportant features (
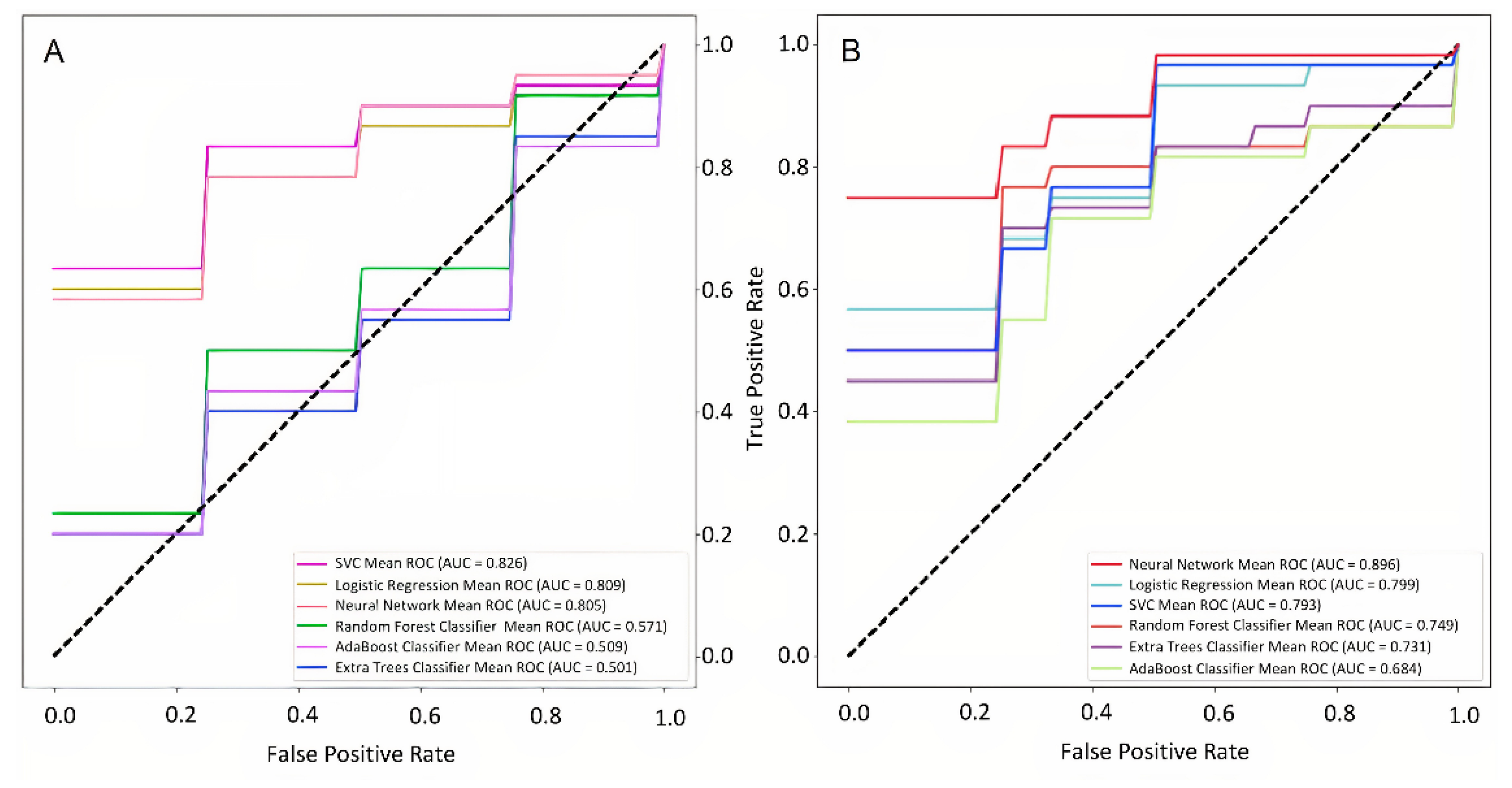
Figure 3d). The performances of these ML models for predicting the early stroke outcome from the clinicodemographic risk factors alone and in combination with weather parameters are shown in
Figure 6a,b.
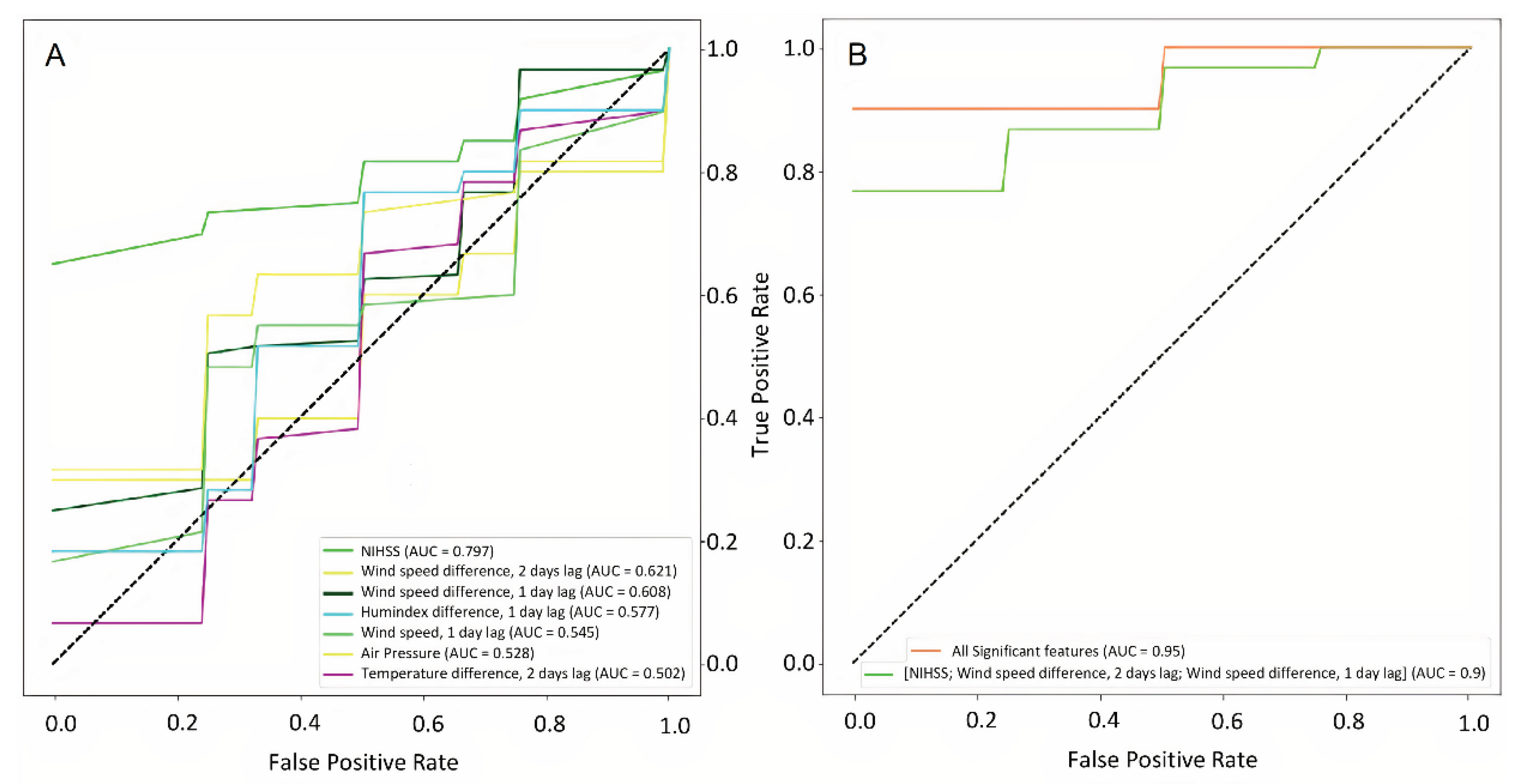
Figure 7a presents the binary classification accuracy of models trained on each predictor separately.
