Introduction
Bacteria can grow either as planktonic cells or as communities within biofilms. Harrell et al. (2021) defines biofilm as a collection of cells connected to a surface and enclosed by self-produced extracellular polymeric substances (EPS) on living or non-living surfaces. They stated that biofilm formation is frequently triggered by many adverse environmental factors such alterations in pH, oxygen levels, temperature, and nutrient supply. Biofilms provide protection against external stressors such the host immune system, harmful chemicals, and antibiotics (Yuyama et al., 2020; Zakaria et al. 2023). Chronic infections induced by bacteria organised in a biofilm are difficult to cure because they have a high tolerance to antibiotic concentrations. Various studies have examined the effectiveness of current antibiotics, antifungals, disinfectants, and medicinal herbs on microbial biofilms (Rashid et al. 2022, Isa et al. 2022, Kamaruzzaman et al. 2022, Johari et al. 2023, Amran et al. 2022).
Salmonella is a prevalent bacterium responsible for causing foodborne illnesses. Pang et al. (2017) identified Salmonella as the primary bacterium responsible for causing foodborne illnesses in the United States. Salmonella typhimurium is the predominant serovar responsible for approximately 20.2% of reported cases of salmonellosis in Europe, posing a serious public health risk and potential economic repercussions. Previous studies demonstrated that Salmonella could form biofilms and may adhere to various surfaces such plastics, rubbers, and stainless steel (Pang et al. 2007; Othman & Yahya 2019). Therefore, this leads to an increase in several foodborne outbreaks.
Actinomycetes originates from the Greek terms “aktis” and “mykes,” meaning “ray” and “fungus” respectively. Faja et al. (2017) classified actinomycetes as Gram-positive bacteria that typically inhabit marine and soil sediments. Budhathoki and Shrestha (2020) suggested that actinomycetes play a significant role in creating bioactive chemicals. Actinomycetes are responsible for creating over 50% of the bioactive secondary metabolites identified to date, including immunosuppressive drugs, anticancer agents, enzymes, and notably antibiotics (Budhathoki & Shrestha, 2020). Actinomycetes in the field of microbiology are emerging as a promising source of antibiotics. Recent studies have suggested that certain bioactive compounds produced by actinomycetes exhibit antifungal, antimicrobial, anti-inflammatory, and other pharmacological properties (Budhathoki & Shrestha, 2020). Faja et al. (2017) stated that actinomycetes are currently recognised as significant antibiotic producers, generating a large number of antibiotics and other physiologically active secondary metabolites, accounting for approximately 80% of total antibiotic products. The antibiofilm activity of actinomycetes against S. typhimurium has not been well studied thus far.
Methodology
Test Microorganism and Actinomycete Extracts
The bacterial strain tested in this study was S. typhimurium ATCC 14028, obtained from the Microbiology Lab, Faculty of Applied Sciences, UiTM Shah Alam, Selangor, while ethyl acetate extracts of actinomycetes isolated from BRIS soil, Terengganu (EAS5, EAS7, EAA141, and EAAA11), were obtained from the Microbiology Laboratory, Faculty of Science and Mathematics, Universiti Pendidikan Sultan Idris, Tanjung Malim, Perak.
Pellicle Assay
Pellicles were grown in sterile test tubes. After incubation at 37 °C for 24 h, the nutrient medium was discarded while the pellicle fractions were rinsed with distilled water twice, heat-fixed at 37 °C for 30 min, stained with 0.5% crystal violet and 25% methanol for 5 min, and gently destained with distilled water. The pellicle formed at the air–liquid interface was inspected visually.
Microplate Biofilm Assay
Biofilms were formed in the wells of 96-well microplate as previously reported (Man et al. 2022). 100 μl of EA extracts (10% (v/v)-50% (v/v)) and 100 μl of bacterial inoculum (OD600: 0.5) were loaded into microplate wells in triplicates. The mixture was then incubated at 37 ºC for 6 h, 12 h, and 18 h. Meanwhile, 100 μl of inoculum and 100 μl of fresh broth were loaded into microplate well as the negative control.
Crystal Violet Assay
After incubation at 37 °C for 24 h, the nutrient medium was discarded while the biofilm fractions were rinsed with distilled water twice, heat-fixed at 60 °C for 30 min, stained with 0.5% (w/v) crystal violet and 25% (v/v) methanol for 5 min, and gently destained with distilled water. Stained biofilms were then dissolved in absolute ethanol, and the absorbance was measured at 600 nm using a BioTek Synergy H1 Hybrid microplate reader.
