Submitted:
19 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods and Materials
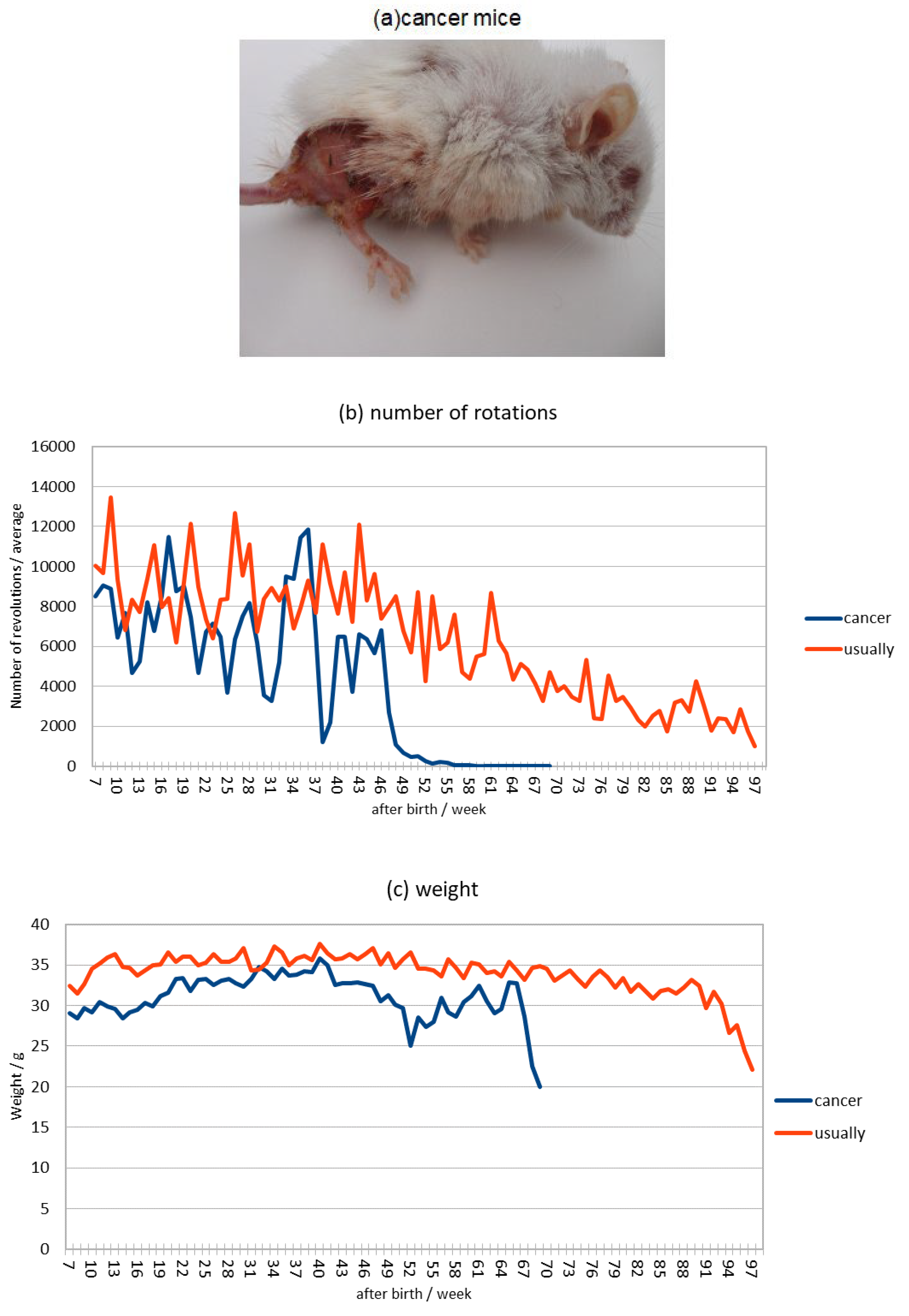
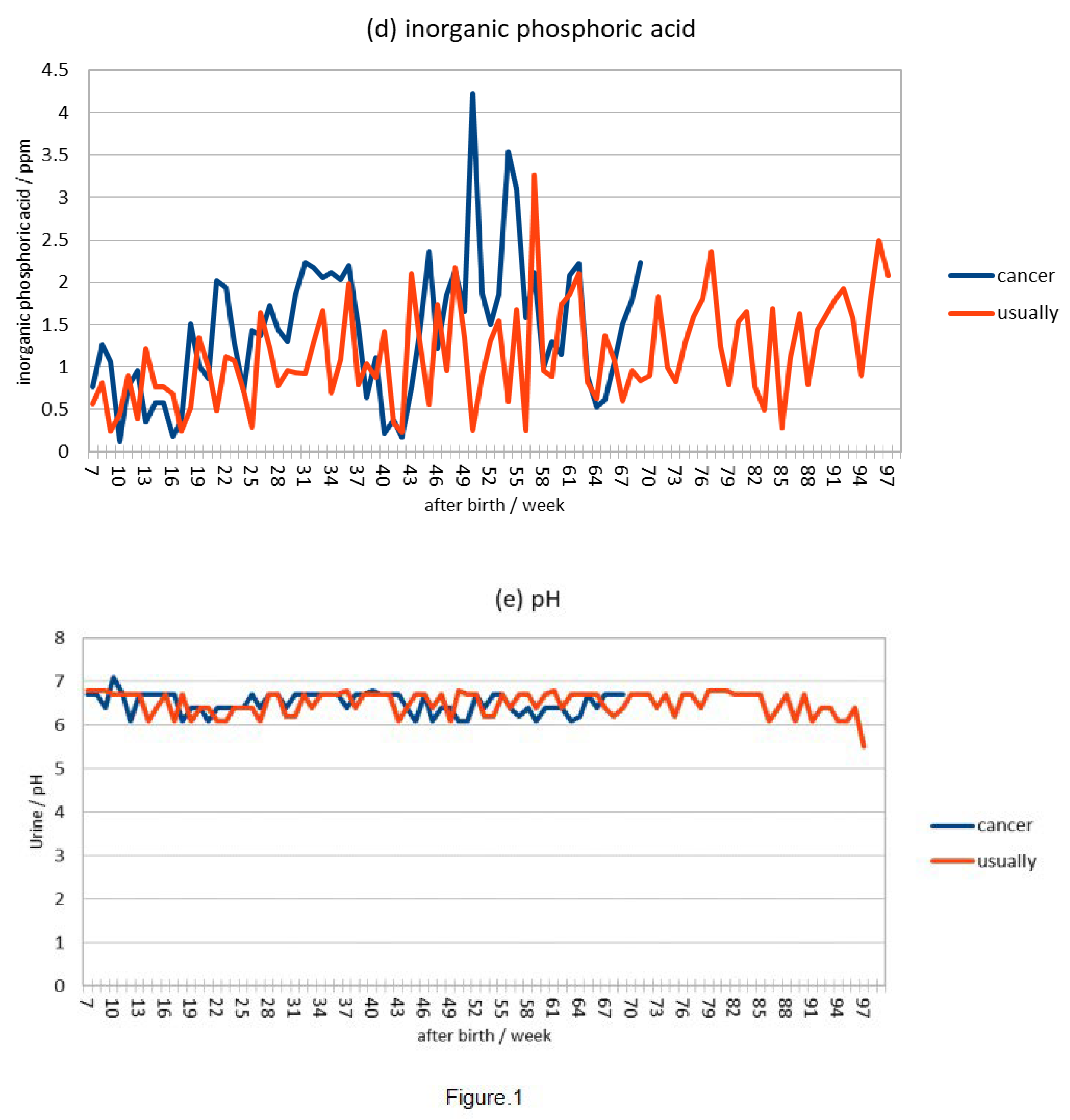
Results
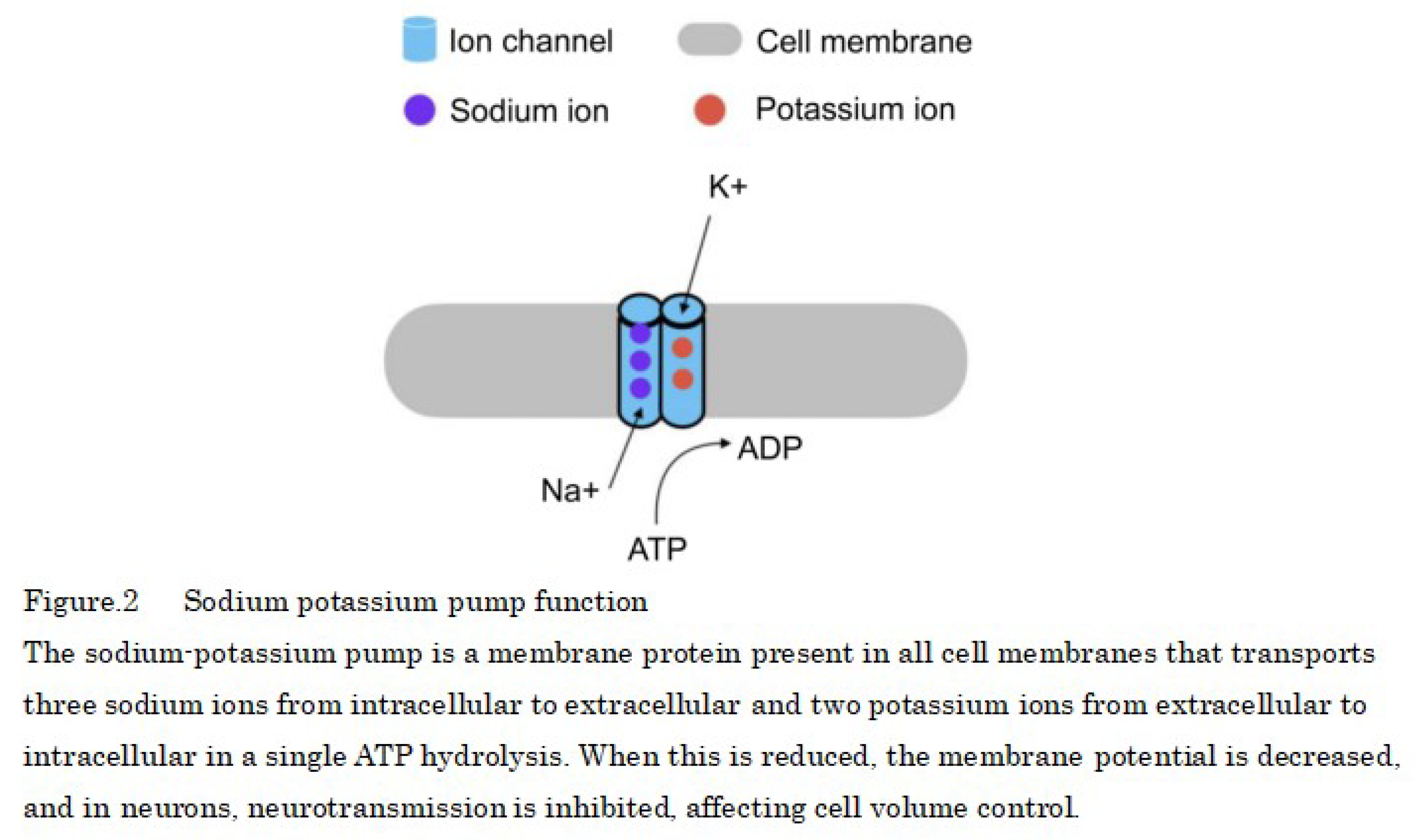
Discussion
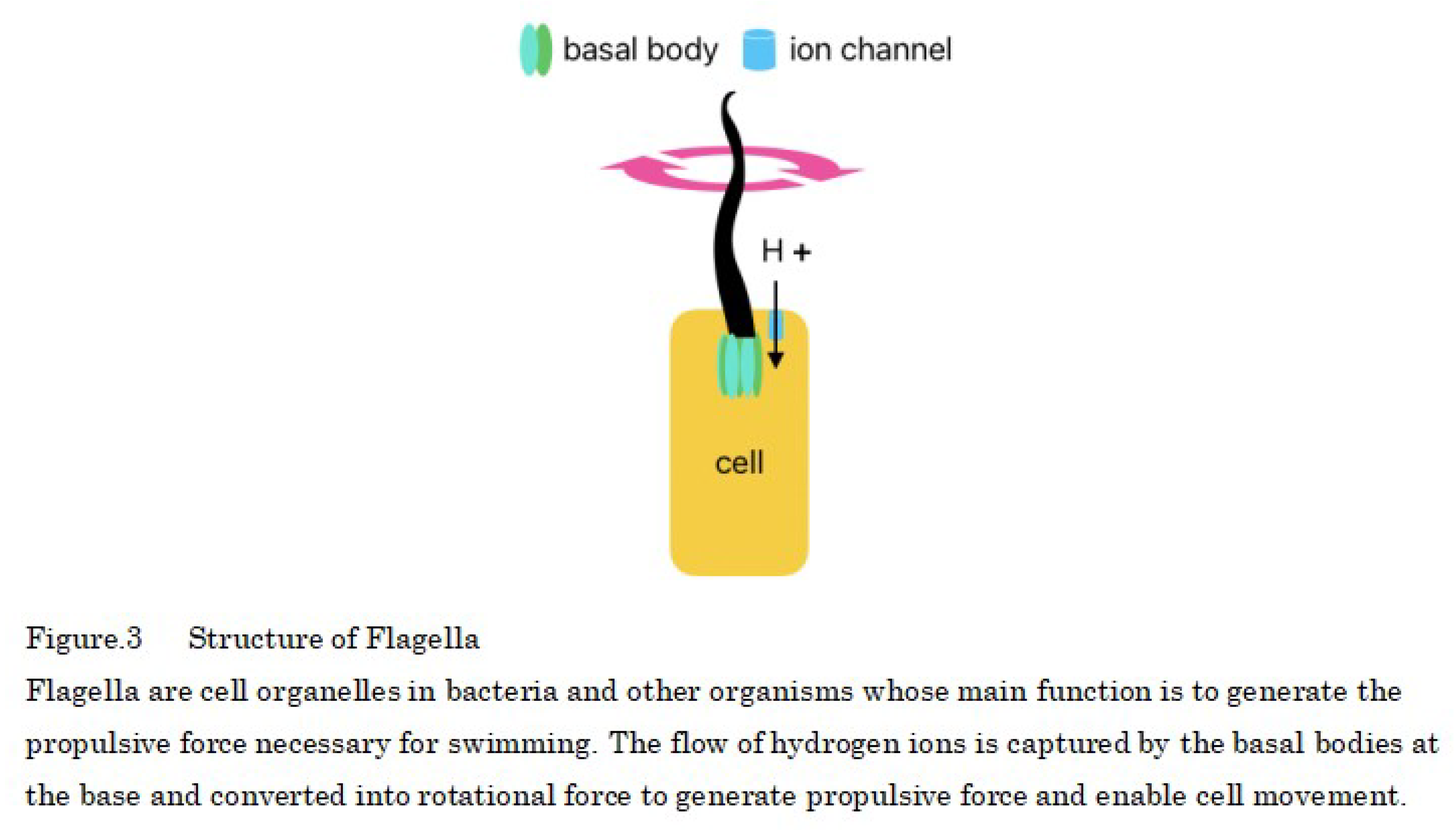
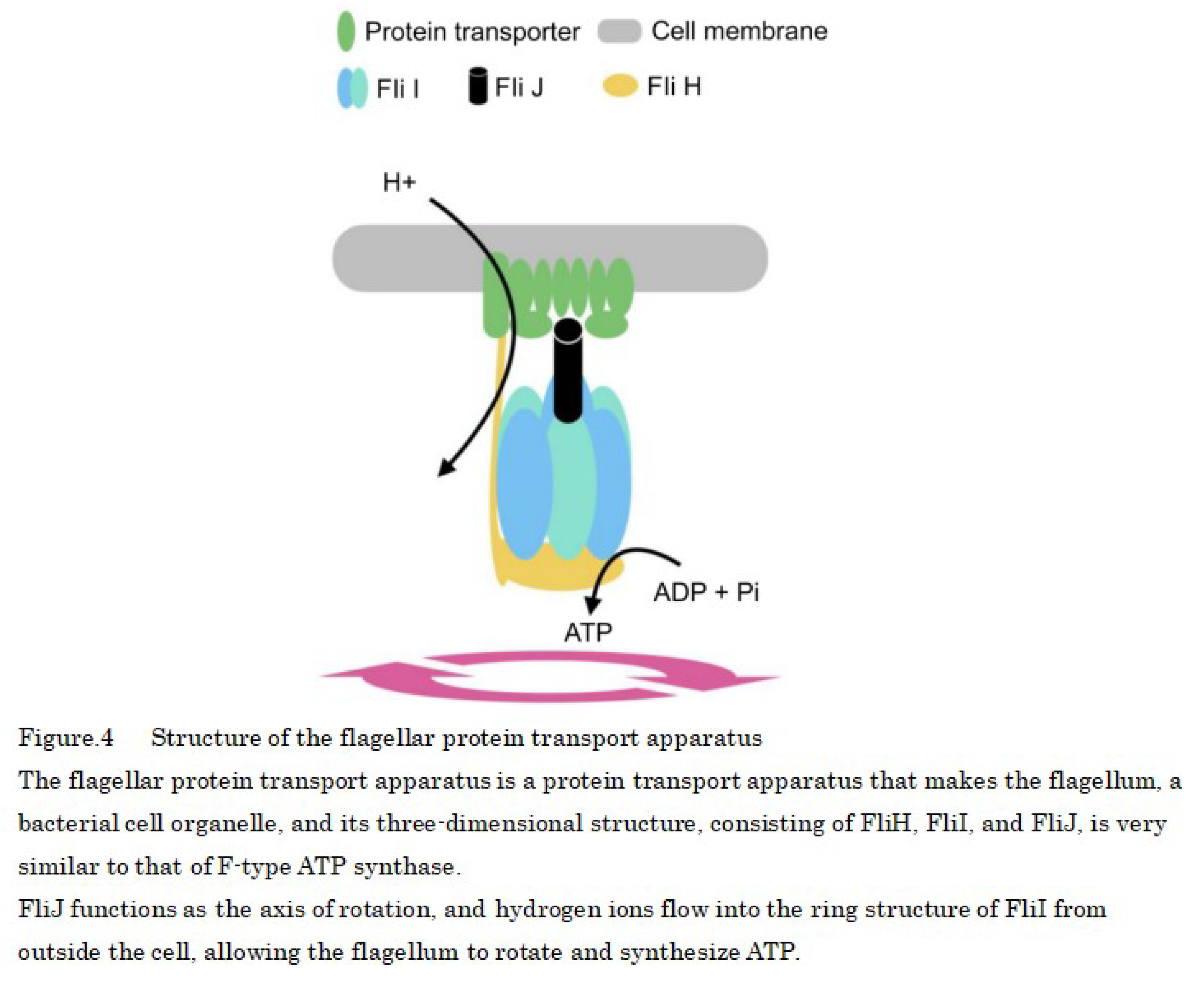
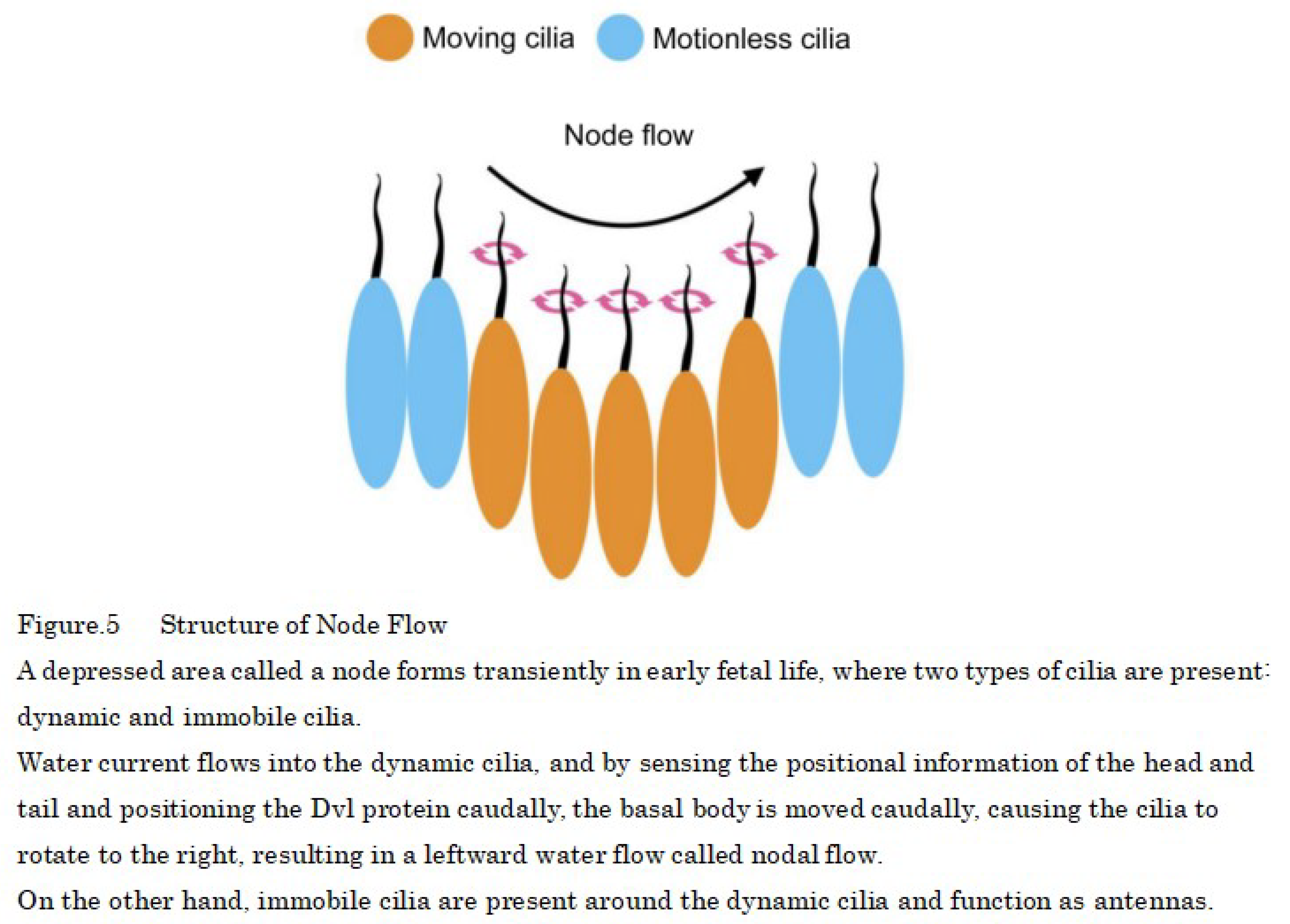
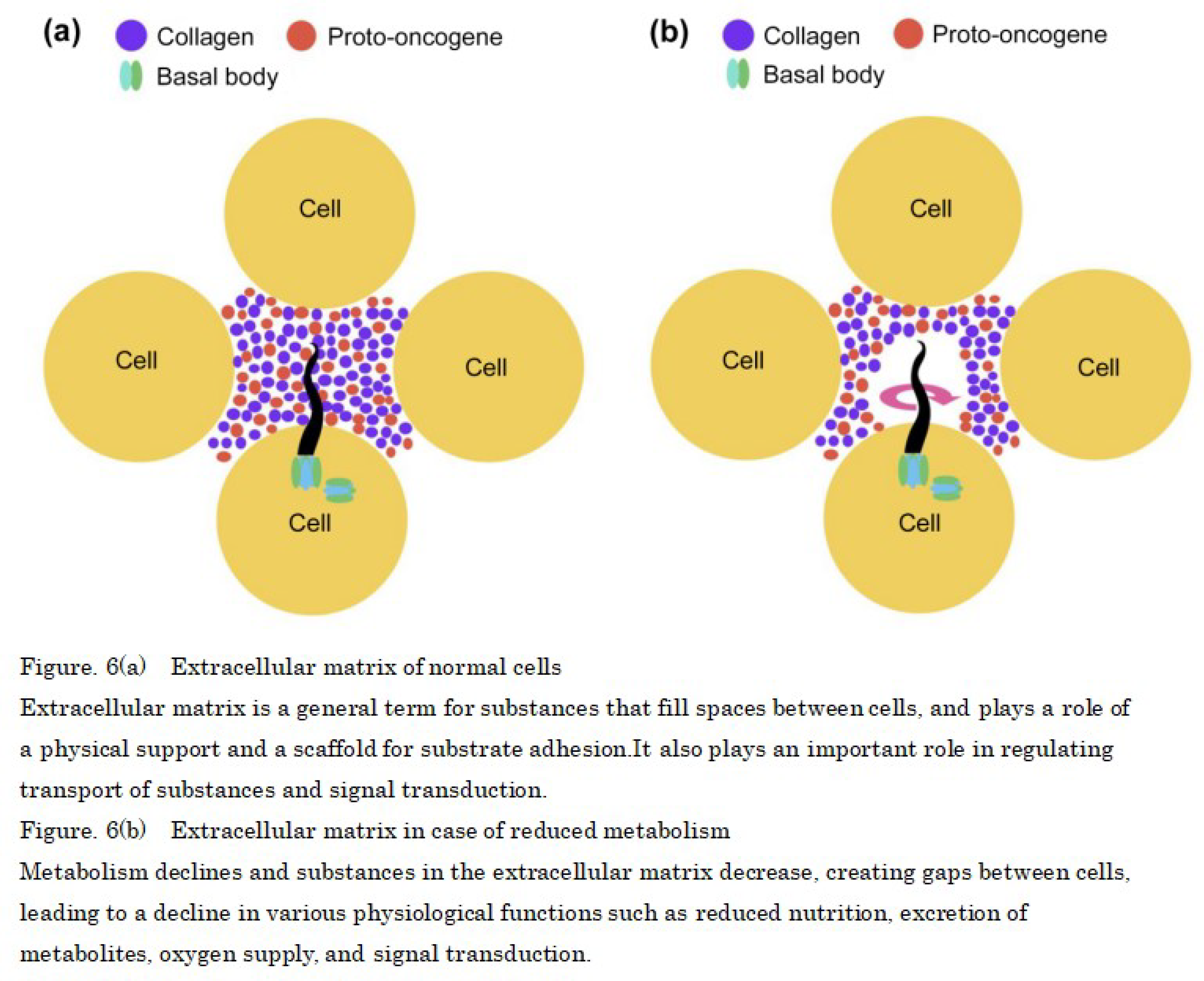
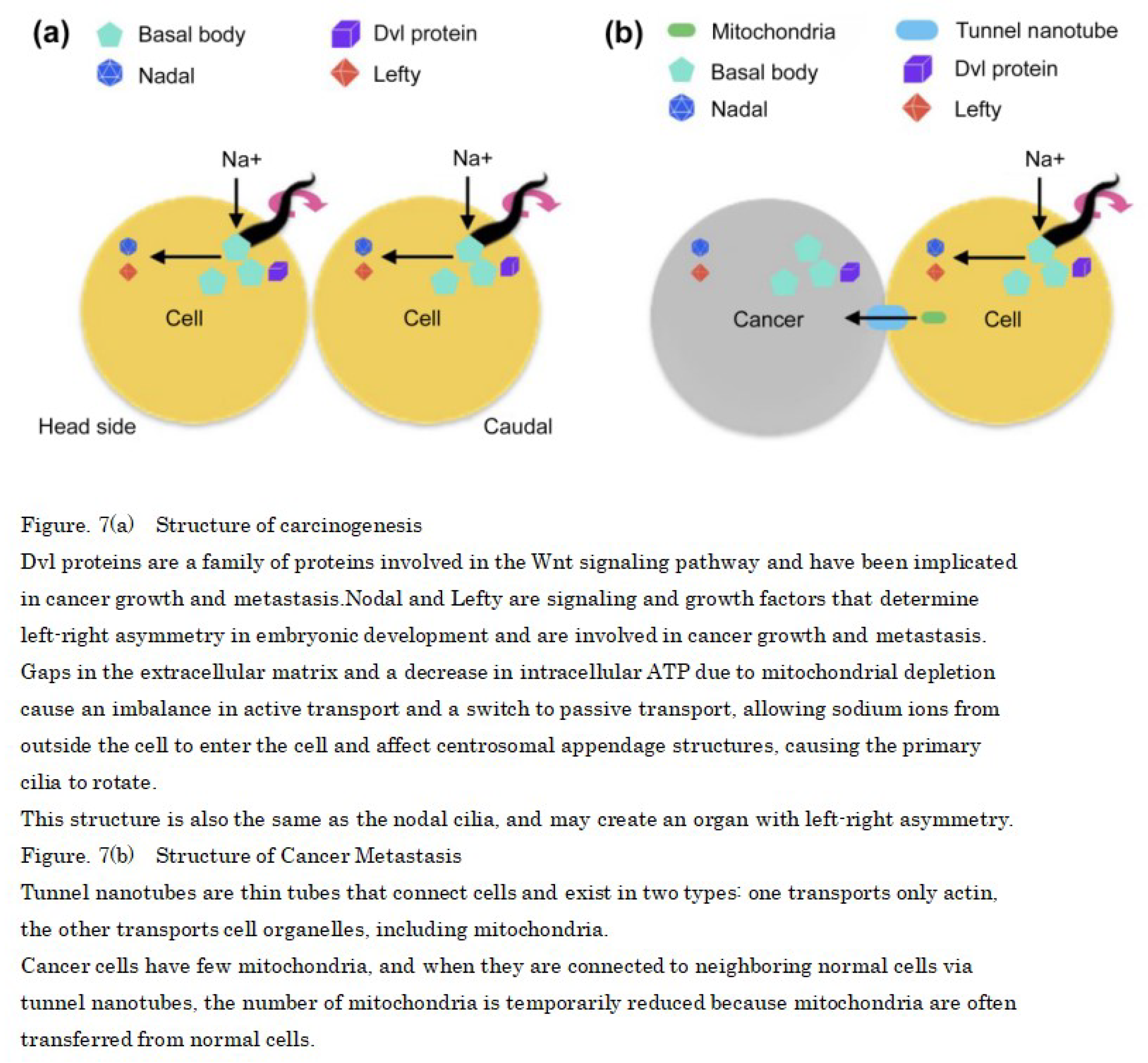
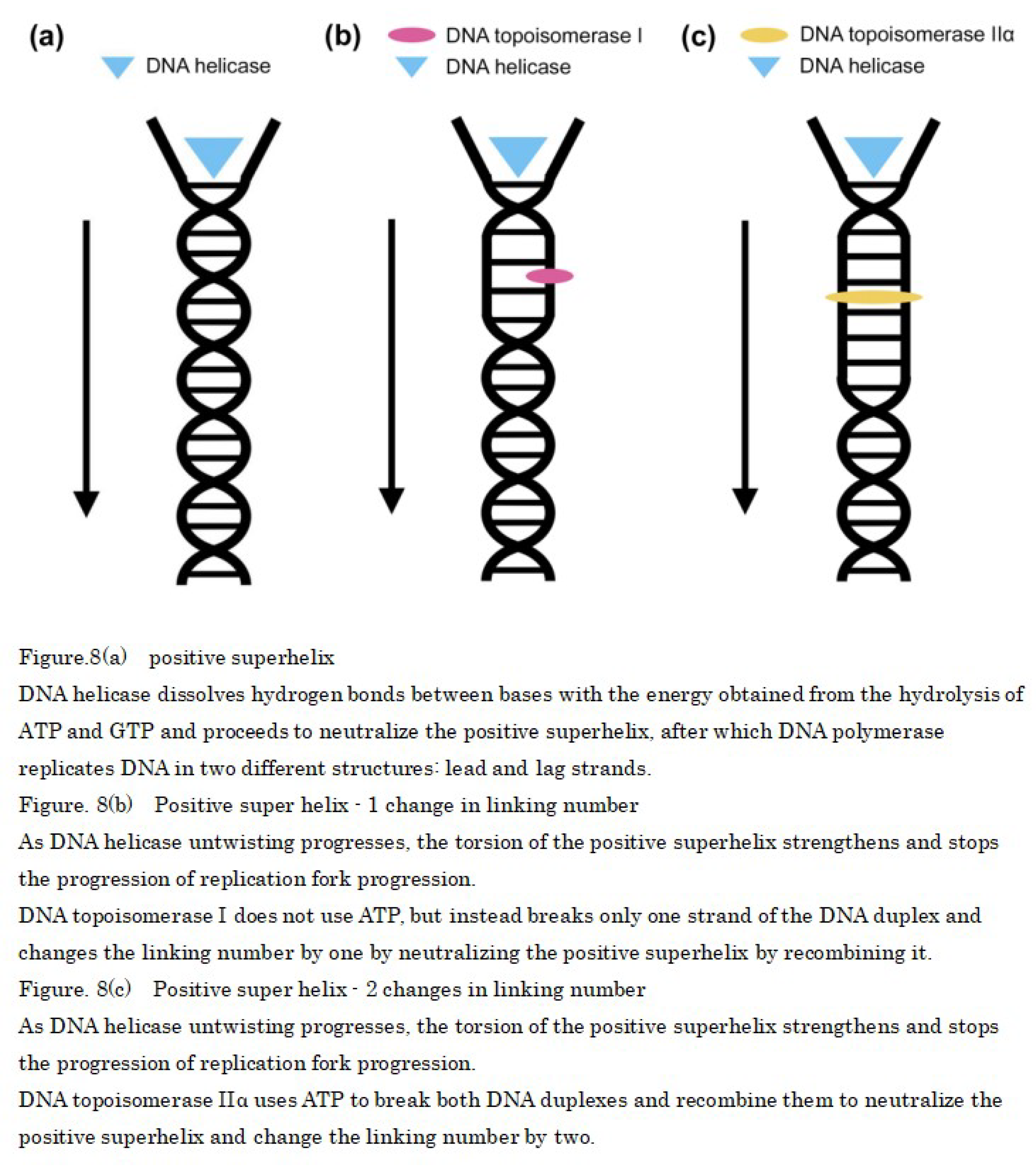
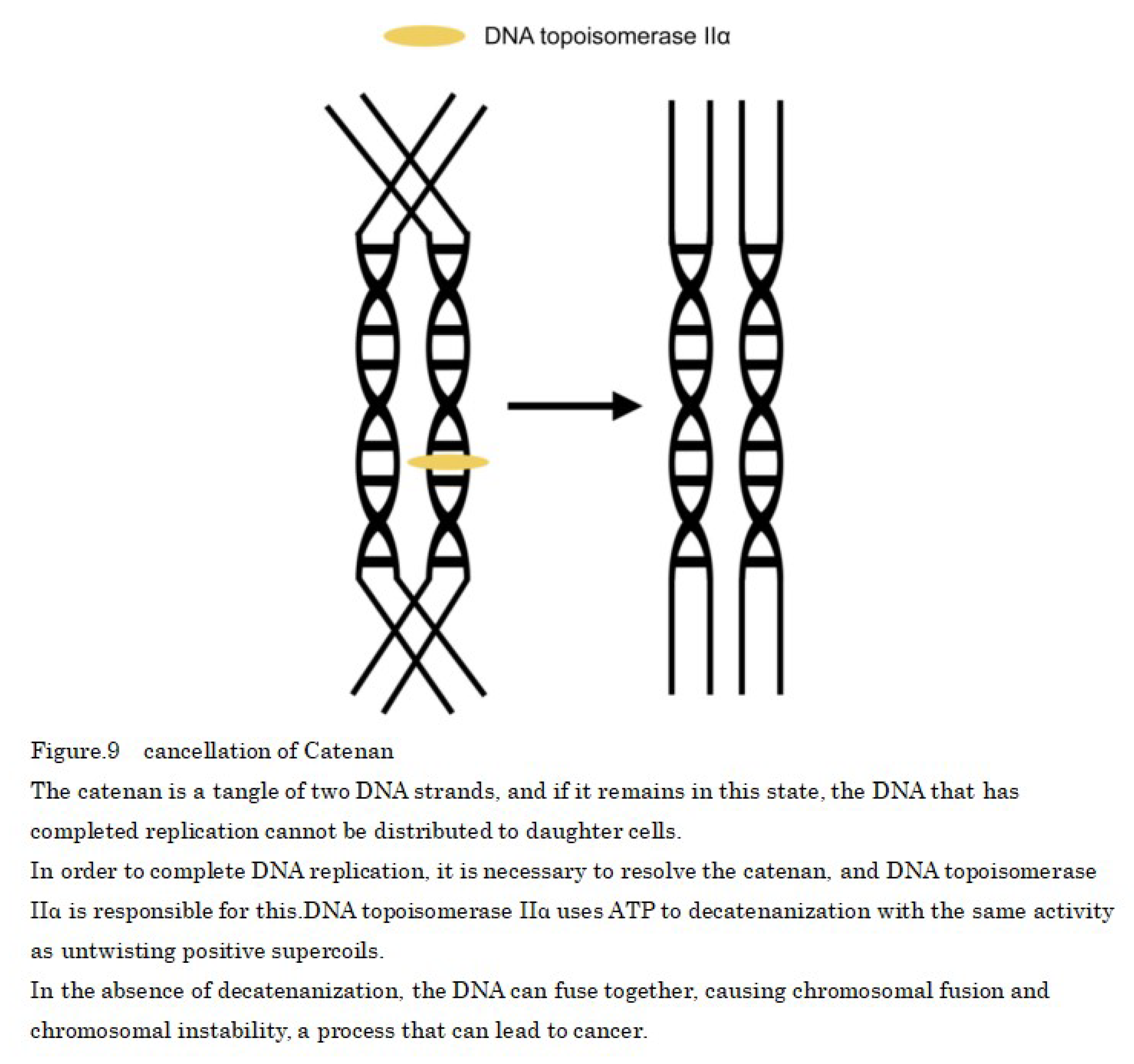
Conclusion
Competing interests
Funding