Figure 7b illustrates the characteristics of the models based on the most valuable features in comparison to all significant features.
6. Discussion
6.1. Atmospheric and Individual Risk Factors of HS Incidence
6.1.1. Association of HS Incidence with Weather
The association between meteorological change and HS risk is still debated. In contrast to the previous studies, we examined daily changes and the mean values of various weather parameters, e.g., temperature, AP, RH, WS, and humidex. Our study followed the recommendation of researchers to consider AT and humidity in combination [
33,
34]. Furthermore, we conducted advanced distributed lag nonlinear model analyses to reveal less obvious temporal associations with clinical risk factors and outcome measures, such as delayed effects of weather on stroke risk.
Association of HS incidence with ambient temperature. Our study showed associations between HS incidence and average mean changes in temperature, AP, RH, and humidex. Generally, the lower mean monthly temperature was linked with a higher HS risk: case counts were higher in colder months. Similar findings were reported by other authors [
35,
36]. Exposure to cold temperatures can disrupt normal homeostatic mechanisms and increase heart rate, blood pressure, platelet count, blood viscosity, serum low-density lipoprotein-cholesterol concentration, and other cardiac biomarkers, thereby elevating cardiovascular event risk [
37,
38]. We showed the adverse effect of low temperatures on ICH incidence, which is consistent with the findings of several previous studies [
18,
20,
31,
39,
40,
41,
42,
43]. However, lower or unchanged HS risk was also reported [
44]. A possible reason for the inconsistent findings is that the impact of AT on ICH incidence could differ among age groups [
45]. The AT-to-ICH association is stronger after 60 years of age compared to 20-59 years [
46]. Good compliance with antihypertensive treatment can reduce the risk of ICH associated with the change in daily mean or maximal AT [
47].
Temperature variance. A 1°C day-to-day variation of AT increased ICH risk by 7.5% [
48]. The increased ICH incidence is associated with a daily AT decline, especially in patients older than 65 [
49]. The SAH risk is associated with a decrease in AT which either occurred on the day of onset or a day before [
50]. In particular, the incidence of aneurysmal SAH correlates with lower intraday AT difference [
51] and a decrease in AT below 16°C [
52]. Our distributed lag nonlinear model analysis identified differences in lag effects between cold and hot temperature exposure. Similar to Polcaro-Pichet et al. [
53], the effect of low temperatures (below 20°C) was noted immediately, with little effect a week after the exposure. In contrast, the effect of hot temperatures was delayed, with the highest relative risk (>3) between two and four days after the weather event. Although our observations on extreme cold weather were limited due to the temperature range in Al Ain, these findings indicate that extreme heat also increases HS risk (
Figure 1a). Daily temperature change was not significantly associated with HS risk. However, a greater relative risk (>10) was found for a substantial temperature difference of -10°C three days after the weather event. The incidence rate ratio increased in parallel with temperature change.
Association of HS incidence with atmospheric pressure. Changes in AP affect the homeostatic regulatory mechanisms of the human body, including blood pressure. Higher systolic and diastolic blood pressure and more frequent hypertension complications are common during winter, especially among the elderly [
54]. Also, AP depends on the temperature, and colder seasons are usually accompanied by higher and more variable AP values than warmer seasons [
55]. Moreover, extensive AP fluctuations and vasoconstriction caused by lower mean temperature may contribute to higher ICH incidence in winter [
56]. This was confirmed as we found a positive correlation between monthly change in AP and HS frequency, which is consistent with previous publications [
18,
56,
57,
58]. An increased incidence of SAH correlates with the daily minimum and maximum AP [
59] and with the mean daily variation of AP over 3.9 hPa [
60]. A rise in the daily mean AP by 1 hPa heightens the ICH risk by 2.4% [
48], and an increase in AP by 11.5 hPa raises the SAH risk by 15% [
61]. The maximum daily AP is associated with the SAH onset [
52]. Contrarily, other authors reported a close association of the AP decline with the elevated SAH risk (p=0.021) [
62]. The references listed above reveal inconsistent findings by different authors. The discrepancy may attribute to the fluctuating effect of AP and its changes on the ICH incidence. Our research supports this through the distributed lag nonlinear model. However, our results were contradicting to those of Mukai et al. who reported a strong association of HS count with daily AP changes [
57]. In comparison, the association found in our research was weak (p=0.59). This discrepancy may be related to the unique desert climate of Al Ain and the smaller AP variation.
Association of HS incidence with humidity. Previous studies on the association between RH and HS yielded inconsistent results [
58,
63]. Some of them demonstrated no direct relationship between them [
18,
50,
61,
64,
65,
66]. We found a weak positive correlation with the mean monthly change in RH. We attempted to overcome the shortcomings of the previous studies by examining the effects of humidex which is strongly associated with the level of discomfort [
67]. We found that high humidex values were associated with greater HS risk. Exposure to a very high humidex (49.95°C) immediately increased the relative risk sixfold (
Figure 1b). High temperature combined with high humidity may slow or stop sweat evaporation, increasing body temperature and inducing heat-related illness. According to Morris and Patel, extreme heat may also disrupt the function of heat-shock proteins which are responsible for repairing temperature-related damage to the body [
68]. This leads to electrolyte abnormalities (hypernatremia) and more severe conditions (hemorrhage, brain edema, and permanent brain damage) [
68]. In a study by Chen et al., the authors resorted to another temperature-humidity index. They found a high ICH risk on days with a low and moderate index, and a risk reduction when the index reaches 30.88°C. In the humidex range from 24 to 27 °C, lower humidity could reverse the protective effect of temperature into a harmful effect [
69]. The general consensus is that the AT effect on ICH should be interpreted in conjunction with humidity.
Association of HS incidence with wind speed. The impact of WS on the ICH occurrence is questionable. From our results, HS incidence is not significantly associated with WS (
Figure 1d). A study by Li et al., supports our findings [
58].
Models predicting HS incidence from multiple weather parameters. We showed that the acute and delayed weather effects may differ. This overcomes the limitations of recent studies that produced contradictory results because they did not consider the cumulative effects of multiple weather factors. A research team presented deep-learning-based prediction models trained on 221 meteorological and calendar factors, including daytime of ICH onset. The team reported high accuracy for the models: 0.714-0.988 AUC [
70]. Other researchers did not justify their results [
71].