Determination of Biofilm Inhibition
The mean absorbance of the samples was determined, and the percentage inhibition of biofilm was calculated using the equation as shown below:
Percentage (%) inhibition = (OD negative control ˗ OD experimental)/(OD negative control) ×100
Results and Discussion
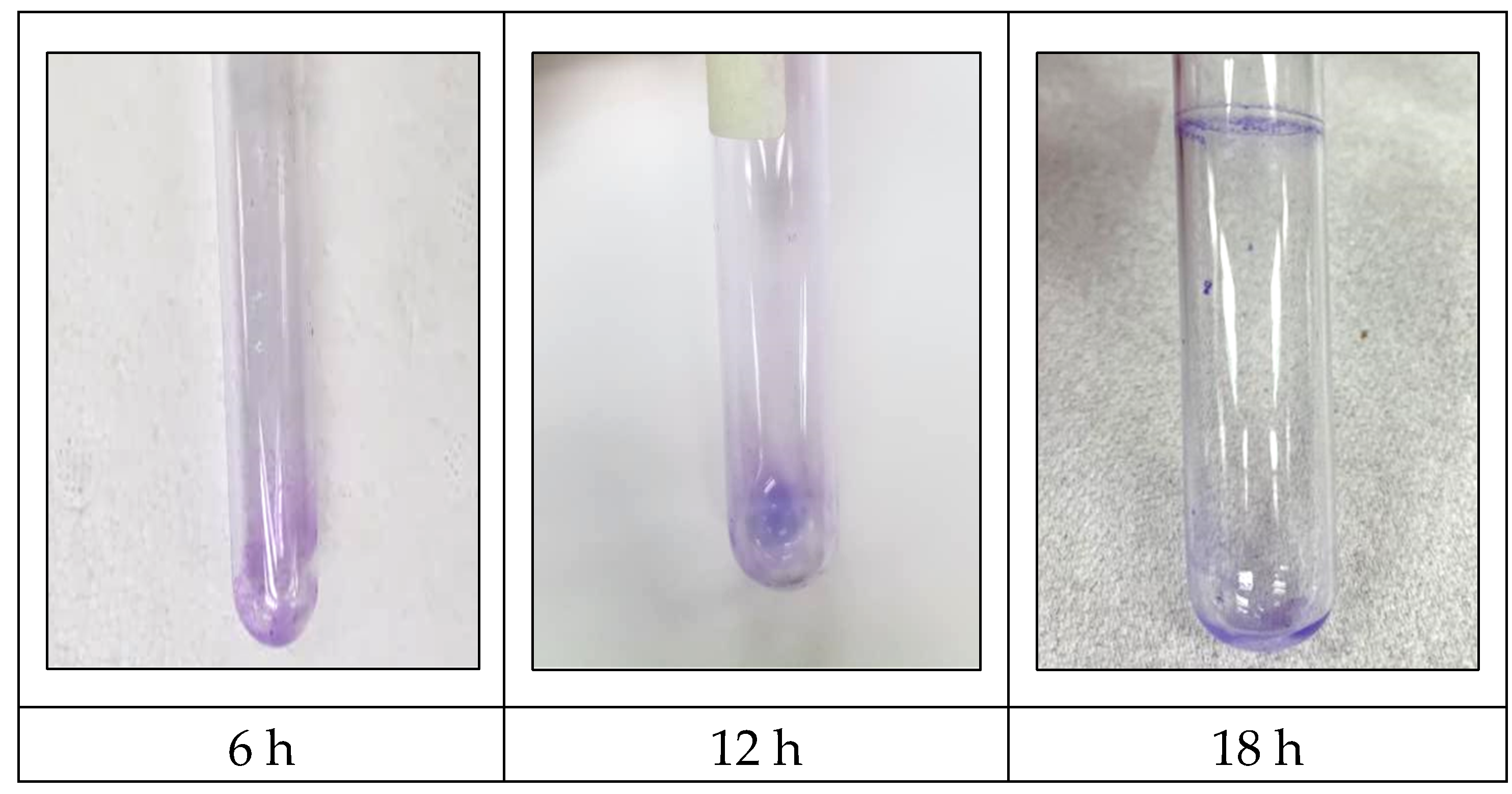
Figure 1 displays the detection of
S. typhimurium biofilm using the pellicle assay. The pellicle intensity increased from 6 h to 18 h, indicating the capability of