Acknowledgements
References
- Nichols, C.A.; Gibson, W.J.; Brown, M.S.; Kosmicki, J.A.; Busanovich, J.P.; Wei, H.; Urbanski, L.M.; Curimjee, N.; Berger, A.C.; Gao, G.F.; et al. Loss of heterozygosity of essential genes represents a widespread class of potential cancer vulnerabilities. Nat. Commun. 2020, 11, 2517. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Wu, C.-F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell. Physiol. Biochem. 2018, 51, 2647–2693. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Baart, V.M.; Houvast, R.D.; de Geus-Oei, L.F.; Quax, P.H.A.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Sier, C.F.M. Molecular imaging of the urokinase plasminogen activator receptor: opportunities beyond cancer. EJNMMI Res. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Zhang, H.; Menzies, K.J.; Auwerx, J. The role of mitochondria in stem cell fate and aging. Development 2018, 145, dev143420. [Google Scholar] [CrossRef] [PubMed]
- van der Reest, J.; Cecchino, G.N.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2017, 592, 728–742. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Tamrakar, P.; Rosenthal, R.E.; Fiskum, G. Calcium uptake and cytochrome c release from normal and ischemic brain mitochondria. Neurochem. Int. 2018, 117, 15–22. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Bazylianska, V.; Kalpage, H.A.; Wan, J.; Vaishnav, A.; Mahapatra, G.; Turner, A.A.; Chowdhury, D.D.; Kim, K.; Morse, P.T.; Lee, I.; et al. Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis. Cells 2021, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Rong, N.; Mistriotis, P.; Wang, X.; Tseropoulos, G.; Rajabian, N.; Zhang, Y.; Wang, J.; Liu, S.; Andreadis, S.T. Restoring