6.1.2. Effects of Ethnicity and Sex on HS Incidence
Recent studies reported that ethnicity and sex are risk factors for HS [
72,
73,
74,
75], which we also justified.
Ethnicity and HS incidence. In our investigation, HS cases were higher among Arabs than Asians under identical environmental conditions (AT, AP, RH, and humidex). While the effects of genetics were not assessed [
76,
77,
78], we speculate that this disparity partly results from different dietary practices and other cultural factors. For instance, vitamin D deficiency is common among Arabs, and it contributes to stroke development [
79,
80] by increasing the effect of atmospheric conditions on HS risk. Alternatively, the Asian population of the UAE is primarily composed of younger working expatriates who present fewer lifestyle risk factors than the Arabs [81]. However, it was reported that expatriate populations are at higher stroke risk due to socioeconomic challenges, e.g., inaccessibility of medical care or reduced adaptability to climatic conditions (82, 83, 84). Some argue that a genetic predisposition differs among nations (73, 85, 86), but this opinion requires additional support.
Sex and HS incidence. According to our results, HS was more prevalent in males than females under the same environmental conditions. In agreement with our findings, other authors reported that cold-related relative risk for ICH is higher in men [
45]. Male sex is a risk for non-lobar ICH rather than lobar ICH [
15]. From other sources, the male sex also elevates the ICH risk to the same extent as arterial hypertension and previous stroke with mRS 3-5 [
49]. In general, females are at a lower risk of developing stroke (87, 88), potentially due to the cardio- and neuroprotective effects of female sex hormones. However, the studies had common limitations. They did not consider other factors associated with the female sex and HS risk, such as migraine with aura, contraceptives or hormone replacement therapy, genetics, and vitamin D deficiency. These unresolved issues warrant further research.
6.2. Effect of Clinicodemographic Risk Factors and Weather Parameters on HS Severity
Through modeling analysis, we found that age, BMI, and time of day at HS onset were the strongest clinical predictors of HS severity (
Figure 3a). The results revealed the well-established associations between disease severity, its outcomes, and the primary individual risk factors such as age (89, 90), abnormality of body weight (91, 92), and comorbidities.
Age and body weight. Age is well recognized as a strong predictor of in-hospital mortality (89) and 3-month neurologic disability (90) following HS. Increased BMI is also associated with an increased risk of ischemic stroke, but, curiously, high BMI was associated with a lower HS incidence and severity among females (92). Further, researchers concluded that in contrast to age, BMI is not an independent predictor of SAH or IPH outcome (93, 94). This phenomenon was termed the “obesity paradox.” Being underweight or obese can serve as predictors of poor outcomes. It was found that both low and high BMI are associated with deep IPH rather than lobar IPH. This suggests that BMI plays a role in the vascular pathologies underlying deep IPH rather than in cerebral angiopathy [
14]. Underweight patients have almost double the chance of in-hospital mortality and higher rates of such complications as pneumonia, dysphagia and urinary tract infection. At the same time, obese patients have higher rates of in-hospital complications, such as hematoma expansion, deep vein thrombosis and gastrointestinal bleeding (91).
Comorbidities. Researchers investigated clinicodemographic parameters associated with increased incidence of non-lobar and lobar ICH. They highlighted that diabetes predisposes patients to non-lobar ICH, while arterial hypertension elevates the risk of both non-lobar and lobar ICH [
15]. The current study reported data on the incidence of the most common comorbidities among ICH patients (
Table 3,
Figure 3). Future studies are supposed to clarify the input of concomitant disease in the ICH severity.
Weather parameters. From the results obtained, the atmospheric factors that predict HS with highest accuracy were not absolute values of humidex, WS, AP, and temperature but their monthly changes. These results were consistent with previous statistical analysis [
31] and justified the use of a distributed lag nonlinear model in this study. Furthermore, we demonstrated that HS incidence is affected by air masses, i.e. large volumes of air uniform in temperature and moisture ,b,
Table 6).
6.3. Combined Influence of Clinicodemographic Factors and Weather Parameters on Disease Outcome
The models built in the present study accurately classify patients by early outcomes (mild and moderate vs. severe disability). The advantage of this study is a reliable performance of the prediction model based on a relatively small number of individual and environmental risks (AUC 0.95). Previous studies on the same issue either failed to achieve reputable performance (AUC 0.52) [
71], or considered an extensive list of atmospheric factors without revealing personal risks (AUC 0.714-0.988) [
70]. The models without clinicodemographic factors are limited to only providing academic value but are non-applicable to clinical usage. The results of this study justified the critical importance of NIHSS score at admission for predicting HS outcomes. Other researchers reported similar findings when they screened 206 clinical variables to identify 22 essential features from a HS dataset (95). This correlates with the results of our previous study on ML methods to predict ischemic stroke outcomes [
13]. Our findings are at odds with a publication which reported that comorbidities influenced in-hospital mortality and outcome at discharge (89). A possible explanation comes from another study in which antihypertensive treatment was associated with a reduced risk of recurrent IPH (96). In our cohort, comorbidities were effectively detected and managed by drug treatment, that is why they might not have affected HS outcomes.
7. Strength and Limitations
This study identified several atmospheric parameters that influence the incidence rate, severity, and early outcome of ICH. We addressed the limitations of previous studies by analyzing a complete set of atmospheric parameters, including AT, RH, humidex, AP, and WS, as well as their changes at various times preceding HS onset. Our results highlighted the impact of weather changes over several preceding days on HS incidence compared to known clinicodemographic risk factors such as BMI, sex, age, and ethnicity.
The present study also has several limitations. First, we were unable to research the effects of extremely cold temperatures due to the desert climate of Al Ain city. Similar studies should be conducted in regions with highly variable weather conditions to address the relative effects of extreme heat and cold. Second, we did not include the full set of environmental parameters that influence cardiovascular function (e.g., air and water pollution, etc.). A recent study reported an association of IPH occurrence with air pollution levels [
63]. The air quality index should be included in future predictive models of HS.
8. Conclusion
This study identified associations between multiple atmospheric parameters on HS incidence, severity, and early outcome. On average, the risk ratio of HS increases over days following a significant rise or drop in AT, humidex or AP. The risk may remain elevated throughout the adjustment to the environmental change. Humidex was a stronger predictor than AT, indicating that temperature and humidity should be considered together. For accurate predictions, it is crucial to analyze the full range of meteorological data over several preceding days in combination. These associations may provide clues to the pathophysiological mechanisms triggering HS.
In predicting HS severity, the strongest clinicodemographic were BMI, age, and daytime at onset. These factors outperformed atmospheric parameters.
In prognosticating the level of disability at discharge, the top informative factor was NIHSS score at admission.
Atmospheric conditions should be included in stroke prediction applications. The weather-dependent risk stratification models may help stroke units to operate more effectively.