S. typhimurium to form a biofilm in this investigation.
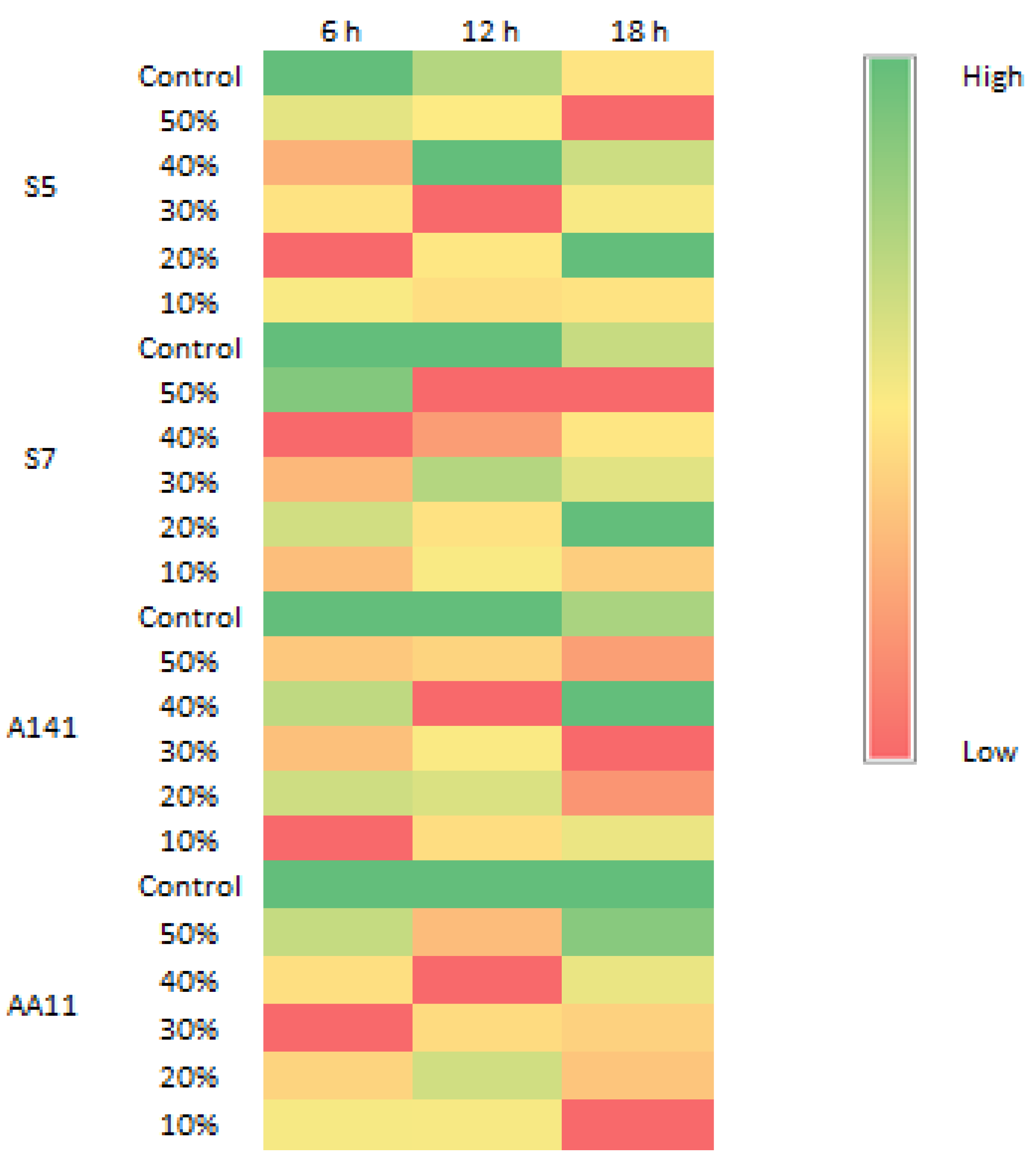
Figure 2 displays the biomass of
S. typhimurium biofilm at 6 h, 12 h, and 18 h. The biomass of
S. typhimurium biofilm decreased when exposed to all EA extracts.
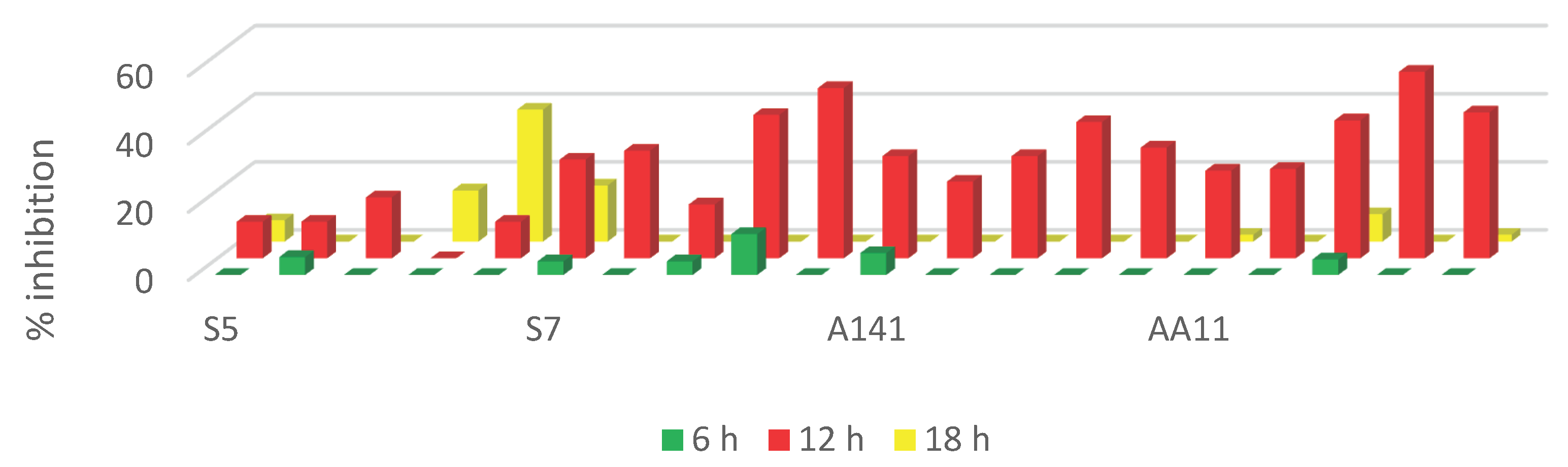
Figure 3 displays the percentage of biofilm inhibition by all EA extracts. Application of all EA extracts marginally suppressed
S. typhimurium biofilm at 6 h. EAS7, EAA141, and EAAA11 significantly reduced biofilm formation at 12 h. EAS5 was the only extract that effectively prevented the formation of
S. typhimurium biofilm at 18 h.
It is understood that juvenile biofilm and planktonic cells are more vulnerable to antimicrobial treatments than mature biofilm. According to Huan et al. (2019), initial biofilm development promotes the infiltration of antimicrobial agents. These contradict our existing data, which demonstrated weak inhibition at 6 h. It is possible that S. typhimurium may not have fully developed a biofilm at 6 h, and thus, the antibiofilm activities of EA extracts could not be determined. In the present study, most of the biofilm inhibition by EA extracts occurred at 12 h. This is strongly believed to be due to the mixture of antimicrobial compounds produced by actinomycetes (Faja et al., 2017; Diarra et al., 2024). Majhool et al. (2021) reported that actinomycetes isolated from the BRIS