extracellular matrix synthesis in senescent stem cells. FASEB J. 2019, 33, 10954–10965. [Google Scholar] [CrossRef]
- Higgins, M.; Obaidi, I.; McMorrow, T. Primary cilia and their role in cancer (Review). Oncol. Lett. 2019, 17, 3041–3047. [Google Scholar] [CrossRef]
- Cao, M.; Zou, X.; Li, C.; Lin, Z.; Wang, N.; Zou, Z.; Ye, Y.; Seemann, J.; Levine, B.; Tang, Z.; et al. An actin filament branching surveillance system regulates cell cycle progression, cytokinesis and primary ciliogenesis. Nat. Commun. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Daggubati V, Vykunta A, Choudhury A, et al. Hedgehog target genes regulate lipid metabolism to drive basal cell carcinoma and medulloblastoma.Research Square. 2023;1.
- Kim, D.H.; Ahn, J.S.; Han, H.J.; Kim, H.-M.; Hwang, J.; Lee, K.H.; Cha-Molstad, H.; Ryoo, I.-J.; Jang, J.-H.; Ko, S.-K.; et al. Cep131 overexpression promotes centrosome amplification and colon cancer progression by regulating Plk4 stability. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Hoffmann, I. Role of Polo-like Kinases Plk1 and Plk4 in the Initiation of Centriole Duplication—Impact on Cancer. Cells 2022, 11, 786. [Google Scholar] [CrossRef]
- Wu, Q.; Li, B.; Liu, L.; Sun, S.; Sun, S. Centrosome dysfunction: a link between senescence and tumor immunity. Signal Transduct. Target. Ther. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Adachi N. koutou sinkaku seibutu no ni gata DNA topoisomera-ze no kouzou to kinou[Structure and function of type II DNA topoisomerase from higher eukaryotes]. Seikagaku J Jpn Biochem Soc. 1995;67(1):36–40. Japanese.