Author Contributions
Conceptualization was done by Y.S., E.F. and J.AM.; writing (original draft preparation) – by E.F., T.T. and Y.S.; study methodology was selected and formulated by J.AK. and Y.S.; data analysis and visualization were performed by V.L.; data curation - by J.AK.; writing (review and editing) - by Y.S.; validation of study results - by F.AZ., M.S. and T.AM., KNVG; problem investigation - by D.S., G.L.S., T.T.; supervision - by Y.S. and J.AK.; project administration - by K.NVG.; funding acquisition - by M.L. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
The study is supported by ASPIRE, the technology program management pillar of Abu Dhabi’s Advanced Technology Research Council (ATRC), via the ASPIRE Precision Medicine Research Institute Abu Dhabi (ASPIREPMRIAD) award grant number VRI-20-10.
Institutional Review Board Statement
The study was reviewed by the Al Ain Hospital Research Ethics Governance Committee (reference number AAHEC-12-19-033) and approved for the retrospective analysis of the data obtained as standard of care; the procedures followed were in accordance with institutional guidelines.
Data Availability Statement
Abbreviations
| AP |
atmospheric pressure; |
| AT |
ambient temperature; |
| AUC |
area under the curve; |
| BMI |
body mass index; |
| CT |
computed tomography; |
| DM |
diabetes mellitus; |
| HI |
humidity index; |
| HS |
hemorrhagic stroke; |
| ICD |
International Classification of Diseases; |
| ICH |
intracranial hemorrhage; |
| IPH |
intraparenchymal hemorrhage; |
| ML |
machine learning; |
| mRS |
modified Ranking Score; |
| NIHSS |
National Institute of Health Stroke Scale; |
| OR |
odds ratio; |
| RH |
relative humidity; |
| RR |
relative risk; |
| SAH |
subarachnoid hemorrhage; |
| UAE |
United Arab Emirates; |
| WS |
wind speed. |
References
- El-Hajj M, Salameh P, Rachidi S, Hosseini H. The epidemiology of stroke in the middle east. European Stroke Journal 1 (2016) 180–198. [CrossRef]
- Wei JW, Heeley EL, Wang JG, Huang Y, Wong LK, Li Z, et al. Comparison of recovery patterns and prognostic indicators for ischemic and hemorrhagic stroke in china: the chinaquest (quality evaluation of stroke care and treatment) registry study. Stroke 41 (2010) 1877–1883. [CrossRef]
- Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, De Angelis D, et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: a matched comparison. Stroke 34 (2003) 2861–2865. [CrossRef]
- John S, Hussain SI, Piechowski-Jozwiak B, Dibu J, Kesav P, Bayrlee A, et al. Clinical characteristics and admission patterns of stroke patients during the covid 19 pandemic: a single center retrospective, observational study from the abu dhabi, united arab emirates. Clinical Neurology and Neurosurgery 199 (2020) 106227. [CrossRef]
- Tenny S, Thorell W. Intracranial hemorrhage. StatPearls [Internet] (2021).
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurologic clinics 26 (2008) 871–895. [CrossRef]
- Statsenko Y, Habuza T, Smetanina D, Simiyu GL, Uzianbaeva L, Neidl-Van Gorkom K, et al. Brain morphometry and cognitive performance in normal brain aging: Age-and sex-related structural and functional changes. Frontiers in aging neuroscience 13 (2021). [CrossRef]
- Statsenko Y, Habuza T, Uzianbaeva L, Gorkom K, Belghali M, Charykova I, et al. Correlation between lifelong dynamics of psychophysiological performance and brain morphology. esnr 2021. Neuroradiology 63 (2021) 41–42. [CrossRef]
- Gorkom K, Statsenko Y, Habuza T, Uzianbaeva L, Belghali M, Charykova I, et al. Comparison of brain volumetric changes with functional outcomes in physiologic brain aging. esnr 2021. Neuroradiology 63 (2021) 43–44. [CrossRef]
- Uzianbaeva L, Statsenko Y, Habuza T, Gorkom K, Belghali M, Charykova I, et al. Effects of sex age-related changes in brain morphology. esnr 2021. Neuroradiology 63 (2021) 42–43. [CrossRef]
- Statsenko Y, Fursa E, Laver V, Altakarli N, Almansoori T, Al Zahmi F, et al. Risk stratification and prediction of severity of hemorrhagic stroke in dry desert climate-a retrospective cohort study in eastern region of abu dhabi emirate. Journal of the neurological sciences 429 (2021). https://doi.org/10.1016/j.jns.2021. 117760. [CrossRef]
- Al Zahmi F, Habuza T, Awawdeh R, Elshekhali H, Lee M, Salamin N, et al. Ethnicity-specific features of covid-19 among arabs, africans, south asians, east asians, and caucasians in the united arab emirates. Frontiers in Cellular and Infection Microbiology (2022) 1241. [CrossRef]
- Statsenko Y, Habuza T, Fursa E, Ponomareva A, Almansoori TM, Al Zahmi F, et al. Prognostication of incidence and severity of ischemic stroke in hot dry climate from environmental and non-environmental predictors. IEEE Access (2022). [CrossRef]
- Biffi A, Cortellini L, Nearnberg CM, Ayres AM, Schwab K, Gilson AJ, et al. Body mass index and etiology of intracerebral hemorrhage. Stroke 42 (2011) 2526–2530. [CrossRef]
- Jolink WM, Wiegertjes K, Rinkel GJ, Algra A, De Leeuw FE, Klijn CJ. Location-specific risk factors for intracerebral hemorrhage: Systematic review and meta-analysis. Neurology 95 (2020) e1807–e1818. [CrossRef]
- Lindgren A. Stroke genetics: a review and update. Journal of stroke 16 (2014) 114. [CrossRef]
- Falcone GJ, Malik R, Dichgans M, Rosand J. Current concepts and clinical applications of stroke genetics. The Lancet Neurology 13 (2014) 405–418. [CrossRef]
- Jimenez-Conde J, Ois A, Gomis M, Rodriguez-Campello A, Cuadrado-Godia E, Subirana I, et al. Weather as a trigger of stroke. Cerebrovascular Diseases 26 (2008) 348–354. [CrossRef]
- Buxton N, Liu C, Dasic D, Moody P, Hope DT. Relationship of aneurysmal subarachnoid hemorrhage to changes in atmospheric pressure: results of a prospective study. Journal of neurosurgery 95 (2001) 391–39. [CrossRef]
- Zheng D, Arima H, Sato S, Gasparrini A, Heeley E, Delcourt C, et al. Low ambient temperature and intracerebral hemorrhage: the interact2 study. PLoS One 11 (2016) e0149040. [CrossRef]
- abuza T, Navaz AN, Hashim F, Alnajjar F, Zaki N, Serhani MA, et al. Ai applications in robotics, precision medicine, and medical image analysis: an overview and future trends. Informatics in Medicine Unlocked (2021) 100596–100596. [CrossRef]