soil, Terengganu, contained various antimicrobial compounds including hexadecanoic acid, actinomycin C2, and methyl stearate. The heterogeneous mature stage of biofilm often results in reduced sensitivity of bacteria to antimicrobial treatment (Li et al., 2020). This corroborates our discovery that most EA extracts exhibited minimal suppression of biofilm formation at 18 h.
Conclusions
Ethyl acetate extracts of actinomycetes isolated from BRIS soil in Terengganu exhibited antibiofilm properties against S. typhimurium. EAAA11 was the most effective against the 12-hour biofilm, whereas EAS5 was the most effective against the 18-hour biofilm among these extracts. These actinomycetes demonstrate promise in managing Salmonella infection.
References
- Amran, S. S. D., Jalil, M. T. M., Aziz, A. A., & Yahya, M. F. Z. R. (2023). Methanolic Extract of Swietenia macrophylla Exhibits Antibacterial and Antibiofilm Efficacy Against Gram-Positive Pathogens. Malaysian Applied Biology, 52(2), 129-138. [CrossRef]
- Budhathoki, S. & Shrestha, A. (2020). Screening of Actinomycetes from soil for antibacterial activity. Nepal Journal of Biotechnology, 8(3), 102-110. [CrossRef]
- Faja, O., Sharad, A. A., Younis, K. M., Usup, G., & Ahmad, A. (2017). Isolation, screening and antibiotic profiling of marine Actinomycetes extracts from coastal of Peninsular Malaysia. International Journal of ChemTech Research, 10(3), 212-224.