- Madabhushi, R. The Roles of DNA Topoisomerase IIβ in Transcription. Int. J. Mol. Sci. 2018, 19, 1917. [Google Scholar] [CrossRef]
- Kumiko, T. Temperature-dependent gene expression in hyperthermia. Japanese journal of health & research. 2018;39:46-53. [CrossRef]
- Mielczarek-Lewandowska, A.; Hartman, M.L.; Czyz, M. Inhibitors of HSP90 in melanoma. Apoptosis 2019, 25, 12–28. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 379–397. [Google Scholar] [CrossRef]
- Chen, W. Thermometry and interpretation of body temperature. Biomed. Eng. Lett. 2019, 9, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ichise, H.; Tsukamoto, S.; Hirashima, T.; Konishi, Y.; Oki, C.; Tsukiji, S.; Iwano, S.; Miyawaki, A.; Sumiyama, K.; Terai, K.; et al. Functional visualization of NK cell-mediated killing of metastatic single tumor cells. eLife 2022, 11. [Google Scholar] [CrossRef]
- Glenn, A.; Armstrong, C.E. Physiology of red and white blood cells. Anaesth. Intensiv. Care Med. 2019, 20, 170–174. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.-E.; Hwang, H.-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Krapf, R. Phosphate intake, hyperphosphatemia, and kidney function. Pfl?gers Arch. Eur. J. Physiol. 2022, 474, 935–947. [Google Scholar] [CrossRef]
- Rajkumar, P.; Pluznick, J.L. Acid-base regulation in the renal proximal tubules: using novel pH sensors to maintain homeostasis. Am. J. Physiol. Physiol. 2018, 315, F1187–F1190. [Google Scholar] [CrossRef]
- Matzke, N.J.; Lin, A.; Stone, M.; Baker, M.A.B. Flagellar export apparatus and ATP synthetase: Homology evidenced by synteny predating the Last Universal Common Ancestor. BioEssays 2021, 43, 2100004. [Google Scholar] [CrossRef]
- Guo, H.; Suzuki, T.; Rubinstein, J.L. ; Japan Structure of a bacterial ATP synthase. eLife 2019, 8. [Google Scholar] [CrossRef]
- Nakamura, S.; Minamino, T. Flagella-Driven Motility of Bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Shiozawa, K.; Katoh, T.A.; Minegishi, K.; Ide, T.; Ikawa, Y.; Nishimura, H.; Takaoka, K.; Itabashi, T.; Iwane, A.H.; et al. Role of Ca 2+ transients at the node of the mouse embryo in breaking of left-right symmetry. Sci. Adv. 2020, 6, eaba1195. [Google Scholar] [CrossRef]
- Little, R.B.; Norris, D.P. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021, 110, 11–18. [Google Scholar] [CrossRef]
- Szenker-Ravi, E.; Ott, T.; Khatoo, M.; de Bellaing, A.M.; Goh, W.X.; Chong, Y.L.; Beckers, A.; Kannesan, D.; Louvel, G.; Anujan, P.; et al. Discovery of a genetic module essential for assigning left–right asymmetry in humans and ancestral vertebrates. Nat. Genet. 2021, 54, 62–72. [Google Scholar] [CrossRef]
- Petri, N.D. Evolutionary Diversity of the Mechanisms Providing the Establishment of Left-Right Asymmetry in Metazoans. Russ. J. Dev. Biol. 2020, 51, 84–98. [Google Scholar] [CrossRef]
- Ide, T.; Twan, W.K.; Lu, H.; Ikawa, Y.; Lim, L.-X.; Henninger, N.; Nishimura, H.; Takaoka, K.; Narasimhan, V.; Yan, X.; et al. CFAP53 regulates mammalian cilia-type motility patterns through differential localization and recruitment of axonemal dynein components. PLOS Genet. 2020, 16, e1009232. [Google Scholar] [CrossRef]
- Xie, H.; Kang, Y.; Liu, J.; Huang, M.; Dai, Z.; Shi, J.; Wang, S.; Li, L.; Li, Y.; Zheng, P.; et al. Ependymal polarity defects coupled with disorganized ciliary beating drive abnormal cerebrospinal fluid flow and spine curvature in zebrafish. PLOS Biol. 2023, 21, e3002008. [Google Scholar] [CrossRef]
- Mehmet V. Cell-Extracellular Matrix Adhesion Assay. Epidermal Cells. 2019;2109:209-217. [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Mei, J.; Yang, X.; Xia, D.; Zhou, W.; Gu, D.; Wang, H.; Liu, C. Systematic summarization of the expression profiles and prognostic roles of the dishevelled gene family in hepatocellular carcinoma. Mol. Genet. Genom. Med. 2020, 8, e1384. [Google Scholar] [CrossRef]
- Gambichler, T.; Ardabili, S.; Lang, K.; Dreißigacker, M.; Scheel, C.; Brand-Saberi, B.; Skrygan, M.; Stockfleth, E.; Käfferlein, H.; Brüning, T.; et al. Expression of Lefty predicts Merkel cell carcinoma-specific death. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Hekmatshoar, Y.; Nakhle, J.; Galloni, M.; Vignais, M.-L. The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem. J. 2018, 475, 2305–2328. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 1–18. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Dröge, P. Chromatin Architectural Factors as Safeguards against Excessive Supercoiling during DNA Replication. Int. J. Mol. Sci. 2020, 21, 4504. [Google Scholar] [CrossRef] [PubMed]
- Spakman, D.; Bakx, J.A.M.; Biebricher, A.S.; Peterman, E.J.G.; Wuite, G.J.L.; A King, G. Unravelling the mechanisms of Type 1A topoisomerases using single-molecule approaches. Nucleic Acids Res. 2021, 49, 5470–5492. [Google Scholar] [CrossRef]
- Soren, B.C.; Dasari, J.B.; Ottaviani, A.; Lacovelli, F.; Fiorani, P. Topoisomerase IB: a relaxing enzyme for stressed DNA. Cancer Drug Resist. 2019, 3, 18–25. [Google Scholar] [CrossRef]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. BioEssays 2021, 43, 2000286–e2000286. [Google Scholar] [CrossRef]
- Karol S, Arkadi M, Heather M R. Topoisomerase II contributes to DNA secondary structure-mediated double-stranded breaks. Nucleic Acids Research. 2020;48(12):6654–6671. [CrossRef]
- Broeck, A.V.; Lotz, C.; Drillien, R.; Haas, L.; Bedez, C.; Lamour, V. Structural basis for allosteric regulation of Human Topoisomerase IIα. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Miklós S, János G. Chapter 23 - Thermoregulation and age. Handbook of Clinical Neurology. 2018;156:377-395. [CrossRef]
- Geneva, I.I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis. 2019, 6, ofz032. [Google Scholar] [CrossRef]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Liu, B. Microtubule nucleation for the assembly of acentrosomal microtubule arrays in plant cells. New Phytol. 2019, 222, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