- Statsenko Y, Habuza T, Charykova I, Gorkom KNV, Zaki N, Almansoori TM, et al. Predicting age from behavioral test performance for screening early onset of cognitive decline. Frontiers in Aging Neuroscience (2021) 282. [CrossRef]
- Habuza T, Statsenko Y, Uzianbaeva L, Gorkom K, Zaki N, Belghali M, et al. Models of brain cognitive and morphological changes across the life: machine learning-based approach. esnr 2021. Neuroradiology 63 (2021) 42. [CrossRef]
- Statsenko Y, Fursa E, Laver V, Altakarli N, Almansoori T, Al Zahmi F, et al. Prediction of early functional outcomes of hemorrhagic stroke. Journal of the neurological sciences 429 (2021). [CrossRef]
- Habuza T, Zaki N, Statsenko Y, Elyassami S. Mri and cognitive tests-based screening tool for dementia. Journal of the Neurological Sciences 429 (2021) 118964. [CrossRef]
- Statsenko Y, Habuza T, Charykova I, Gorkom K, Zaki N, Almansoori T, et al. Predicting cognitive age for screening for neurodegeneration. Journal of the Neurological Sciences 429 (2021). [CrossRef]
- Statsenko Y, Al Zahmi F, Habuza T, Neidl-Van Gorkom K, Zaki N. Prediction of covid-19 severity using laboratory findings on admission: informative values, thresholds, ml model performance. BMJ open 11 (2021) e044500. [CrossRef]
- Habuza T, Zaki N, Statsenko Y, Alnajjar F, Elyassami S. Predicting the diagnosis of dementia from mri data: added value to cognitive tests. The 7th Annual International Conference on Arab Women in Computing in Conjunction with the 2nd Forum of Women in Research (2021), 1–7. [CrossRef]
- Habuza T, Zaki N, Statsenko Y, Alnajjar F, Elyassami S. Deep learning for predicting cognitive gap as a reliable biomarker of dementia. medRxiv (2021). [CrossRef]
- Habuza T, Zaki N, Mohamed EA, Statsenko Y. Deviation from model of normal aging in alzheimer’s disease: Application of deep learning to structural mri data and cognitive tests. IEEE Access (2022). [CrossRef]
- Ertl M, Beck C, Kühlbach B, Hartmann J, Hammel G, Straub A, et al. New insights into weather and stroke: influences of specific air masses and temperature changes on stroke incidence. Cerebrovascular Diseases 47 (2019) 275–284. [CrossRef]
- Gasparrini A. Distributed lag linear and non-linear models in r: the package dlnm. Journal of statistical software 43 (2011) 1. [CrossRef]
- McGregor GR, Bessmoulin P, Ebi K, Menne B. Heatwaves and health: guidance on warning-system development. (WMOP) (2015).
- Alfano FRD, Palella BI, Riccio G. Thermal environment assessment reliability using temperature—humidity indices. Industrial health 49 (2011) 95–106. [CrossRef]
- Kuzmenko N, Galagudza M. Dependence of seasonal dynamics of hemorrhagic and ischemic strokes on the climate of a region: A meta-analysis. International Journal of Stroke 17 (2022) 226–235. [CrossRef]
- Sayore C, Ouambi LI, Bechri H, Kaoukou F, Oudrhiri M, Boutarbouch M, et al. Influence of seasonal factors on the incidence of ruptured intracranial aneurysms: Moroccan fifteen years’ experience. Interdisciplinary Neurosurgery 26 (2021) 101344. [CrossRef]
- Hong YC, Kim H, Oh SY, Lim YH, Kim SY, Yoon HJ, et al. Association of cold ambient temperature and cardiovascular markers. Science of the total environment 435 (2012) 74–79. [CrossRef]
- Matsumoto M, Ishikawa S, Kajii E. Cumulative effects of weather on stroke incidence: a multi- community cohort study in japan. Journal of Epidemiology 20 (2010) 136–142. [CrossRef]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environmental health perspectives 120 (2012) 19–28. [CrossRef]
- Hori A, Hashizume M, Tsuda Y, Tsukahara T, Nomiyama T. Effects of weather variability and air pollutants on emergency admissions for cardiovascular and cerebrovascular diseases. International Journal of Environmental Health Research 22 (2012) 416–430. [CrossRef]
- Wang X, Cao Y, Hong D, Zheng D, Richtering S, Sandset EC, et al. Ambient temperature and stroke occurrence: a systematic review and meta-analysis. International journal of environmental research and public health 13 (2016) 698. [CrossRef]
- Morabito M, Crisci A, Vallorani R, Modesti PA, Gensini GF, Orlandini S. Innovative approaches helpful to enhance knowledge on weather-related stroke events over a wide geographical area and a large population. Stroke 42 (2011) 593–600. [CrossRef]
- Fang CW, Ma MC, Lin HJ, Chen CH. Ambient temperature and spontaneous intracerebral haemorrhage: a cross-sectional analysis in tainan, taiwan. BMJ open 2 (2012) e000842. [CrossRef]
- Lavados PM, Olavarría VV, Hoffmeister L. Ambient temperature and stroke risk: evidence supporting a short-term effect at a population level from acute environmental exposures. Stroke 49 (2018) 255–261. [CrossRef]
- Li L, Huang S, Duan Y, Liu P, Lei L, Tian Y, et al. Effect of ambient temperature on stroke onset: a time-series analysis between 2003 and 2014 in shenzhen, china. Occupational and Environmental Medicine 78 (2021) 355–363. [CrossRef]
- Lin CW, Chen PW, Liu WM, Hsu JY, Huang YL, Cheng Y, et al. Dynamic changes and temporal association with ambient temperatures: Nonlinear analyses of stroke events from a national health insurance database. Journal of Clinical Medicine 10 (2021) 5041. [CrossRef]
- 47. Wang P, Luo S, Cheng S, Li Y, Song W. Optimal antihypertensive medication adherence reduces the effect of ambient temperature on intracerebral hemorrhage occurrence: A case-crossover study. Patient preference and adherence. [CrossRef]
- Wang P, Cheng S, Song W, Li Y, Liu J, Zhao Q, et al. Daily meteorological parameters influence the risk of intracerebral hemorrhage in a subtropical monsoon basin climate. Risk Management and Healthcare Policy 14 (2021) 4833. [CrossRef]
- Yamamoto S, Koh M, Matsumura K, Hamazaki K, Inadera H, Kuroda S. Impact of low ambient temperature on the occurrence of spontaneous intracerebral hemorrhage-analysis of population-based stroke registry in toyama, japan. Journal of Stroke and Cerebrovascular Diseases 31 (2022) 106156. [CrossRef]
- Yao D, Liu Y, Wu Q, Guo N, Pan F, Yu H. Temperature decline is a trigger of subarachnoid hemorrhage: Case-crossover study with distributed lag model. Eur Rev Med Pharmacol Sci 24 (2020) 5633–43. [CrossRef]
- Rivera-Lara L, Kowalski RG, Schneider EB, Tamargo RJ, Nyquist P. Elevated relative risk of aneurysmal subarachnoid hemorrhage with colder weather in the mid-atlantic region. Journal of Clinical Neuroscience 22 (2015) 1582–1587. [CrossRef]