- Diarra, U., Osborne-Naikatini, T., & Subramani, R. (2024). Actinomycetes associated with hymenopteran insects: a promising source of bioactive natural products. Frontiers in Microbiology, 15, 1303010. [CrossRef]
- Faja, O., Sharad, A. A., Younis, K. M., Usup, G., & Ahmad, A. (2017). Isolation, screening and antibiotic profiling of marine Actinomycetes extracts from coastal of Peninsular Malaysia. International Journal of ChemTech Research, 10(3), 212-224.
- Harrell, J. E., Hahn, M. M., D’Souza, S. J., Vasicek, E. M., Sandala, J. L., Gunn, J. S., & McLachlan, J. B. (2021). Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Frontier in Cellular and Infection Microbiology, 10, 1-17. [CrossRef]
- Huan, Gu., Won, L..., Joseph, C., Zhaowei, J., & Dacheng R. (2019). Antibiotic susceptibility of Escherichia colicells during early-stage biofilm formation. . [CrossRef]
- Isa, S. F. M., Abdul Hamid, U. M., & Zaman Raja Yahya, M. F. (2022). Treatment with the combined antimicrobials triggers proteomic changes in P. aeruginosa-C. albicans polyspecies biofilms. ScienceAsia, 48(2). [CrossRef]
- Johari, N. A., Aazmi, M. S., & Yahya, M. F. Z. R. (2023). FTIR Spectroscopic Study of Inhibition of Chloroxylenol-Based Disinfectant Against Salmonella enterica serovar Thyphimurium Biofilm. Malaysian Applied Biology, 52(2), 97-107. [CrossRef]
- Majhool, A. A., Idris, H., Hakimi, W. M. N., & Abdullah, M. D. D. (2021). Chemical Compounds and Antimicrobial Activities of Actinomycetes Isolates from BRIS Soil. Research Journal of Pharmacy and Technology, 14(9), 4783-4788. [CrossRef]
- Man, C. A. I. C., Razak, W. R. W. A., & Yahya, M. F. Z. R. (2022). Antibacterial and antibiofilm activities of Swietenia macrophylla King ethanolic extract against foodborne pathogens. Malaysian Applied Biology, 51(4), 45-56. [CrossRef]
- Kamaruzzaman, A.N.A., Mulok, T.E.T.Z., Nor, N.H.M. & Yahya, M.F.Z.R. 2022a. FTIR spectral changes in Candida albicans biofilm following exposure to antifungals. Malaysian Applied Biology, 51(4): 57-66. [CrossRef]
- Li, Y., Xiao, P., Wang, Y., & Hao, Y. (2020). Mechanisms and control measures of mature biofilm resistance to antimicrobial agents in the clinical context. ACS omega, 5(36), 22684-22690. [CrossRef]
- Othman, N.A. & Yahya, M.F.Z.R. 2019. In silico analysis of essential gene and non-homologous proteins in
Salmonella typhimurium biofilm. Journal of Physics: Conference Series, 1349: 012133. [CrossRef]
- Pang, X., Yang, Y., & Yuk, H. (2017). Biofilm formation and disinfectant resistance of Salmonella sp. in mono- and dual-species with Pseudomonas aeruginosa. Journal of Applied Microbiology, 123(3), 651-660. [CrossRef]
- Rashid, S.A.A., Yaacob, M.F., Aazmi, M.S., Jesse, F.F.A., & Yahya, M.F.Z.R. 2022. Inhibition of Corynebacterium pseudotuberculosis biofilm by DNA synthesis and protein synthesis inhibitors. Journal of Sustainability Science and Management, 17(4): 49-56. [CrossRef]
- Yuyama, K. T., Rohde, M., Molinari, G., Stadler, M., & Abraham, W. (2020). Unsaturated fatty acids control biofilm formation of Staphylococcus aureus and other gram-positive bacteria. Antibiotics, 9(11), 1-11. [CrossRef]
- Zakaria, N.F.S., Yahya, M.F.Z.R., & Jamil, N.M. (2023). Multiple Bacterial Strategies to Survive Antibiotic Pressure: A Review. Preprints 2023, 2023040591. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).