- Huang Q, Lin Sw, Hu WP, Li HY, Yao PS, Sun Y, et al. Meteorological variation is a predisposing factor for aneurismal subarachnoid hemorrhage: a 5-year multicenter study in fuzhou, china. World Neurosurgery 132 (2019) e687–e695. [CrossRef]
- Polcaro-Pichet S, Kosatsky T, Potter BJ, Bilodeau-Bertrand M, Auger N. Effects of cold temperature and snowfall on stroke mortality: a case-crossover analysis. Environment international 126 (2019) 89–95. [CrossRef]
- Charach G, Rabinovich PD, Weintraub M. Seasonal changes in blood pressure and frequency of related complications in elderly israeli patients with essential hypertension. Gerontology 50 (2004) 315–321. [CrossRef]
- Helsper M, Agarwal A, Aker A, Herten A, Darkwah-Oppong M, Gembruch O, et al. The subarachnoid hemorrhage–weather myth: A long-term big data and deep learning analysis. Frontiers in Neurology 12 (2021) 653483. [CrossRef]
- Luft A, Katan L. Global burden of stroke. Semin Neurol 38 (2018) 208–211. [CrossRef]
- Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circulation research 120 (2017) 472–495. [CrossRef]
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circulation research 120 (2017) 439–448. [CrossRef]
- Narayanaswamy V, Woo Y, Pandian J, Navarro J. Stroke epidemiology in south, east, and south-east asia: A review. Journal of Stroke [Internet] (2017). [CrossRef]
- Salhab HA, Salameh P, Hajj H, Hosseini H. Stroke in the arab world: A bibliometric analysis of research activity (2002–2016). Eneurologicalsci 13 (2018) 40–45. [CrossRef]
- Al Zarooni AAR, Al Marzouqi FI, Al Darmaki SH, Prinsloo EAM, Nagelkerke N. Prevalence of vitamin d deficiency and associated comorbidities among abu dhabi emirates population. BMC research notes 12 (2019) 1–6. [CrossRef]
- Ibrahim O, Al-Tameemi N, Dawoud D. Knowledge and perceptions of vitamin d deficiency among the united arab emirates population. Asian J. Pharmaceut. Clin. Res 12 (2019) 183–186. [CrossRef]
- Judd SE, Morgan CJ, Panwar B, Howard VJ, Wadley VG, Jenny NS, et al. Vitamin d deficiency and incident stroke risk in community-living black and white adults. International Journal of Stroke 11 (2016) 93–102. [CrossRef]
- Leung RY, Han Y, Sing CW, Cheung BM, Wong IC, Tan KC, et al. Serum 25-hydroxyvitamin d and the risk of stroke in hong kong chinese. Thrombosis and haemostasis 26 (2017) 158–163. [CrossRef]
- Vyas MV, Laupacis A, Austin PC, Fang J, Silver FL, Kapral MK. Association between immigration status and acute stroke care: a retrospective study. Stroke 51 (2020) 1555–1562. [CrossRef]
- Singh V, Dhamoon MS, Alladi S. Stroke risk and vascular dementia in south asians. Current Atherosclerosis Reports 20 (2018) 1–7. [CrossRef]
- Ramadan H, Patterson C, Maguire S, Melvin I, Kain K, Teale E, et al. Incidence of first stroke and ethnic differences in stroke pattern in bradford, uk: Bradford stroke study. International Journal of Stroke 13 (2018) 374–378. [CrossRef]
- Fedeli U, Pigato M, Avossa F, Ferroni E, Nardetto L, Giometto B, et al. Large variations in stroke hospitalization rates across immigrant groups in italy. Journal of neurology 263 (2016) 449–454. [CrossRef]
- Chauhan G, Debette S. Genetic risk factors for ischemic and hemorrhagic stroke. Current cardiology reports 18 (2016) 1–11. [CrossRef]
- Nandeesh BN, Bindu PS, Narayanappa G, Chickabasaviah Yasha T, Mahadevan A, Kulanthaivelu K, et al. Cerebral small vessel disease with hemorrhagic stroke related to col4a1 mutation: A case report. Neuropathology 40 (2020) 93–98. [CrossRef]
- Meirhaeghe A, Cottel D, Cousin B, Dumont MP, Marécaux N, Amouyel P, et al. Sex differences in stroke attack, incidence, and mortality rates in northern france. Journal of Stroke and Cerebrovascular Diseases 27 (2018) 1368–1374. [CrossRef]
- Liu CT, Wu BY, Hu WL, Hung YC. Gender-based differences in mortality and complementary therapies for patients with stroke in taiwan. Complementary Therapies in Medicine 30 (2017) 113–117. [CrossRef]
- Bir SC, Maiti TK, Ambekar S, Nanda A. Incidence, hospital costs and in-hospital mortality rates of epidural hematoma in the united states. Clinical neurology and neurosurgery 138 (2015) 99–103. [CrossRef]
- Woo D, Comeau ME, Venema SU, Anderson CD, Flaherty M, Testai F, et al. Risk factors associated with mortality and neurologic disability after intracerebral hemorrhage in a racially and ethnically diverse cohort. JAMA network open 5 (2022) e221103–e221103. [CrossRef]
- Cao Z, Liu X, Li Z, Gu H, Jiang Y, Zhao X, et al. Body mass index and clinical outcomes in patients with intracerebral haemorrhage: results from the china stroke center alliance. Stroke and vascular neurology 6 (2021). [CrossRef]
- Canfell K, Kroll M, Green J, Beral V, Sudlow C, Brown A, et al. Adiposity and ischemic and hemorrhagic stroke: Prospective study in women and meta-analysis. (2016). [CrossRef]
- Platz J, Güresir E, Schuss P, Konczalla J, Seifert V, Vatter H. The impact of the body mass index on outcome after subarachnoid hemorrhage: is there an obesity paradox in sah? a retrospective analysis. Neurosurgery 73 (2013) 201–208. [CrossRef]
- Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Archives of Neurology 52 (1995) 1193–1200. [CrossRef]
- Lin CH, Hsu KC, Johnson KR, Fann YC, Tsai CH, Sun Y, et al. Evaluation of machine learning methods to stroke outcome prediction using a nationwide disease registry. Computer methods and programs in biomedicine 190 (2020) 105381. [CrossRef]
- Schmidt LB, Goertz S, Wohlfahrt J, Melbye M, Munch TN. Recurrent intracerebral hemorrhage: associations with comorbidities and medicine with antithrombotic effects. PloS one 11 (2016) e0166223. [CrossRef]
Figure 1.
Contour exposure–lag-response plots and three-dimensional exposure–lag-response plots of hemorrhagic stroke risk versus ambient temperature (a, b), perceived temperature (c, d), atmosphere pressure (e, f), wind speed (g, h) and daily changes in them (lag = 7 days).
Figure 1.
Contour exposure–lag-response plots and three-dimensional exposure–lag-response plots of hemorrhagic stroke risk versus ambient temperature (a, b), perceived temperature (c, d), atmosphere pressure (e, f), wind speed (g, h) and daily changes in them (lag = 7 days).
Figure 2.
(a) Variation of weather parameters on the day of hemorrhagic stroke (HS) and within 2 days before HS. Cases are stratified according to severity: minor [National Institutes of Health Stroke Scale (NIHSS) score ≤ 4]; moderate and severe (NIHSS score > 4). (b) Variations of body mass index (BMI), age, and modified Ranking Score (mRS) at discharge in patients with minor hemorrhagic stroke [NIHSS score ≤ 4] and moderate–severe hemorrhagic stroke (NIHSS score > 4). (c) Variations of body mass index (BMI), age, and NIHSS score at admission among patients with hemorrhagic stroke and slight to moderate disability at discharge (mRS ≤ 3) or severe disability/death (mRS > 3). (d) Variation in time of day at hemorrhagic stroke onset stratified by severity. (e) Variation in time of day at hemorrhagic stroke onset stratified by early outcome.
Figure 2.
(a) Variation of weather parameters on the day of hemorrhagic stroke (HS) and within 2 days before HS. Cases are stratified according to severity: minor [National Institutes of Health Stroke Scale (NIHSS) score ≤ 4]; moderate and severe (NIHSS score > 4). (b) Variations of body mass index (BMI), age, and modified Ranking Score (mRS) at discharge in patients with minor hemorrhagic stroke [NIHSS score ≤ 4] and moderate–severe hemorrhagic stroke (NIHSS score > 4). (c) Variations of body mass index (BMI), age, and NIHSS score at admission among patients with hemorrhagic stroke and slight to moderate disability at discharge (mRS ≤ 3) or severe disability/death (mRS > 3). (d) Variation in time of day at hemorrhagic stroke onset stratified by severity. (e) Variation in time of day at hemorrhagic stroke onset stratified by early outcome.
Figure 3.
(a) Ranked informative values of clinicodemographic factors for predicting hemorrhagic stroke severity. (b) Feature selection for predicting hemorrhagic stroke severity from both weather factors and clinicodemographic risk factors (bold). (c) Ranked informative values of clinicodemographic factors for predicting disability level after hemorrhagic stroke (short-term outcome). (d) Feature selection for predicting disability outcome, including both weather and clinicodemographic factors (bold).
Figure 3.
(a) Ranked informative values of clinicodemographic factors for predicting hemorrhagic stroke severity. (b) Feature selection for predicting hemorrhagic stroke severity from both weather factors and clinicodemographic risk factors (bold). (c) Ranked informative values of clinicodemographic factors for predicting disability level after hemorrhagic stroke (short-term outcome). (d) Feature selection for predicting disability outcome, including both weather and clinicodemographic factors (bold).
Figure 4.
Barplots of average monthly air temperature, average daily changes, mean relative humidity, and incidence of hemorrhagic stroke by month.
Figure 4.
Barplots of average monthly air temperature, average daily changes, mean relative humidity, and incidence of hemorrhagic stroke by month.
Figure 5.
Performance of neural network classification models for predicting hemorrhagic stroke severity. The input values are the clinicodemographic (nonweather) risk factors (a) and the combination of clinicodemographic and weather parameters. (b).
Figure 5.
Performance of neural network classification models for predicting hemorrhagic stroke severity. The input values are the clinicodemographic (nonweather) risk factors (a) and the combination of clinicodemographic and weather parameters. (b).
Figure 6.
Performance of neural network classification models for predicting disability level at discharge. The input values are the clinicodemographic risk factors (a) and the combination of clinicodemographic risk factors and weather parameters (b).
Figure 6.
Performance of neural network classification models for predicting disability level at discharge. The input values are the clinicodemographic risk factors (a) and the combination of clinicodemographic risk factors and weather parameters (b).
Figure 7.
ROC curves for the significant weather and clinicodemographic features used as input to neural network models separately (a) and in different combinations (b).
Figure 7.
ROC curves for the significant weather and clinicodemographic features used as input to neural network models separately (a) and in different combinations (b).
Table 1.
Diagnoses of the study cohort.
Table 1.
Diagnoses of the study cohort.
| ICD codes |
Nosology |
Number of cases |
| Nontraumatic subarachnoid hemorrhage |
2 (1.25%)
1 (0.62%)
1 (0.62%) |
I60.4
I60.9 |
From basilar artery
Unspecified |
| Nontraumatic intracerebral hemorrhage with a specified location |
51 (31.88%)
17 (10.62%)
11 (6.88%)
2 (1.25%)
7 (4.38%)
8 (5%)
4 (2.5%)
2 (1.25%) |
I61.0
I61.1 I61.2 I61.3 I61.4 I61.5 I61.6 |
In hemisphere, subcortical
In hemisphere, cortical
In hemisphere, unspecified
Hemorrhage in brain stem
Hemorrhage in cerebellum
Hemorrhage, intraventricular
Hemorrhage, multiple localized |
| Nontraumatic intracerebral hemorrhage with an unspecified location |
95 (59.38%)
12 (7.5%)
83 (51.88%) |
I61.8
I61.9, 431 |
Other nontraumatic intracerebral hemorrhage
Unspecified |
| Nontraumatic intracranial hemorrhage |
12 (7.5%)
12 (7.5%) |
| I62.9, 432.9 |
- Unspecified |
| Total: |
160 (100%) |
Table 2.
Incidences of intracranial hemorrhage in Al Ain stratified by sex and age group.
Table 2.
Incidences of intracranial hemorrhage in Al Ain stratified by sex and age group.
| Variable |
Total
number |
Mean number
per annum |
Mean per
100,000 people |
City
population |
HS cases |
Female |
44 |
11.0 |
5.00 |
249,940 |
| Male |
116 |
29.0 |
9.67 |
315,310 |
| Total |
160 |
40.0 |
7.60 |
565,250 |
| in 2016 |
38 |
- |
7.25 |
524,000 |
| in 2017 |
21 |
- |
3.79 |
554,000 |
| in 2018 |
50 |
- |
8.54 |
585,000 |
| in 2019 |
51 |
- |
8.52 |
598,000 |
Age groups |
0‒34 years |
7 |
1.75 |
1.80 |
389,057 |
| 35‒44 years |
42 |
10.50 |
40.15 |
104,595 |
| 45‒54 years |
40 |
10.00 |
82.57 |
48,443 |
| 55‒64 years |
32 |
8.00 |
190.41 |
16,806 |
| 65‒74 years |
19 |
4.75 |
400.33 |
4,746 |
|
≥75 years |
20 |
5.00 |
1247.66 |
1,603 |
Table 3.
Comparisons of clinicodemographic parameters between ethnic groups and sexes within the study cohort.
Table 3.
Comparisons of clinicodemographic parameters between ethnic groups and sexes within the study cohort.
| |
Arabs
n1 = 74 |
Asians
n2 = 85 |
p1−2
|
Female
n3 = 44 |
Male
n4 = 116 |
p3−4
|
| Male |
50 (43.5%) |
65 (56.5%) |
0.283 |
- |
- |
- |
| Female |
24 (54.5%) |
20 (45.5%) |
| Age |
62.31 ± 14.88 |
47.63 ± 9.90 |
<0.001 |
53.18 ± 13.52 |
57.81 ± 16.28 |
0.098 |
| Current smoking |
6 (8.11%) |
9 (10.59%) |
0.593 |
- |
15 (12.93%) |
<0.001 |
| Arterial fibrillation |
2 (2.70%) |
1 (1.18%) |
0.496 |
2 (4.55%) |
1 (0.86%) |
0.269 |
| Hypertension |
61 (82.43%) |
63 (74.12%) |
0.205 |
38 (86.36%) |
87 (75.00%) |
0.089 |
| Hyperlipidemia |
8 (10.81%) |
0 |
0.004 |
2 (4.55%) |
6 (5.17%) |
0.869 |
| Diabetes mellitus |
48 (64.86%) |
25 (29.41%) |
<0.001 |
21 (47.73%) |
52 (44.83%) |
0.746 |
| Ischemic heart disease |
10 (13.51%) |
3 (3.53%) |
0.028 |
5 (11.36%) |
8 (6.90%) |
0.410 |
Table 4.
Associations between meteorological factors and hemorrhagic stroke incidence.
Table 4.
Associations between meteorological factors and hemorrhagic stroke incidence.
| Variable |
Mean ± SD |
Median [IQR] |
Min - Max |
Correlation with HS Incidence |
| r-coefficient |
p-value |
| Temperature, ◦C
|
absolute value
daily change |
29.36 ± 7.16
-0.002 ± 1.6 |
30.39
0.06 |
[22.74‒35.93]
[-0.78 to 0.89] |
11.67
-11.61 |
41.89
5.5 |
-0.1123
-0.02817 |
0.000058
0.3146 |
| Atmospheric pressure, mbar
|
absolute value
daily change |
979.42 ± 7.02
-0.0008 ± 1.41 |
980.5
0 |
[973.23‒980.5]
[-0.8 to 0.8] |
964.1
-4.7 |
993.5
7.5 |
0.08889
0.01519 |
0.00148
0.5878 |
| Wind speed, knot
|
absolute value
daily change |
7.74 ± 1.96
0.0007 ± 1.89 |
7.5
0 |
[6.5‒8.7]
[-1 to 1] |
3.7
-11.9 |
21.1
12.1 |
-0.03227
0.00722 |
0.24935
0.7968 |
| Relative humidity, %
|
absolute value |
37.89 ± 14.96
0.019 ± 9.18 |
36.2
-0.11 |
[25.25‒48.51]
[-5.33 to 5.07] |
10.86
-37.83 |
87.73
48.94 |
0.07537
0.02289 |
0.007
0.4139 |
| daily change |
| Humidex, ◦C
|
absolute value
daily change |
32.08 ± 8.5
-0.00096 ±1.86 |
32.54
0.05 |
[24.73‒39.6]
[-0.92 to 1.11] |
10.19
-11.07 |
49.95
7.57 |
-0.10847
0 |
0.0001
0.9984 |
Table 5.
Associations of meteorological factors with hemorrhagic stroke incidence by ethnicity and sex.
Table 5.
Associations of meteorological factors with hemorrhagic stroke incidence by ethnicity and sex.
| Variable |
Years |
Correlation with HS incidence |
2016
n1 = 366 |
2017
n2 = 363 |
2018
n3 = 356 |
2019
n4 = 365 |
p |
Arab,
n = 75 |
Asian,
n = 85 |
Female,
n = 44 |
Male,
n = 117 |
| r |
p |
r |
p |
r |
p |
r |
p |
| AT, ◦C
|
absolute |
28.9±7.1 |
29.7±7.3 |
29.7±7.1 |
29.1±7.2 |
0.344 |
−0.128 |
0.001 |
−0.093 |
0.032 |
−0.229 |
<0.001 |
−0.07 |
0.034 |
| change |
−0.0±1.6 |
0.0±1.7 |
0.0±1.5 |
−0.0±1.6 |
1.000 |
|
|
|
|
|
|
|
|
| AP, mbar
|
absolute |
979.7±6.9 |
979.5±6.9 |
979.0±7.1 |
979.4±7.1 |
0.620 |
0.109 |
0.003 |
0.065 |
0.133 |
0.191 |
<0.001 |
0.052 |
0.111 |
| change |
−0.0±1.4 |
−0.0±1.4 |
0.0±1.4 |
−0.0±1.4 |
0.999 |
| WS, knot
|
absolute |
7.4±1.6 |
7.8±2.0 |
7.7±1.8 |
8.0±2.3 |
0.001 |
−0.033 |
0.374 |
−0.027 |
0.540 |
0.034 |
0.522 |
−0.061 |
0.064 |
| change |
0.0±1.6 |
−0.0±2.0 |
0.0±1.9 |
−0.0±2.1 |
0.999 |
| RH, %
|
absolute |
39.9±15.5 |
36.9±15.2 |
35.6±13.9 |
39.1±14.9 |
<0.001 |
0.095 |
0.010 |
0.063 |
0.145 |
0.142 |
0.007 |
0.054 |
0.103 |
| change |
0.1±9.4 |
−0.1±9.5 |
0.0±8.5 |
0.1±9.3 |
0.996 |
| HI, ◦C
|
absolute |
31.9±8.4 |
32.2±8.4 |
32.1±8.4 |
32.0±8.8 |
0.962 |
−0.121 |
0.001 |
−0.089 |
0.040 |
−0.224 |
<0.001 |
−0.065 |
0.049 |
| change |
–0.0±1.7 |
−0.0±1.9 |
0.0±1.7 |
−0.0±2.1 |
1.000 |
Table 6.
Top performance models for predicting severity and early outcome of hemorrhagic stroke.
Table 6.
Top performance models for predicting severity and early outcome of hemorrhagic stroke.
| Prediction |
Predictors |
AUC |
F1 Score |
Sensitivity |
Specificity |
p |
| NIHSS |
Individual risk factors (BMI, age, daytime) |
0,805 |
0.732807 |
0.750 |
0.617 |
0.30 |
| Weather and individual risk factors |
0.804 |
0.875065 |
0.875 |
0.892 |
0.23 |
| mRS |
Individual risk factors (NIHSS, BMI, age) |
0.805 |
0.641190 |
0.667 |
0.750 |
0.37 |
| Weather and individual risk factors |
0.896 |
0.915714 |
0.900 |
0.975 |
0.05 |
| Top informative features |
|
|
|
|
|
| NIHSS score at admission |
0.797 |
0.517143 |
0.500 |
0.833 |
0.02 |
| Wind speed difference, 2 day lag |
0.621 |
0.440 |
0.367 |
0.900 |
0.89 |
| Wind speed difference, 1 day lag |
0.608 |
0.437143 |
0.500 |
0.617 |
0.56 |
| Less informative features |
|
|
|
|
|
| Humidex difference, 1 day lag |
0.577 |
0.463810 |
0.450 |
0.683 |
0.31 |
| Wind speed, 1 day lag |
0.545 |
0.386667 |
0.383 |
0.650 |
0.20 |
| Atmospheric pressure |
0.528 |
0.311032 |
0.367 |
0.550 |
0.78 |
| Temperature difference, 2 day lag |
0.502 |
0.532381 |
0.583 |
0.533 |
0.50 |
| Three top informative features |
0.900 |
0.790952 |
0.800 |
0.850 |
0.13 |
| All significant features |
0.950 |
0.827143 |
0.833 |
0.900 |
0.44 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).